Small Airways Disease as a Novel Target for Mepolizumab in Asthma—The SASAM Prospective Real-Life Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
- -
- Pulmonary function tests and body plethysmography were performed using the Q-Box (COSMED—The Metabolic Company, Rome, Italy) [20];
- -
- -
- Airway inflammatory markers (FeNO); FeNO measurements were performed in accordance with ATS/ERS recommendations at a flow rate of 50 mL/s [22], using a chemiluminescence analyzer (NIOX Flex, Aerocrine AB, Solna, Sweden).
2.2. Study Objectives
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACT | Asthma Control Test |
| AE | Acute Exacerbations |
| BEC | Blood Eosinophil Count |
| BMI | Body Mass Index |
| COVID-19 | Coronavirus disease-19 |
| FEF25-75% | Forced Expiratory Flow between 25% and 75% of vital capacity; |
| FeNO | Fractional Exhaled Nitric Oxide |
| FEV1/FVC | Forced Expiratory Volume in 1 s/Forced Vital Capacity |
| FEV1 | Forced Expiratory Volume in 1 s % of predicted |
| FVC | Forced Vital Capacity |
| ICS/LABA | Inhaled Corticosteroids/Long-Acting Beta Agonists |
| IOS | Impulse Oscillometry |
| IQR | Interquartile Range |
| LAMA | Long-acting Muscarinic Agonists |
| OCS | Oral Corticosteroids |
| mOCS | Maintenance oral corticosteroids |
| PROs | Patient Reported Outcomes |
| R5–R20 | Resistance at 5 Hz and 20 Hz |
| RV/TLC | Residual Volume/Total Lung Capacity |
| RCT | Randomized Clinical Trial |
| SA | Small Airways |
| SAD | Small Airway Disease |
| SD | Standard Deviation |
| SEM | Standard Error of the Mean |
| TLC | Total Lung Capacity |
References
- Chung, K.F. Diagnosis and Management of Severe Asthma. Semin. Respir. Crit. Care Med. 2018, 39, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Israel, E.; Reddel, H.K. Severe and Difficult-to-Treat Asthma in Adults. N. Engl. J. Med. 2017, 377, 965–976. [Google Scholar] [CrossRef]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef]
- Chung, K.F.; Adcock, I.M. Precision medicine for the discovery of treatable mechanisms in severe asthma. Allergy 2019, 74, 1649–1659. [Google Scholar] [CrossRef]
- Siddiqui, S.; Usmani, O.S. Small airways, big challenge: Measuring the unseen? Nat. Med. 2012, 18, 1619–1621. [Google Scholar] [CrossRef] [PubMed]
- Lipworth, B.; Manoharan, A.; Anderson, W. Unlocking the quiet zone: The small airway asthma phenotype. Lancet Respir. Med. 2014, 2, 497–506. [Google Scholar] [CrossRef]
- Usmani, O.S.; Singh, D.; Spinola, M.; Bizzi, A.; Barnes, P.J. The prevalence of small airways disease in adult asthma: A systematic literature review. Respir. Med. 2016, 116, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Carr, T.F.; Altisheh, R.; Zitt, M. Small airways disease and severe asthma. World Allergy Organ. J. 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Bonini, M.; Usmani, O.S. The role of the small airways in the pathophysiology of asthma and chronic obstructive pulmonary disease. Ther. Adv. Respir. Dis. 2015, 9, 281–293. [Google Scholar] [CrossRef]
- McNulty, W.; Usmani, O.S. Techniques of assessing small airways dysfunction. Eur. Clin. Respir. J. 2014, 1, 25898. [Google Scholar] [CrossRef]
- Paredi, P.; Kharitonov, S.A.; Meah, S.; Barnes, P.J.; Usmani, O.S. A novel approach to partition central and peripheral airway nitric oxide. Chest 2014, 145, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wu, F.; Tian, H.; Yang, H.; Zheng, Y.; Deng, Z.; Wang, Z.; Xiao, S.; Wen, X.; Huang, P.; et al. Clinical characteristics of and risk factors for small airway dysfunction detected by impulse oscillometry. Respir. Med. 2021, 190, 106681. [Google Scholar] [CrossRef] [PubMed]
- Postma, D.S.; Brightling, C.; Baldi, S.; Berge, M.V.D.; Fabbri, L.M.; Gagnatelli, A.; Papi, A.; Van der Molen, T.; Rabe, K.F.; Siddiqui, S.; et al. Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): Baseline data from a prospective cohort study. Lancet Respir. Med. 2019, 7, 402–416. [Google Scholar] [CrossRef]
- Postma, D.S.; Brightling, C.; Fabbri, L.; van der Molen, T.; Nicolini, G.; Papi, A.; Rabe, K.F.; Siddiqui, S.; Singh, D.; Berge, M.v.D.; et al. Unmet needs for the assessment of small airways dysfunction in asthma: Introduction to the ATLANTIS study. Eur. Respir. J. 2015, 45, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, C.; Cottini, M.; Berti, A.; Comberiati, P. Monoclonal antibodies targeting small airways: A new perspective for biological therapies in severe asthma. Asthma Res. Pract. 2022, 8, 1–11. [Google Scholar] [CrossRef]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet. 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; Fitzgerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab Treatment in Patients with Severe Eosinophilic Asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef]
- Gyawali, B.; Georas, S.N.; Khurana, S. Biologics in severe asthma: A state-of-the-art review. Eur. Respir. Rev. 2025, 34, 240088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2023. 2023. Available online: www.ginasthma.org (accessed on 1 April 2025).
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2022, 60, 2101499. [Google Scholar] [CrossRef]
- Oostveen, E.; MacLeod, D.; Lorino, H.; Farré, R.; Hantos, Z.; Desager, K.; Marchal, F. The forced oscillation technique in clinical practice: Methodology, recommendations and future developments. Eur. Respir. J. 2003, 22, 1026–1041. [Google Scholar] [CrossRef]
- Khatri, S.B.; Iaccarino, J.M.; Barochia, A.; Farré, R.; Hantos, Z.; Desager, K.; Marchal, F. Use of Fractional Exhaled Nitric Oxide to Guide the Treatment of Asthma: An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2021, 204, E97–E109. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, A.; Anderson, W.J.; Lipworth, J. Small airway dysfunction is associated with poorer asthma control. Eur. Respir. J. 2014, 44, 1353–1355. [Google Scholar] [CrossRef] [PubMed]
- Usmani, O.S. Small airways dysfunction in asthma: Evaluation and management to improve asthma control. Allergy Asthma Immunol. Res. 2014, 6, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.; Richardson, M.; Hallmark, B.; Billheimer, D.; Berge, M.V.D.; Fabbri, L.M.; Van der Molen, T.; Nicolini, G.; Papi, A.; Rabe, K.F.; et al. The role of small airway dysfunction in asthma control and exacerbations: A longitudinal, observational analysis using data from the ATLANTIS study. Lancet Respir. Med. 2022, 10, 661–668. [Google Scholar] [CrossRef]
- Farah, C.S.; Badal, T.; Reed, N.; Rogers, P.G.; King, G.G.; Thamrin, C.; Peters, M.J.; Seccombe, L.M. Mepolizumab improves small airway function in severe eosinophilic asthma. Respir. Med. 2019, 148, 49–53. [Google Scholar] [CrossRef]
- Braido, F.; Scichilone, N.; Lavorini, F.; Usmani, O.S.; Dubuske, L.; Boulet, L.P.; Mosges, R.; Nunes, C.; Sánchez-Borges, M.; Ansotegui, I.J.; et al. Manifesto on small airway involvement and management in asthma and chronic obstructive pulmonary disease: An Interasma (Global Asthma Association—GAA) and World Allergy Organization (WAO) document endorsed by Allergic Rhinitis and its Impact on Asthma (ARIA) and Global Allergy and Asthma European Network (GA2LEN). Asthma Res. Pract. 2016, 2, 12. [Google Scholar] [CrossRef]
- Lavorini, F.; Pedersen, S.; Usmani, O.S.; Barnes, P.; Corbetta, L.; Corrigan, C.; Chawes, B.; Dekhuijzen, P.; Hausen, T.; Levy, M.; et al. Dilemmas, Confusion, and Misconceptions Related to Small Airways Directed Therapy. Chest 2017, 151, 1345–1355. [Google Scholar] [CrossRef]
- Siora, A.; Vontetsianos, A.; Chynkiamis, N.; Anagnostopoulou, C.; Bartziokas, K.; Anagnostopoulos, N.; Rovina, N.; Bakakos, P.; Papaioannou, A.I. Small airways in asthma: From inflammation and pathophysiology to treatment response. Respir. Med. 2024, 222, 107532. [Google Scholar] [CrossRef]
- Kaminsky, D.A.; Simpson, S.J.; Berger, K.I.; Calverley, P.; de Melo, P.L.; Dandurand, R.; Dellacà, R.L.; Farah, C.S.; Farré, R.; Hall, G.L.; et al. Clinical significance and applications of oscillometry. Eur. Respir. Rev. 2022, 31, 210208. [Google Scholar] [CrossRef]
- King, G.G.; Bates, J.; Berger, K.I.; Calverley, P.; De Melo, P.L.; Dellacà, R.L.; Farre, R.; Hall, G.; Ioan, I.; Irvin, C.G.; et al. Technical standards for respiratory oscillometry. Eur. Respir. J. 2020, 55, 1900753. [Google Scholar] [CrossRef]
- Menzella, F.; Antonicelli, L.; Cottini, M.; Imeri, G.; Corsi, L.; Di Marco, F. Oscillometry in severe asthma: The state of the art and future perspectives. Expert. Rev. Respir. Med. 2023, 17, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Antonicelli, L.; Tontini, C.; Marchionni, A.; Lucchetti, B.; Garritani, M.S.; Bilò, M.B. Forced oscillation technique as method to document and monitor the efficacy of mepolizumab in treating severe eosinophilic asthma. Allergy 2020, 75, 433–436. [Google Scholar] [CrossRef]
- Sposato, B.; Camiciottoli, G.; Bacci, E.; Scalese, M.; Carpagnano, G.E.; Pelaia, C.; Santus, P.; Maniscalco, M.; Masieri, S.; Corsico, A.; et al. Mepolizumab effectiveness on small airway obstruction, corticosteroid sparing and maintenance therapy step-down in real life. Pulm. Pharmacol. Ther. 2020, 61, 101899. [Google Scholar] [CrossRef]
- Abdo, M.; Watz, H.; Veith, V.; Kirsten, A.-M.; Biller, H.; Pedersen, F.; von Mutius, E.; Kopp, M.V.; Hansen, G.; Waschki, B.; et al. Small airway dysfunction as predictor and marker for clinical response to biological therapy in severe eosinophilic asthma: A longitudinal observational study. Respir. Res. 2020, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bonini, M.; Di Paolo, M.; Bagnasco, D.; Baiardini, I.; Braido, F.; Caminati, M.; Carpagnano, E.; Contoli, M.; Corsico, A.; Del Giacco, S.; et al. Minimal clinically important difference for asthma endpoints: An expert consensus report. Eur. Respir. Rev. 2020, 29, 190137. [Google Scholar] [CrossRef]
- De Lange, E.E.; Altes, T.A.; Patrie, J.T.; Gaare, J.D.; Knake, J.J.; Mugler, J.P.; Platts-Mills, T.A. Evaluation of asthma with hyperpolarized helium-3 MRI: Correlation with clinical severity and spirometry. Chest 2006, 130, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, S.; Schuermans, D.; Vincken, W. Inflammation and airway function in the lung periphery of patients with stable asthma. J. Allergy Clin. Immunol. 2010, 125, 611–616. [Google Scholar] [CrossRef]
- Hamid, Q.; Song, Y.; Kotsimbos, T.C.; Minshall, E.; Bai, T.R.; Hegele, R.G.; Hogg, J.C. Inflammation of small airways in asthma. J. Allergy Clin. Immunol. 1997, 100, 44–51. [Google Scholar] [CrossRef]
- Farah, C.S.; King, G.G.; Brown, N.J.; Peters, M.J.; Berend, N.; Salome, C.M. Ventilation heterogeneity predicts asthma control in adults following inhaled corticosteroid dose titration. J. Allergy Clin. Immunol. 2012, 130, 61–68. [Google Scholar] [CrossRef]
- Thompson, B.R.; Douglass, J.A.; Ellis, M.J.; Kelly, V.J.; O’Hehir, R.E.; King, G.G.; Verbanck, S. Peripheral lung function in patients with stable and unstable asthma. J. Allergy Clin. Immunol. 2013, 131, 1322–1328. [Google Scholar] [CrossRef]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.-C.; Plummer, A.L.; Taylor, D.R. An Official ATS Clinical Practice Guideline: Interpretation of Exhaled Nitric Oxide Levels (FeNO) for Clinical Applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602. [Google Scholar] [CrossRef] [PubMed]
- National Asthma Education. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J. Allergy Clin. Immunol. 2007, 120 (Suppl. 5), S94–S138. [Google Scholar] [CrossRef]
- Shirai, T.; Akamatsu, T.; Hirai, K.; Watanabe, H.; Tamura, K.; Kishimoto, Y.; Saigusa, M. Oscillometry improves earlier than spirometry after benralizumab initiation in severe asthma. Allergy 2020, 75, 2678–2680. [Google Scholar] [CrossRef] [PubMed]
| Age (years) | 61.1 (±12.0) | |
| BMI | 27.1 (±5.10) | |
| Gender | Female | 10 (55.5) |
| Male | 8 (44.5) | |
| Smoking History | Never | 13 (72.2) |
| Former | 4 (22.2) | |
| Current | 1 (5.6) | |
| R5–R20 > 0.07 kPa/L/s (SAD) | 15 (83.3) | |
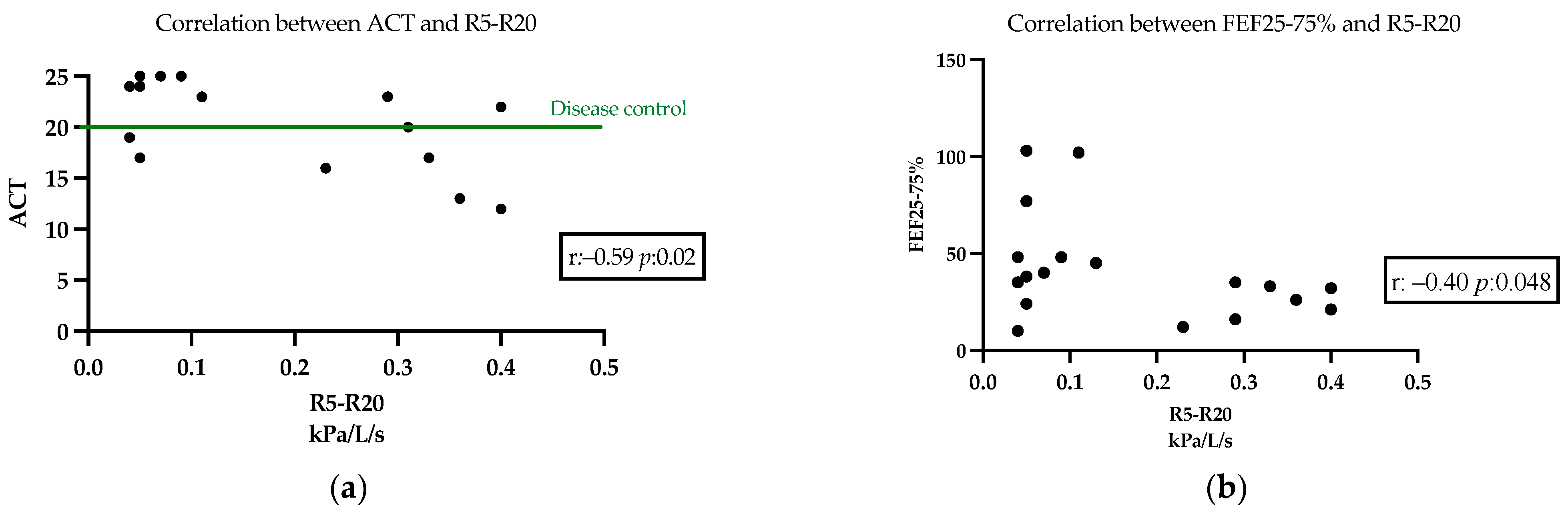
| R5–R20 (kPa/L/s) | 0.25 (±0.15) | |
| FEV1L | 1.70 (±0.78) | |
| FEV1%pred. | 68.0 (±24.1) | |
| FEV1/FVC% | 59 (±21.6) | |
| FEF25-75% pred. | 31.9 (±23.0) | |
| TLC% pred. | 96.4 (±16.2) | |
| RV/TLC% | 45.0 (±10.7) | |
| ACT | 14.7 (±5.6) | |
| BEC (cell/mm3) | 261 (220–520) | |
| FeNO (ppb) | 47.8 (±36.5) | |
| Medium–high dose ICS/LABAs | 18 (100) | |
| LAMA | 17 (94) | |
| Patients ≥ 1 AE requiring OCS/previous year | 15 (83.3) | |
| mOCS | 4 (22) |
| T0 | T3 | p-Value T0–T3 | T6 | p-Value T0–T6 | T12 | p-Value T0–T12 | |
|---|---|---|---|---|---|---|---|
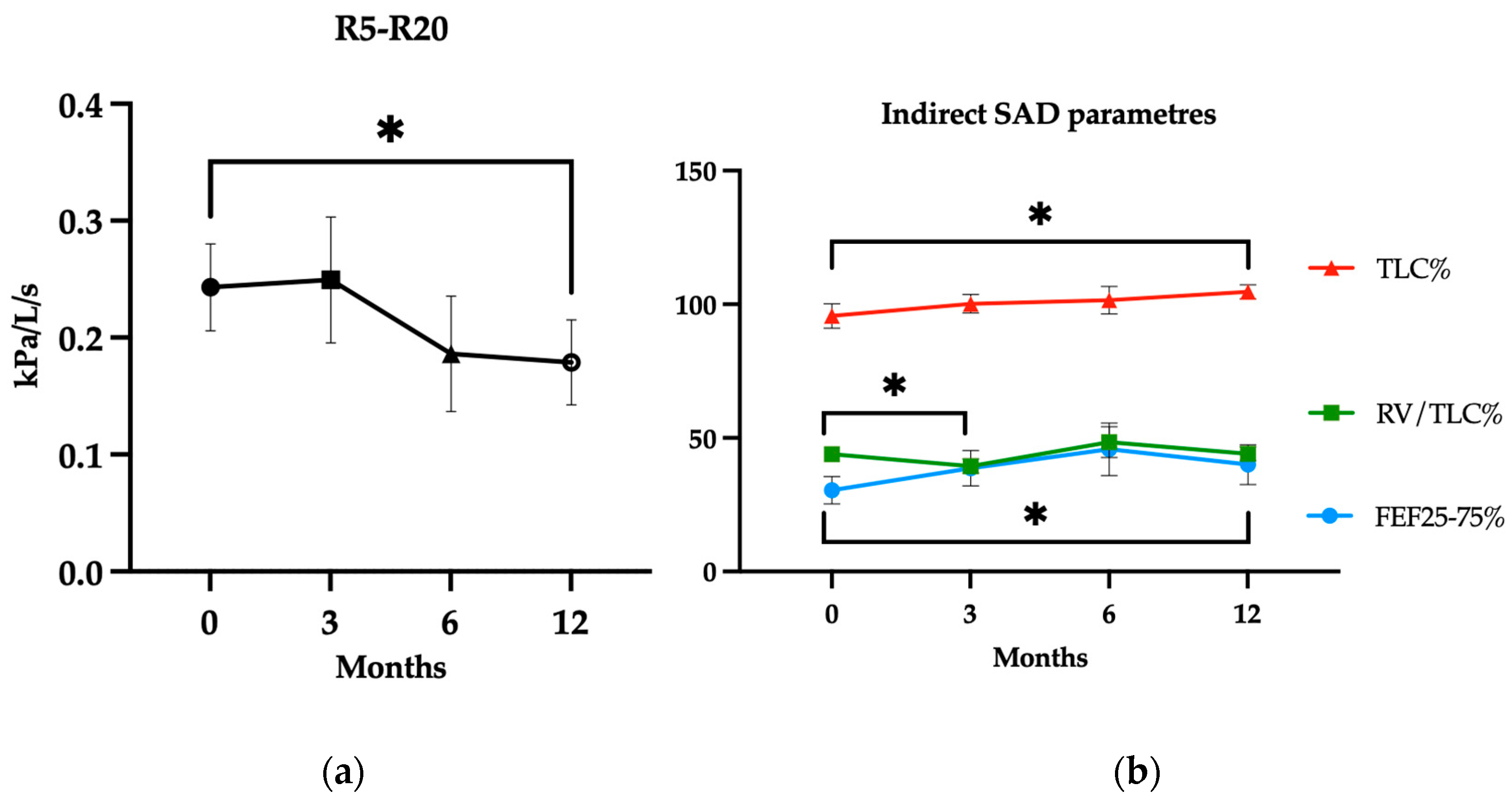
| R5–R20 (kPa/L/s) | 0.24 (±0.15) | 0.25 (±0.21) | 0.45 | 0.19 (±0.18) | 0.05 | 0.18 (±0.18) | 0.03 * |
| SAD—n (%) | 13 (81) | 10 (62) | 0.43 | 10 (62) | 0.43 | 9 (56) | 0.13 |
| FEF25-75%pred. | 30.4 (±20.6) | 38.7 (±25.6) | 0.09 | 47.5 (±34.1) | 0.06 | 41.4 (±28.8) | 0.04 * |
| TLC%pred. | 95.6 (±17.1) | 100.2 (±12.3) | 0.21 | 101.5 (±17.1) | 0.26 | 104.7 (±9.3) | 0.04 * |
| RV/TLC% | 43.9 (±9.55) | 39.5 (±10.1) | 0.01 * | 48.4 (±19.0) | 0.26 | 44.9 (±9.3) | 0.12 |
| FEV1%pred. | 71.4 (±22.9) | 82.3 (±34.3) | 0.05 | 83.1 (±33.4) | 0.06 | 80.8 (±26.7) | 0.03 * |
| ACT | 15.1 (5.8) | 19.0 (±4.6) | <0.01 * | 19.6 (±3.5) | <0.01 * | 20.3 (±4.4) | <0.01 * |
| Patients ≥ 1 AE requiring OCS/previous year | 15 (94%) | 1 (6.2%) | <0.01 * | 2 (12.5%) | <0.01 * | 3 (18.7%) | <0.01 * |
| Patients with mOCS | 4 (25%) | 0 (0%) | 0.10 | 0 (0%) | 0.10 | 0 (0%) | 0.10 |
| BEC (cell/mm3) | 250 (220–477) | 70 (40–130) | <0.01 * | 40 (20–111) | <0.01 * | 50 (40–90) | <0.01 * |
| FeNO (ppb) | 49.2 (±39.4) | 44.5 (±43.6) | 0.35 | 52.8 (±48.5) | 0.24 | 43.1 (±26.4) | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonini, M.; Boccabella, C.; Cefaloni, F.; De Corso, E.; Donfrancesco, F.; Schiavi, E.; Richeldi, L. Small Airways Disease as a Novel Target for Mepolizumab in Asthma—The SASAM Prospective Real-Life Study. J. Clin. Med. 2025, 14, 2928. https://doi.org/10.3390/jcm14092928
Bonini M, Boccabella C, Cefaloni F, De Corso E, Donfrancesco F, Schiavi E, Richeldi L. Small Airways Disease as a Novel Target for Mepolizumab in Asthma—The SASAM Prospective Real-Life Study. Journal of Clinical Medicine. 2025; 14(9):2928. https://doi.org/10.3390/jcm14092928
Chicago/Turabian StyleBonini, Matteo, Cristina Boccabella, Francesca Cefaloni, Eugenio De Corso, Federico Donfrancesco, Enrico Schiavi, and Luca Richeldi. 2025. "Small Airways Disease as a Novel Target for Mepolizumab in Asthma—The SASAM Prospective Real-Life Study" Journal of Clinical Medicine 14, no. 9: 2928. https://doi.org/10.3390/jcm14092928
APA StyleBonini, M., Boccabella, C., Cefaloni, F., De Corso, E., Donfrancesco, F., Schiavi, E., & Richeldi, L. (2025). Small Airways Disease as a Novel Target for Mepolizumab in Asthma—The SASAM Prospective Real-Life Study. Journal of Clinical Medicine, 14(9), 2928. https://doi.org/10.3390/jcm14092928









