Positron Emission Tomography in Takayasu Arteritis: A Review Including Patterns of Vascular Involvement Across Modalities and Regions
Abstract
1. Introduction
2. FDG-PET/CT as a Diagnostic Tool
3. Activity Assessment
4. Monitoring Activity
5. Prediction for Prognosis
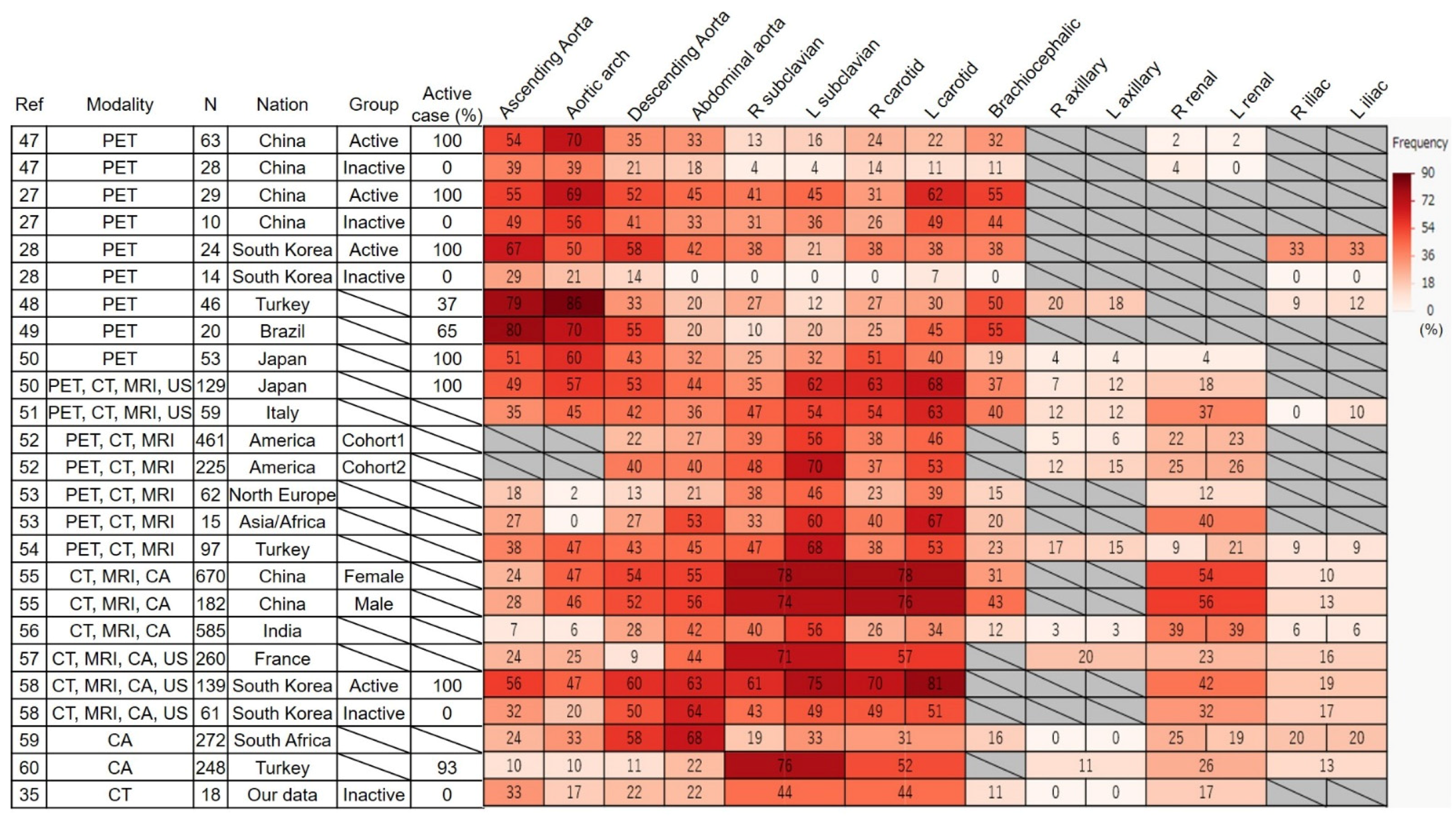
6. Frequency and Distribution of Affected Vessels in FDG-PET
6.1. Frequency
6.2. Distribution
7. Differences in Affected Lesions Between Countries
8. New Technology
8.1. PET/MRI
8.2. Somatostatin Receptor 2
8.3. Fibroblast
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheumatol. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T. Common Autoantibody among Takayasu Arteritis and Ulcerative Colitis: A Possible Pathophysiology that Includes Gut-Vessel Connection in Vascular Inflammation. JMA J. 2023, 6, 265–273. [Google Scholar] [CrossRef]
- Danda, D.; Manikuppam, P.; Tian, X.; Harigai, M. Advances in Takayasu arteritis: An Asia Pacific perspective. Front. Med. 2022, 9, 952972. [Google Scholar] [CrossRef]
- Yoshifuji, H.; Nakaoka, Y.; Uchida, H.A.; Sugihara, T.; Watanabe, Y.; Funakoshi, S.; Isobe, M.; Harigai, M.; Japan Research Committee of the Ministry of Health, Labour, and Welfare for Intractable Vasculitis (JPVAS). Organ Damage and Quality of Life in Takayasu Arteritis—Evidence From a National Registry Analysis. Circ. J. 2024, 88, 285–294. [Google Scholar] [CrossRef]
- Dejaco, C.; Ramiro, S.; Bond, M.; Bosch, P.; Ponte, C.; Mackie, S.L.; Bley, T.A.; Blockmans, D.; Brolin, S.; Bolek, E.C.; et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice: 2023 update. Ann. Rheum. Dis. 2024, 83, 741–751. [Google Scholar] [CrossRef]
- Peverelli, M.; Tarkin, J.M. Emerging PET radiotracers for vascular imaging. Rheumatology 2025, 64, i33–i37. [Google Scholar] [CrossRef]
- Tawakol, A.; Migrino, R.Q.; Bashian, G.G.; Bedri, S.; Vermylen, D.; Cury, R.C.; Yates, D.; LaMuraglia, G.M.; Furie, K.; Houser, S.; et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J. Am. Coll. Cardiol. 2006, 48, 1818–1824. [Google Scholar] [CrossRef]
- Hara, M.; Goodman, P.C.; Leder, R.A. FDG-PET finding in early-phase Takayasu arteritis. J. Comput. Assist. Tomogr. 1999, 23, 16–18. [Google Scholar] [CrossRef]
- Grayson, P.C.; Alehashemi, S.; Bagheri, A.A.; Civelek, A.C.; Cupps, T.R.; Kaplan, M.J.; Malayeri, A.A.; Merkel, P.A.; Novakovich, E.; Bluemke, D.A.; et al. (18) F-Fluorodeoxyglucose-Positron Emission Tomography as an Imaging Biomarker in a Prospective, Longitudinal Cohort of Patients with Large Vessel Vasculitis. Arthritis Rheumatol. 2018, 70, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Choi, S.J.; Ji, J.D.; Song, G.G. Diagnostic accuracy of 18F-FDG PET or PET/CT for large vessel vasculitis: A meta-analysis. Z. Rheumatol. 2016, 75, 924–931. [Google Scholar] [CrossRef]
- Soussan, M.; Nicolas, P.; Schramm, C.; Katsahian, S.; Pop, G.; Fain, O.; Mekinian, A. Management of large-vessel vasculitis with FDG-PET: A systematic literature review and meta-analysis. Medicine 2015, 94, e622. [Google Scholar] [CrossRef] [PubMed]
- Barra, L.; Kanji, T.; Malette, J.; Pagnoux, C. Imaging modalities for the diagnosis and disease activity assessment of Takayasu’s arteritis: A systematic review and meta-analysis. Autoimmun. Rev. 2018, 17, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Espitia, O.; Schanus, J.; Agard, C.; Kraeber-Bodéré, F.; Hersant, J.; Serfaty, J.M.; Jamet, B. Specific features to differentiate Giant cell arteritis aortitis from aortic atheroma using FDG-PET/CT. Sci. Rep. 2021, 11, 17389. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Komatsu, H.; Sato, H.; Fujii, H.; Ishii, T.; Harigae, H. Migratory Aortitis Associated with Granulocyte-colony-stimulating Factor. Intern. Med. 2020, 59, 1559–1563. [Google Scholar] [CrossRef]
- Nielsen, B.D.; Gormsen, L.C.; Hansen, I.T.; Keller, K.K.; Therkildsen, P.; Hauge, E.M. Three days of high-dose glucocorticoid treatment attenuates large-vessel 18F-FDG uptake in large-vessel giant cell arteritis but with a limited impact on diagnostic accuracy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1119–1128. [Google Scholar] [CrossRef]
- Narváez, J.; Estrada, P.; Vidal-Montal, P.; Sánchez-Rodríguez, I.; Sabaté-Llobera, A.; Nolla, J.M.; Cortés-Romera, M. Impact of previous glucocorticoid therapy on diagnostic accuracy of [18F] FDG PET-CT in giant cell arteritis. Semin. Arthritis Rheum. 2023, 60, 152183. [Google Scholar] [CrossRef]
- Fagart, A.; Machet, T.; Collet, G.; Quemeneur, T.; Ben Ticha, R.; Verstraete, M.; Le Gouellec, N.; Demailly, F.; Rousselin, C. Fluorodeoxyglucose positron emission tomography-computed tomography findings in a first series of 10 patients with polyarteritis nodosa. Rheumatology 2022, 61, 1663–1668. [Google Scholar] [CrossRef]
- Kemna, M.J.; Vandergheynst, F.; Voo, S.; Blocklet, D.; Nguyen, T.; Timmermans, S.; van Paassen, P.; Cogan, E.; van Kroonenburgh, M.; Tervaert, J.W.C. Positron emission tomography scanning in anti-neutrophil cytoplasmic antibodies-associated vasculitis. Medicine 2015, 94, e747. [Google Scholar] [CrossRef]
- Delaval, L.; Samson, M.; Schein, F.; Agard, C.; Trefond, L.; Deroux, A.; Dupuy, H.; Garrouste, C.; Godmer, P.; Landron, C.; et al. Temporal Arteritis Revealing Antineutrophil Cytoplasmic Antibody-Associated Vasculitides: A Case-Control Study. Arthritis Rheumatol. 2021, 73, 286–294. [Google Scholar] [CrossRef]
- Kaymakci, M.S.; Elfishawi, M.M.; Langenfeld, H.E.; Hanson, A.C.; Crowson, C.S.; Bois, M.C.; Ghaffar, U.; Koster, M.J.; Specks, U.; Warrington, K.J. Large vessel involvement in antineutrophil cytoplasmic antibody-associated vasculitis. Rheumatology 2024, 63, 1682–1689. [Google Scholar] [CrossRef]
- Soussan, M.; Abisror, N.; Abad, S.; Nunes, H.; Terrier, B.; Pop, G.; Eder, V.; Valeyre, D.; Sberro-Soussan, R.; Guillevin, L.; et al. FDG-PET/CT in patients with ANCA-associated vasculitis: Case-series and literature review. Autoimmun Rev. 2014, 13, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Minamimoto, R.; Yamashita, H.; Yoshida, S.; Morooka, M.; Okasaki, M.; Mimori, A.; Kubota, K. Evaluation of Wegener’s granulomatosis using 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Ann. Nucl. Med. 2013, 27, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Frary, E.C.; Hess, S.; Gerke, O.; Laustrup, H. 18F-fluoro-deoxy-glucose positron emission tomography combined with computed tomography can reliably rule-out infection and cancer in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis suspected of disease relapse. Medicine 2017, 96, e7613. [Google Scholar] [CrossRef] [PubMed]
- Kerr, G.S.; Hallahan, C.W.; Giordano, J.; Leavitt, R.Y.; Fauci, A.S.; Rottem, M.; Hoffman, G.S. Takayasu arteritis. Ann. Intern. Med. 1994, 120, 919–929. [Google Scholar] [CrossRef]
- Han, Q.; Liang, Q.; Kang, F.; Wang, J.; Wu, Z.; Zhu, P. An increased major vessel uptake by 18F-FDG-PET/CT in NIH criteria inactive patients with Takayasu’s arteritis. Clin. Exp. Rheumatol. 2018, 36, 88–92. [Google Scholar]
- Tezuka, D.; Haraguchi, G.; Ishihara, T.; Ohigashi, H.; Inagaki, H.; Suzuki, J.; Hirao, K.; Isobe, M. Role of FDG PET-CT in Takayasu arteritis: Sensitive detection of recurrences. JACC Cardiovasc. Imaging 2012, 5, 422–429. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, J.; Sun, Y.; Shi, H.; Ji, Z.; Jiang, L. (18)F-FDG-PET/CT: An accurate method to assess the activity of Takayasu’s arteritis. Clin. Rheumatol. 2018, 37, 1927–1935. [Google Scholar] [CrossRef]
- Lee, K.H.; Cho, A.; Choi, Y.J.; Lee, S.W.; Ha, Y.J.; Jung, S.J.; Park, M.C.; Lee, J.D.; Lee, S.K.; Park, Y.B. The role of (18)F-fluorodeoxyglucose-positron emission tomography in the assessment of disease activity in patients with takayasu arteritis. Arthritis Rheumatol. 2012, 64, 866–875. [Google Scholar] [CrossRef]
- Blockmans, D.; de Ceuninck, L.; Vanderschueren, S.; Knockaert, D.; Mortelmans, L.; Bobbaers, H. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: A prospective study of 35 patients. Arthritis Rheumatol. 2006, 55, 131–137. [Google Scholar] [CrossRef]
- Ora, M.; Misra, D.P.; Kavadichanda, C.G.; Singh, K.; Rathore, U.; Jain, N.; Agarwal, V.; Gambhir, S. Metabolic inflammatory volume and total inflammatory glycolysis: Novel parameters to evaluate PET-CT disease activity in Takayasu arteritis. Clin. Rheumatol. 2023, 42, 1855–1861. [Google Scholar] [CrossRef]
- Rimland, C.A.; Quinn, K.A.; Rosenblum, J.S.; Schwartz, M.N.; Bates Gribbons, K.; Novakovich, E.; Sreih, A.G.; Merkel, P.A.; Ahlman, M.A.; Grayson, P.C. Outcome Measures in Large Vessel Vasculitis: Relationship Between Patient-, Physician-, Imaging-, and Laboratory-Based Assessments. Arthritis Care Res. 2020, 72, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Alessi, H.D.; Quinn, K.A.; Ahlman, M.A.; Novakovich, E.; Saboury, B.; Luo, Y.; Grayson, P.C. Longitudinal Characterization of Vascular Inflammation and Disease Activity in Takayasu Arteritis and Giant Cell Arteritis: A Single-Center Prospective Study. Arthritis Care Res. 2023, 75, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Mutoh, T.; Shirai, T.; Fujii, H.; Ishii, T.; Harigae, H. Insufficient Use of Corticosteroids without Immunosuppressants Results in Higher Relapse Rates in Takayasu Arteritis. J. Rheumatol. 2020, 47, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Sato, H.; Fujii, H.; Ishii, T.; Harigae, H. The feasible maintenance dose of corticosteroid in Takayasu arteritis in the era of biologic therapy. Scand. J. Rheumatol. 2021, 50, 462–468. [Google Scholar] [CrossRef]
- Shirai, T.; Ishii, T.; Okazaki, S.; Shirota, Y.; Ishii, Y.; Sato, H.; Fujii, H. Active withdrawal of corticosteroids using tocilizumab and its association with autoantibody profiles in relapsed Takayasu arteritis: A multicentre, single-arm, prospective study (the Ab-TAK study). Front. Immunol. 2024, 15, 1473100. [Google Scholar] [CrossRef]
- Isobe, M.; Maejima, Y.; Saji, M.; Tateishi, U. Evaluation of tocilizumab for intractable Takayasu arteritis and (18)F-fluorodeoxyglucose-positron emission tomography for detecting inflammation under tocilizumab treatment. J. Cardiol. 2021, 77, 539–544. [Google Scholar] [CrossRef]
- Youngstein, T.; Tombetti, E.; Mukherjee, J.; Barwick, T.D.; Al-Nahhas, A.; Humphreys, E.; Nash, J.; Andrews, J.; Incerti, E.; Tombolini, E.; et al. FDG Uptake by Prosthetic Arterial Grafts in Large Vessel Vasculitis Is Not Specific for Active Disease. JACC Cardiovasc. Imaging 2017, 10, 1042–1052. [Google Scholar] [CrossRef]
- Incerti, E.; Tombetti, E.; Fallanca, F.; Baldissera, E.M.; Alongi, P.; Tombolini, E.; Sartorelli, S.; Sabbadini, M.G.; Papa, M.; De Cobelli, F.; et al. (18)F-FDG PET reveals unique features of large vessel inflammation in patients with Takayasu’s arteritis. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1109–1118. [Google Scholar] [CrossRef]
- Galli, E.; Muratore, F.; Mancuso, P.; Boiardi, L.; Marvisi, C.; Besutti, G.; Spaggiari, L.; Casali, M.; Versari, A.; Giorgi Rossi, P.; et al. The role of PET/CT in disease activity assessment in patients with large vessel vasculitis. Rheumatology 2022, 61, 4809–4816. [Google Scholar] [CrossRef]
- Ma, L.; Wu, B.; Sun, Y.; Ding, Z.; Dai, X.; Wang, L.; Dai, X.; Zhang, L.; Chen, H.; Ma, L.; et al. PET vascular activity score for predicting new angiographic lesions in patients with Takayasu arteritis: A Chinese cohort study. Rheumatology 2023, 62, 3310–3316. [Google Scholar] [CrossRef]
- Janes, A.L.F.; Castro, M.F.; Arraes, A.E.D.; Savioli, B.; Sato, E.I.; de Souza, A.W.S. A retrospective cohort study to assess PET-CT findings and clinical outcomes in Takayasu arteritis: Does 18F-fluorodeoxyglucose uptake in arteries predict relapses? Rheumatol. Int. 2020, 40, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.A.; Ahlman, M.A.; Alessi, H.D.; LaValley, M.P.; Neogi, T.; Marko, J.; Novakovich, E.; Grayson, P.C. Association of (18)F-Fluorodeoxyglucose-Positron Emission Tomography Activity With Angiographic Progression of Disease in Large Vessel Vasculitis. Arthritis Rheumatol. 2023, 75, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Besutti, G.; Muratore, F.; Mancuso, P.; Ferrari, M.; Galli, E.; Spaggiari, L.; Monelli, F.; Casali, M.; Versari, A.; Boiardi, L.; et al. Vessel inflammation and morphological changes in patients with large vessel vasculitis: A retrospective study. RMD Open 2022, 8, e001977. [Google Scholar] [CrossRef]
- Terao, C. Revisited HLA and non-HLA genetics of Takayasu arteritis—Where are we? J. Hum. Genet. 2016, 61, 27–32. [Google Scholar] [CrossRef]
- Shirai, T.; Hanaoka, R.; Goto, Y.; Kojima, I.; Ishii, Y.; Hoshi, Y.; Fujita, Y.; Shirota, Y.; Fujii, H.; Ishii, T.; et al. Takayasu Arteritis Coexisting with Sclerosing Osteomyelitis. Intern. Med. 2018, 57, 1929–1934. [Google Scholar] [CrossRef]
- Fan, L.; Chen, J.; Pan, L.; Xin, X.; Geng, B.; Yang, L.; Wang, Q.; Ma, W.; Lou, Y.; Bian, J.; et al. Alterations of Gut Microbiome, Metabolome, and Lipidome in Takayasu Arteritis. Arthritis Rheumatol. 2023, 75, 266–278. [Google Scholar] [CrossRef]
- Ma, L.Y.; Wu, B.; Jin, X.J.; Sun, Y.; Kong, X.F.; Ji, Z.F.; Chen, R.Y.; Cui, X.M.; Shi, H.C.; Jiang, L.D. A novel model to assess disease activity in Takayasu arteritis based on 18F-FDG-PET/CT: A Chinese cohort study. Rheumatology 2022, 61, SI14–SI22. [Google Scholar] [CrossRef]
- Tahra, S.K.; Ozguven, S.; Unal, A.U.; Oner, F.A.; Ones, T.; Erdil, T.Y.; Direskeneli, H. Assessment of Takayasu arteritis in routine practice with PETVAS, an 18F-FDG PET quantitative scoring tool. Turk. J. Med. Sci. 2022, 52, 313–322. [Google Scholar] [CrossRef]
- de Souza Santos, M.P.; Ramos, C.D.; Paixao, M.; Pignaton Naseri, E.; Barros Bertolo, M.; Sachetto, Z. 18F-FDG PET/CT in Late Acquisition Identifies Sites of Active Disease in Treated Takayasu Arteritis. J. Clin. Rheumatol. 2022, 28, 14–20. [Google Scholar] [CrossRef]
- Uchida, H.A.; Nakaoka, Y.; Sugihara, T.; Yoshifuji, H.; Maejima, Y.; Watanabe, Y.; Nagafuchi, H.; Okazaki, T.; Komagata, Y.; Tanaka, Y.; et al. Clinical Characteristics and Treatment Outcomes of Patients With Newly Diagnosed Takayasu Arteritis in Japan During the First 2 Years of Treatment—A Nationwide Retrospective Cohort Study. Circ. J. 2024, CJ-24-0178, in press. [Google Scholar] [CrossRef]
- Boiardi, L.; Galli, E.; Macchioni, P.; Muratore, F.; Klinowski, G.; Hunder, G.G.; Casali, M.; Besutti, G.; Spaggiari, L.; Versari, A.; et al. Takayasu arteritis and large-vessel giant cell arteritis in Italian population. Comprehensive analysis from a single institutional cohort of 184 cases. Semin. Arthritis Rheum. 2023, 59, 152173. [Google Scholar] [CrossRef] [PubMed]
- Gribbons, K.B.; Ponte, C.; Carette, S.; Craven, A.; Cuthbertson, D.; Hoffman, G.S.; Khalidi, N.A.; Koening, C.L.; Langford, C.A.; Maksimowicz-McKinnon, K.; et al. Patterns of Arterial Disease in Takayasu Arteritis and Giant Cell Arteritis. Arthritis Care Res. 2020, 72, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Gudbrandsson, B.; Molberg, O.; Garen, T.; Palm, O. Prevalence, Incidence, and Disease Characteristics of Takayasu Arteritis by Ethnic Background: Data From a Large, Population-Based Cohort Resident in Southern Norway. Arthritis Care Res. 2017, 69, 278–285. [Google Scholar] [CrossRef]
- Kelesoglu Dincer, A.B.; Kilic, L.; Erden, A.; Kalyoncu, U.; Hazirolan, T.; Kiraz, S.; Karada, G.O. Imaging modalities used in diagnosis and follow-up of patients with Takayasu’s arteritis. Turk. J. Med. Sci. 2021, 51, 224–230. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Wang, Y.; Yang, Y.; Zhao, J.; Li, M.; Pang, H.; Wang, T.; Chen, Y.; Tian, X.; et al. Age, sex and angiographic type-related phenotypic differences in inpatients with Takayasu arteritis: A 13-year retrospective study at a national referral center in China. Front. Cardiovasc. Med. 2023, 10, 1099144. [Google Scholar] [CrossRef]
- Danda, D.; Goel, R.; Joseph, G.; Kumar, S.T.; Nair, A.; Ravindran, R.; Jeyaseelan, L.; Merkel, P.A.; Grayson, P.C. Clinical course of 602 patients with Takayasu’s arteritis: Comparison between Childhood-onset versus adult onset disease. Rheumatology 2021, 60, 2246–2255. [Google Scholar] [CrossRef]
- Comarmond, C.; Biard, L.; Lambert, M.; Mekinian, A.; Ferfar, Y.; Kahn, J.E.; Benhamou, Y.; Chiche, L.; Koskas, F.; Cluzel, P.; et al. Long-Term Outcomes and Prognostic Factors of Complications in Takayasu Arteritis: A Multicenter Study of 318 Patients. Circulation 2017, 136, 1114–1122. [Google Scholar] [CrossRef]
- Lee, G.Y.; Jang, S.Y.; Ko, S.M.; Kim, E.K.; Lee, S.H.; Han, H.; Choi, S.H.; Kim, Y.W.; Choe, Y.H.; Kim, D.K. Cardiovascular manifestations of Takayasu arteritis and their relationship to the disease activity: Analysis of 204 Korean patients at a single center. Int. J. Cardiol. 2012, 159, 14–20. [Google Scholar] [CrossRef]
- Mwipatayi, B.P.; Jeffery, P.C.; Beningfield, S.J.; Matley, P.J.; Naidoo, N.G.; Kalla, A.A.; Kahn, D. Takayasu arteritis: Clinical features and management: Report of 272 cases. ANZ J. Surg. 2005, 75, 110–117. [Google Scholar] [CrossRef]
- Bicakcigil, M.; Aksu, K.; Kamali, S.; Ozbalkan, Z.; Ates, A.; Karadag, O.; Ozer, H.T.; Seyahi, E.; Akar, S.; Onen, F.; et al. Takayasu’s arteritis in Turkey—Clinical and angiographic features of 248 patients. Clin. Exp. Rheumatol. 2009, 27, S59–S64. [Google Scholar]
- Blomberg, B.A.; Bashyam, A.; Ramachandran, A.; Gholami, S.; Houshmand, S.; Salavati, A.; Werner, T.; Zaidi, H.; Alavi, A. Quantifying [18F]fluorodeoxyglucose uptake in the arterial wall: The effects of dual time-point imaging and partial volume effect correction. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Grayson, P.C.; Ponte, C.; Suppiah, R.; Robson, J.C.; Gribbons, K.B.; Judge, A.; Craven, A.; Khalid, S.; Hutchings, A.; Danda, D.; et al. 2022 American College of Rheumatology/EULAR classification criteria for Takayasu arteritis. Ann. Rheum. Dis. 2022, 81, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Ogino, H.; Matsuda, H.; Minatoya, K.; Sasaki, H.; Tanaka, H.; Matsumura, Y.; Ishibashi-Ueda, H.; Kobayashi, J.; Yagihara, T.; Kitamura, S. Overview of late outcome of medical and surgical treatment for Takayasu arteritis. Circulation 2008, 118, 2738–2747. [Google Scholar] [CrossRef]
- Quinn, K.A.; Ahlman, M.A.; Malayeri, A.A.; Marko, J.; Civelek, A.C.; Rosenblum, J.S.; Bagheri, A.A.; Merkel, P.A.; Novakovich, E.; Grayson, P.C. Comparison of magnetic resonance angiography and (18)F-fluorodeoxyglucose positron emission tomography in large-vessel vasculitis. Ann. Rheum. Dis. 2018, 77, 1165–1171. [Google Scholar] [CrossRef]
- Isobe, M.; Amano, K.; Arimura, Y.; Ishizu, A.; Ito, S.; Kaname, S.; Kobayashi, S.; Komagata, Y.; Komuro, I.; Komori, K.; et al. JCS 2017 Guideline on Management of Vasculitis Syndrome—Digest Version. Circ. J. 2020, 84, 299–359. [Google Scholar] [CrossRef]
- Hata, A.; Noda, M.; Moriwaki, R.; Numano, F. Angiographic findings of Takayasu arteritis: New classification. Int. J. Cardiol. 1996, 54, S155–163. [Google Scholar] [CrossRef]
- Mutoh, T.; Shirai, T.; Ishii, T.; Shirota, Y.; Fujishima, F.; Takahashi, F.; Kakuta, Y.; Kanazawa, Y.; Masamune, A.; Saiki, Y.; et al. Identification of two major autoantigens negatively regulating endothelial activation in Takayasu arteritis. Nat. Commun. 2020, 11, 1253. [Google Scholar] [CrossRef]
- Laurent, C.; Ricard, L.; Fain, O.; Buvat, I.; Adedjouma, A.; Soussan, M.; Mekinian, A. PET/MRI in large-vessel vasculitis: Clinical value for diagnosis and assessment of disease activity. Sci. Rep. 2019, 9, 12388. [Google Scholar] [CrossRef]
- Einspieler, I.; Thürmel, K.; Pyka, T.; Eiber, M.; Wolfram, S.; Moog, P.; Reeps, C.; Essler, M. Imaging large vessel vasculitis with fully integrated PET/MRI: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1012–1024. [Google Scholar] [CrossRef]
- Ćorović, A.; Wall, C.; Nus, M.; Gopalan, D.; Huang, Y.; Imaz, M.; Zulcinski, M.; Peverelli, M.; Uryga, A.; Lambert, J.; et al. Somatostatin Receptor PET/MR Imaging of Inflammation in Patients with Large Vessel Vasculitis and Atherosclerosis. J. Am. Coll. Cardiol. 2023, 81, 336–354. [Google Scholar] [CrossRef]
- Röhrich, M.; Rosales, J.J.; Hoppner, J.; Kvacskay, P.; Blank, N.; Loi, L.; Paech, D.; Schreckenberger, M.; Giesel, F.; Kauczor, H.U.; et al. Fibroblast activation protein inhibitor-positron emission tomography in aortitis: Fibroblast pathology in active inflammation and remission. Rheumatology 2024, 63, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katakura, T.; Shirai, T. Positron Emission Tomography in Takayasu Arteritis: A Review Including Patterns of Vascular Involvement Across Modalities and Regions. J. Clin. Med. 2025, 14, 2939. https://doi.org/10.3390/jcm14092939
Katakura T, Shirai T. Positron Emission Tomography in Takayasu Arteritis: A Review Including Patterns of Vascular Involvement Across Modalities and Regions. Journal of Clinical Medicine. 2025; 14(9):2939. https://doi.org/10.3390/jcm14092939
Chicago/Turabian StyleKatakura, Tokio, and Tsuyoshi Shirai. 2025. "Positron Emission Tomography in Takayasu Arteritis: A Review Including Patterns of Vascular Involvement Across Modalities and Regions" Journal of Clinical Medicine 14, no. 9: 2939. https://doi.org/10.3390/jcm14092939
APA StyleKatakura, T., & Shirai, T. (2025). Positron Emission Tomography in Takayasu Arteritis: A Review Including Patterns of Vascular Involvement Across Modalities and Regions. Journal of Clinical Medicine, 14(9), 2939. https://doi.org/10.3390/jcm14092939






