The Role of the Genital Tract Microbiome in Human Fertility: A Literature Review
Abstract
:1. Introduction
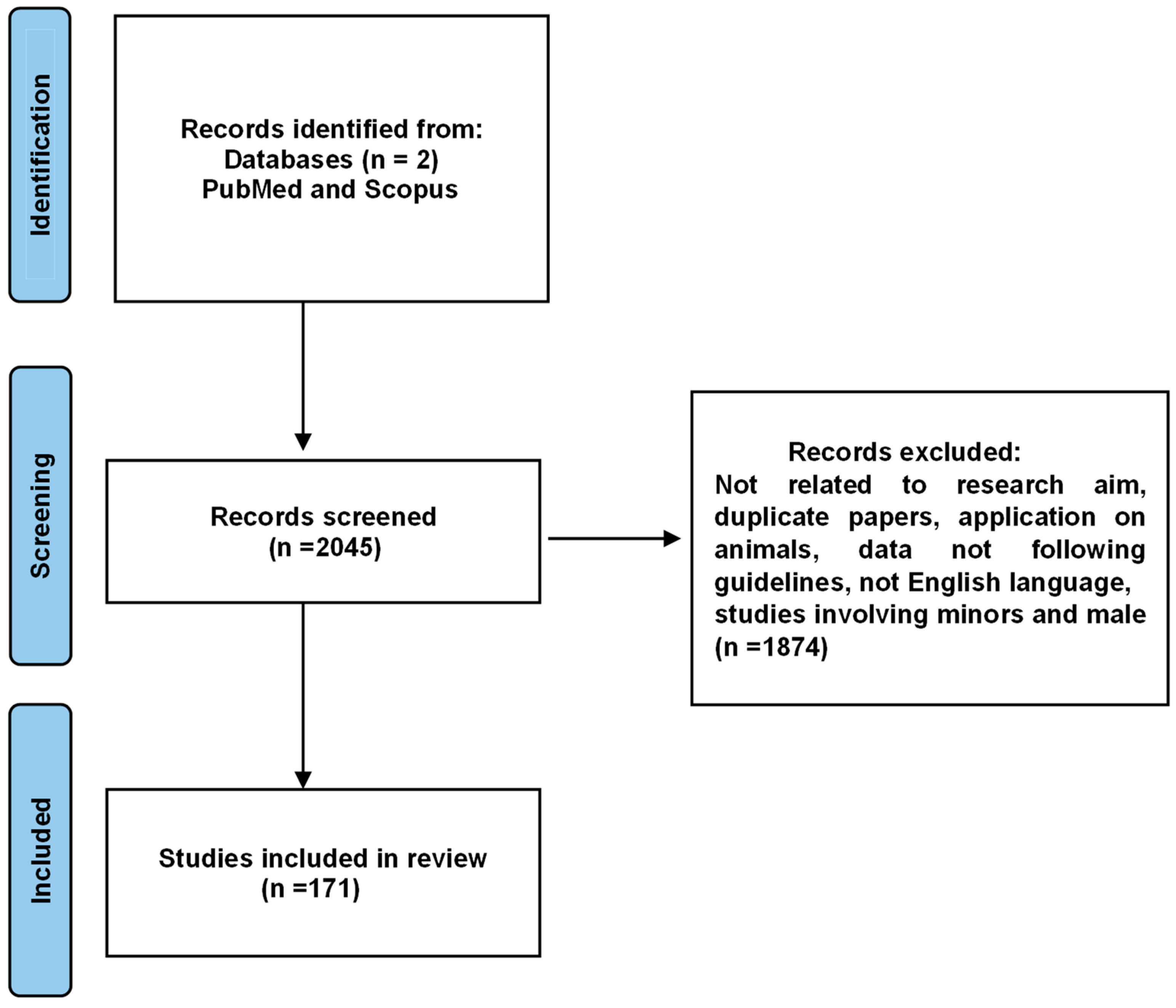
2. Materials and Methods
3. Results
3.1. Composition and Characteristics of the Genital Microbiome in Women of Childbearing Age
3.2. Impact of the Genital Tract Microbiome on Infertility
- Homogeneous, thin, grayish-white vaginal discharge: The discharge should be thin and uniform in appearance, often with an off-white or grayish color.
- pH of the vaginal discharge > 4.5: Normal vaginal pH is typically between 3.8 and 4.5, and a pH greater than 4.5 is indicative of bacterial vaginosis.
- Positive “whiff” test: A strong, fishy odor is released when a small amount of vaginal discharge is mixed with 10% potassium hydroxide (KOH). This odor is due to the production of amines by the anaerobic bacteria associated with BV.
3.3. Impact of Genital Tract Microbiome on Assisted Reproductive Technology
4. Discussion
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Hara, A.M.; Shanahan, F. The Gut Flora as a Forgotten Organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Venneri, M.A.; Franceschini, E.; Sciarra, F.; Rosato, E.; D’Ettorre, G.; Lenzi, A. Human Genital Tracts Microbiota: Dysbiosis Crucial for Infertility. J. Endocrinol. Investig. 2022, 45, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Magill, R.G.; MacDonald, S.M. Male Infertility and the Human Microbiome. Front. Reprod. Health 2023, 5, 1166201. [Google Scholar] [CrossRef] [PubMed]
- Infertility. Available online: https://www.who.int/news-room/fact-sheets/detail/infertility (accessed on 17 March 2025).
- Alimena, S.; Davis, J.; Fichorova, R.N.; Feldman, S. The Vaginal Microbiome: A Complex Milieu Affecting Risk of Human Papillomavirus Persistence and Cervical Cancer. Curr. Probl. Cancer 2022, 46, 100877. [Google Scholar] [CrossRef]
- Balla, B.; Illés, A.; Tobiás, B.; Pikó, H.; Beke, A.; Sipos, M.; Lakatos, P.; Kósa, J.P. The Role of the Vaginal and Endometrial Microbiomes in Infertility and Their Impact on Pregnancy Outcomes in Light of Recent Literature. Int. J. Mol. Sci. 2024, 25, 13227. [Google Scholar] [CrossRef]
- Gullo, G.; Basile, G.; Cucinella, G.; Greco, M.E.; Perino, A.; Chiantera, V.; Marinelli, S. Fresh vs. Frozen Embryo Transfer in Assisted Reproductive Techniques: A Single Center Retrospective Cohort Study and Ethical-Legal Implications. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 6809–6823. [Google Scholar] [CrossRef]
- Sciorio, R.; De Paola, L.; Notari, T.; Ganduscio, S.; Amato, P.; Crifasi, L.; Marotto, D.; Billone, V.; Cucinella, G.; Perino, A.; et al. Decoding the Puzzle of Male Infertility: The Role of Infection, Inflammation, and Autoimmunity. Diagnostics 2025, 15, 547. [Google Scholar] [CrossRef]
- Cucinella, G.; Gullo, G.; Catania, E.; Perino, A.; Billone, V.; Marinelli, S.; Napoletano, G.; Zaami, S. Stem Cells and Infertility: A Review of Clinical Applications and Legal Frameworks. J. Pers. Med. 2024, 14, 135. [Google Scholar] [CrossRef]
- Xiao, L.; Zuo, Z.; Zhao, F. Microbiome in Female Reproductive Health: Implications for Fertility and Assisted Reproductive Technologies. Genom. Proteom. Bioinform. 2024, 22, qzad005. [Google Scholar] [CrossRef]
- Claudiac Recent Insights into the Vaginal Microbiota. Available online: https://www.microbiotajournal.com/article/771 (accessed on 17 March 2025).
- Gullo, G.; Scaglione, M.; Laganà, A.S.; Perino, A.; Andrisani, A.; Chiantera, V.; Cucinella, G.; Gitas, G.; Barra, F.; Riemma, G. Assisted Reproductive Techniques and Risk of Congenital Heart Diseases in Children: A Systematic Review and Meta-Analysis. Reprod. Sci. 2023, 30, 2896–2906. [Google Scholar] [CrossRef]
- Gullo, G.; Scaglione, M.; Cucinella, G.; Perino, A.; Chiantera, V.; D’Anna, R.; Laganà, A.S.; Buzzaccarini, G. Impact of Assisted Reproduction Techniques on the Neuro-Psycho-Motor Outcome of Newborns: A Critical Appraisal. J. Obstet. Gynaecol. 2022, 42, 2583–2587. [Google Scholar] [CrossRef]
- Vergallo, G.M.; Marinelli, S.; Napoletano, G.; De Paola, L.; Treglia, M.; Zaami, S.; Frati, P. 20 Years Since the Enactment of Italian Law No. 40/2004 on Medically Assisted Procreation: How It Has Changed and How It Could Change. Int. J. Environ. Res. Public Health 2025, 22, 296. [Google Scholar] [CrossRef] [PubMed]
- European IVF Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE); Smeenk, J.; Wyns, C.; De Geyter, C.; Kupka, M.; Bergh, C.; Cuevas Saiz, I.; De Neubourg, D.; Rezabek, K.; Tandler-Schneider, A.; et al. ART in Europe, 2019: Results Generated from European Registries by ESHRE. Hum. Reprod. 2023, 38, 2321–2338. [Google Scholar] [CrossRef] [PubMed]
- Tester, R.; Al-Ghazzewi, F.H. Intrinsic and Extrinsic Carbohydrates in the Vagina: A Short Review on Vaginal Glycogen. Int. J. Biol. Macromol. 2018, 112, 203–206. [Google Scholar] [CrossRef]
- France, M.; Alizadeh, M.; Brown, S.; Ma, B.; Ravel, J. Towards a Deeper Understanding of the Vaginal Microbiota. Nat. Microbiol. 2022, 7, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Günther, V.; Allahqoli, L.; Watrowski, R.; Maass, N.; Ackermann, J.; von Otte, S.; Alkatout, I. Vaginal Microbiome in Reproductive Medicine. Diagnostics 2022, 12, 1948. [Google Scholar] [CrossRef]
- Freitas, A.C.; Bocking, A.; Hill, J.E.; Money, D.M.; VOGUE Research Group. Increased Richness and Diversity of the Vaginal Microbiota and Spontaneous Preterm Birth. Microbiome 2018, 6, 117. [Google Scholar] [CrossRef]
- Delgado-Diaz, D.J.; Tyssen, D.; Hayward, J.A.; Gugasyan, R.; Hearps, A.C.; Tachedjian, G. Distinct Immune Responses Elicited from Cervicovaginal Epithelial Cells by Lactic Acid and Short Chain Fatty Acids Associated with Optimal and Non-Optimal Vaginal Microbiota. Front. Cell Infect. Microbiol. 2019, 9, 446. [Google Scholar] [CrossRef]
- Punzón-Jiménez, P.; Labarta, E. The Impact of the Female Genital Tract Microbiome in Women Health and Reproduction: A Review. J. Assist. Reprod. Genet. 2021, 38, 2519–2541. [Google Scholar] [CrossRef]
- Wee, B.A.; Thomas, M.; Sweeney, E.L.; Frentiu, F.D.; Samios, M.; Ravel, J.; Gajer, P.; Myers, G.; Timms, P.; Allan, J.A.; et al. A Retrospective Pilot Study to Determine Whether the Reproductive Tract Microbiota Differs between Women with a History of Infertility and Fertile Women. Aust. N. Z. J. Obstet. Gynaecol. 2018, 58, 341–348. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The Microbiota Continuum along the Female Reproductive Tract and Its Relation to Uterine-Related Diseases. Nat. Commun. 2017, 8, 875. [Google Scholar] [CrossRef] [PubMed]
- Pelzer, E.S.; Willner, D.; Buttini, M.; Huygens, F. A Role for the Endometrial Microbiome in Dysfunctional Menstrual Bleeding. Antonie Van Leeuwenhoek 2018, 111, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Winters, A.D.; Romero, R.; Gervasi, M.T.; Gomez-Lopez, N.; Tran, M.R.; Garcia-Flores, V.; Pacora, P.; Jung, E.; Hassan, S.S.; Hsu, C.-D.; et al. Does the Endometrial Cavity Have a Molecular Microbial Signature? Sci. Rep. 2019, 9, 9905. [Google Scholar] [CrossRef]
- Baker, J.M.; Chase, D.M.; Herbst-Kralovetz, M.M. Uterine Microbiota: Residents, Tourists, or Invaders? Front. Immunol. 2018, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Toson, B.; Simon, C.; Moreno, I. The Endometrial Microbiome and Its Impact on Human Conception. Int. J. Mol. Sci. 2022, 23, 485. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Haick, A.; Nkwopara, E.; Garcia, R.; Rendi, M.; Agnew, K.; Fredricks, D.N.; Eschenbach, D. Colonization of the Upper Genital Tract by Vaginal Bacterial Species in Nonpregnant Women. Am. J. Obstet. Gynecol. 2015, 212, 611.e1–611.e9. [Google Scholar] [CrossRef]
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence That the Endometrial Microbiota Has an Effect on Implantation Success or Failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703. [Google Scholar] [CrossRef]
- Sirota, I.; Zarek, S.M.; Segars, J.H. Potential Influence of the Microbiome on Infertility and Assisted Reproductive Technology. Semin. Reprod. Med. 2014, 32, 35–42. [Google Scholar] [CrossRef]
- Hawes, S.E.; Hillier, S.L.; Benedetti, J.; Stevens, C.E.; Koutsky, L.A.; Wolner-Hanssen, P.; Holmes, K.K. Hydrogen Peroxide-Producing Lactobacilli and Acquisition of Vaginal Infections. J. Infect. Dis. 1996, 174, 1058–1063. [Google Scholar] [CrossRef]
- Aroutcheva, A.A.; Simoes, J.A.; Behbakht, K.; Faro, S. Gardnerella Vaginalis Isolated from Patients with Bacterial Vaginosis and from Patients with Healthy Vaginal Ecosystems. Clin. Infect. Dis. 2001, 33, 1022–1027. [Google Scholar] [CrossRef]
- Ng, S.C.; Hart, A.L.; Kamm, M.A.; Stagg, A.J.; Knight, S.C. Mechanisms of Action of Probiotics: Recent Advances. Inflamm. Bowel Dis. 2009, 15, 300–310. [Google Scholar] [CrossRef]
- Moreno, I.; Garcia-Grau, I.; Perez-Villaroya, D.; Gonzalez-Monfort, M.; Bahçeci, M.; Barrionuevo, M.J.; Taguchi, S.; Puente, E.; Dimattina, M.; Lim, M.W.; et al. Endometrial Microbiota Composition Is Associated with Reproductive Outcome in Infertile Patients. Microbiome 2022, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, C.A.; Amsel, R.; Eschenbach, D.; Schoenknecht, F.; Holmes, K.K. Anaerobic Bacteria in Nonspecific Vaginitis. N. Engl. J. Med. 1980, 303, 601–607. [Google Scholar] [CrossRef]
- Leitich, H.; Kiss, H. Asymptomatic Bacterial Vaginosis and Intermediate Flora as Risk Factors for Adverse Pregnancy Outcome. Best. Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Leitich, H.; Bodner-Adler, B.; Brunbauer, M.; Kaider, A.; Egarter, C.; Husslein, P. Bacterial Vaginosis as a Risk Factor for Preterm Delivery: A Meta-Analysis. Am. J. Obstet. Gynecol. 2003, 189, 139–147. [Google Scholar] [CrossRef]
- Paladine, H.L.; Desai, U.A. Vaginitis: Diagnosis and Treatment. Am. Fam. Physician 2018, 97, 321–329. [Google Scholar]
- Spiegel, C.A.; Amsel, R.; Holmes, K.K. Diagnosis of Bacterial Vaginosis by Direct Gram Stain of Vaginal Fluid. J. Clin. Microbiol. 1983, 18, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Amsel, R.; Totten, P.A.; Spiegel, C.A.; Chen, K.C.; Eschenbach, D.; Holmes, K.K. Nonspecific Vaginitis. Diagnostic Criteria and Microbial and Epidemiologic Associations. Am. J. Med. 1983, 74, 14–22. [Google Scholar] [CrossRef]
- Challa, A.; Sood, S.; Kachhawa, G.; Upadhyay, A.D.; Dwivedi, S.N.; Gupta, S. Diagnostic Concordance between Amsel’s Criteria and the Nugent Scoring Method in the Assessment of Bacterial Vaginosis. Sex. Health 2022, 18, 512–514. [Google Scholar] [CrossRef]
- Borges, S.; Silva, J.; Teixeira, P. The Role of Lactobacilli and Probiotics in Maintaining Vaginal Health. Arch. Gynecol. Obstet. 2014, 289, 479–489. [Google Scholar] [CrossRef]
- Shipitsyna, E.; Roos, A.; Datcu, R.; Hallén, A.; Fredlund, H.; Jensen, J.; Engstrand, L.; Unemo, M. Composition of the Vaginal Microbiota in Women of Reproductive Age—Sensitive and Specific Molecular Diagnosis of Bacterial Vaginosis Is Possible? PLoS ONE 2013, 8, e60670. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Loening-Baucke, V.; Mendling, W.; Dörffel, Y.; Schilling, J.; Halwani, Z.; Jiang, X.; Verstraelen, H.; Swidsinski, S. Infection through Structured Polymicrobial Gardnerella Biofilms (StPM-GB). Histol. Histopathol. 2014, 29, 567–587. [Google Scholar] [CrossRef]
- García-Velasco, J.A.; Menabrito, M.; Catalán, I.B. What Fertility Specialists Should Know about the Vaginal Microbiome: A Review. Reprod. Biomed. Online 2017, 35, 103–112. [Google Scholar] [CrossRef]
- Machado, A.; Cerca, N. Influence of Biofilm Formation by Gardnerella Vaginalis and Other Anaerobes on Bacterial Vaginosis. J. Infect. Dis. 2015, 212, 1856–1861. [Google Scholar] [CrossRef]
- Su, W.; Gong, C.; Zhong, H.; Yang, H.; Chen, Y.; Wu, X.; Jin, J.; Xi, H.; Zhao, J. Vaginal and Endometrial Microbiome Dysbiosis Associated with Adverse Embryo Transfer Outcomes. Reprod. Biol. Endocrinol. 2024, 22, 111. [Google Scholar] [CrossRef] [PubMed]
- Zaami, S. Assisted Heterologous Fertilization and the Right of Donorconceived Children to Know Their Biological Origins. Clin. Ter. 2018, 169, e39–e43. [Google Scholar] [CrossRef]
- Koedooder, R.; Singer, M.; Schoenmakers, S.; Savelkoul, P.H.M.; Morré, S.A.; de Jonge, J.D.; Poort, L.; Cuypers, W.-J.S.S.; Budding, A.E.; Laven, J.S.E.; et al. The ReceptIVFity Cohort Study Protocol to Validate the Urogenital Microbiome as Predictor for IVF or IVF/ICSI Outcome. Reprod. Health 2018, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Brosens, J.J.; Salker, M.S.; Teklenburg, G.; Nautiyal, J.; Salter, S.; Lucas, E.S.; Steel, J.H.; Christian, M.; Chan, Y.-W.; Boomsma, C.M.; et al. Uterine Selection of Human Embryos at Implantation. Sci. Rep. 2014, 4, 3894. [Google Scholar] [CrossRef]
- Templeton, A.; Morris, J.K.; Parslow, W. Factors That Affect Outcome of In-Vitro Fertilisation Treatment. Lancet 1996, 348, 1402–1406. [Google Scholar] [CrossRef]
- Haahr, T.; Jensen, J.S.; Thomsen, L.; Duus, L.; Rygaard, K.; Humaidan, P. Abnormal Vaginal Microbiota May Be Associated with Poor Reproductive Outcomes: A Prospective Study in IVF Patients. Hum. Reprod. 2016, 31, 795–803. [Google Scholar] [CrossRef]
- Graspeuntner, S.; Bohlmann, M.K.; Gillmann, K.; Speer, R.; Kuenzel, S.; Mark, H.; Hoellen, F.; Lettau, R.; Griesinger, G.; König, I.R.; et al. Microbiota-Based Analysis Reveals Specific Bacterial Traits and a Novel Strategy for the Diagnosis of Infectious Infertility. PLoS ONE 2018, 13, e0191047. [Google Scholar] [CrossRef]
- Kyono, K.; Hashimoto, T.; Nagai, Y.; Sakuraba, Y. Analysis of Endometrial Microbiota by 16S Ribosomal RNA Gene Sequencing among Infertile Patients: A Single-Center Pilot Study. Reprod. Med. Biol. 2018, 17, 297–306. [Google Scholar] [CrossRef]
- Mangot-Bertrand, J.; Fenollar, F.; Bretelle, F.; Gamerre, M.; Raoult, D.; Courbiere, B. Molecular Diagnosis of Bacterial Vaginosis: Impact on IVF Outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 535–541. [Google Scholar] [CrossRef]
- Bernabeu, A.; Lledo, B.; Díaz, M.C.; Lozano, F.M.; Ruiz, V.; Fuentes, A.; Lopez-Pineda, A.; Moliner, B.; Castillo, J.C.; Ortiz, J.A.; et al. Effect of the Vaginal Microbiome on the Pregnancy Rate in Women Receiving Assisted Reproductive Treatment. J. Assist. Reprod. Genet. 2019, 36, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Kyono, K. Does Dysbiotic Endometrium Affect Blastocyst Implantation in IVF Patients? J. Assist. Reprod. Genet. 2019, 36, 2471–2479. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, H.; Zhang, C.; Zhang, S. Chronic Endometritis and the Endometrial Microbiota: Implications for Reproductive Success in Patients with Recurrent Implantation Failure. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 49. [Google Scholar] [CrossRef]
- Gullo, G.; Scaglione, M.; Cucinella, G.; Riva, A.; Coldebella, D.; Cavaliere, A.F.; Signore, F.; Buzzaccarini, G.; Spagnol, G.; Laganà, A.S.; et al. Congenital Zika Syndrome: Genetic Avenues for Diagnosis and Therapy, Possible Management and Long-Term Outcomes. J. Clin. Med. 2022, 11, 1351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, C.; Narsing Rao, M.P.; Rafiq, M.; Luo, G.; Li, S.; Kang, Y.-Q. Effects of Vaginal Microbiota on in Vitro Fertilization Outcomes in Women with Different Infertility Causes. Microbiol. Spectr. 2025, 13, e0125524. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhang, X.; Liang, Y.; Lin, S.; Qian, W.; Fan, S. Alterations in Vaginal Microbiota and Associated Metabolome in Women with Recurrent Implantation Failure. mBio 2020, 11, e03242-19. [Google Scholar] [CrossRef]
- Haahr, T.; Humaidan, P.; Elbaek, H.O.; Alsbjerg, B.; Laursen, R.J.; Rygaard, K.; Johannesen, T.B.; Andersen, P.S.; Ng, K.L.; Jensen, J.S. Vaginal Microbiota and In Vitro Fertilization Outcomes: Development of a Simple Diagnostic Tool to Predict Patients at Risk of a Poor Reproductive Outcome. J. Infect. Dis. 2019, 219, 1809–1817. [Google Scholar] [CrossRef]
- Wensel, C.R.; Pluznick, J.L.; Salzberg, S.L.; Sears, C.L. Next-Generation Sequencing: Insights to Advance Clinical Investigations of the Microbiome. J. Clin. Investig. 2022, 132, e154944. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.; Minhas, S.; Jayasena, C.N. Next-Generation Sequencing to Elucidate the Semen Microbiome in Male Reproductive Disorders. Medicina 2023, 60, 25. [Google Scholar] [CrossRef] [PubMed]
- Doroftei, B.; Ilie, O.-D.; Anton, N.; Armeanu, T.; Ilea, C. A Mini-Review Regarding the Clinical Outcomes of In Vitro Fertilization (IVF) Following Pre-Implantation Genetic Testing (PGT)-Next Generation Sequencing (NGS) Approach. Diagnostics 2022, 12, 1911. [Google Scholar] [CrossRef] [PubMed]
- Miloski, B. Opportunities for Artificial Intelligence in Healthcare and in Vitro Fertilization. Fertil. Steril. 2023, 120, 3–7. [Google Scholar] [CrossRef]
- Cheng, X.; Joe, B. Artificial Intelligence in Medicine: Microbiome-Based Machine Learning for Phenotypic Classification. Methods Mol. Biol. 2023, 2649, 281–288. [Google Scholar] [CrossRef]
- Marinelli, S.; De Paola, L.; Stark, M.; Montanari Vergallo, G. Artificial Intelligence in the Service of Medicine: Current Solutions and Future Perspectives, Opportunities, and Challenges. Clin. Ter. 2025, 176, 77–82. [Google Scholar] [CrossRef]
- Jiang, V.S.; Bormann, C.L. Artificial Intelligence in the in Vitro Fertilization Laboratory: A Review of Advancements over the Last Decade. Fertil. Steril. 2023, 120, 17–23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gullo, G.; Satullo, M.; Billone, V.; De Paola, L.; Petousis, S.; Kotlik, Y.; Margioula-Siarkou, C.; Perino, A.; Cucinella, G. The Role of the Genital Tract Microbiome in Human Fertility: A Literature Review. J. Clin. Med. 2025, 14, 2923. https://doi.org/10.3390/jcm14092923
Gullo G, Satullo M, Billone V, De Paola L, Petousis S, Kotlik Y, Margioula-Siarkou C, Perino A, Cucinella G. The Role of the Genital Tract Microbiome in Human Fertility: A Literature Review. Journal of Clinical Medicine. 2025; 14(9):2923. https://doi.org/10.3390/jcm14092923
Chicago/Turabian StyleGullo, Giuseppe, Marini’ Satullo, Valentina Billone, Lina De Paola, Stamatios Petousis, Yuliia Kotlik, Chrysoula Margioula-Siarkou, Antonio Perino, and Gaspare Cucinella. 2025. "The Role of the Genital Tract Microbiome in Human Fertility: A Literature Review" Journal of Clinical Medicine 14, no. 9: 2923. https://doi.org/10.3390/jcm14092923
APA StyleGullo, G., Satullo, M., Billone, V., De Paola, L., Petousis, S., Kotlik, Y., Margioula-Siarkou, C., Perino, A., & Cucinella, G. (2025). The Role of the Genital Tract Microbiome in Human Fertility: A Literature Review. Journal of Clinical Medicine, 14(9), 2923. https://doi.org/10.3390/jcm14092923






