Prevention of Pre-Eclampsia: Modern Strategies and the Role of Early Screening
Abstract
:1. Introduction
2. Methodology
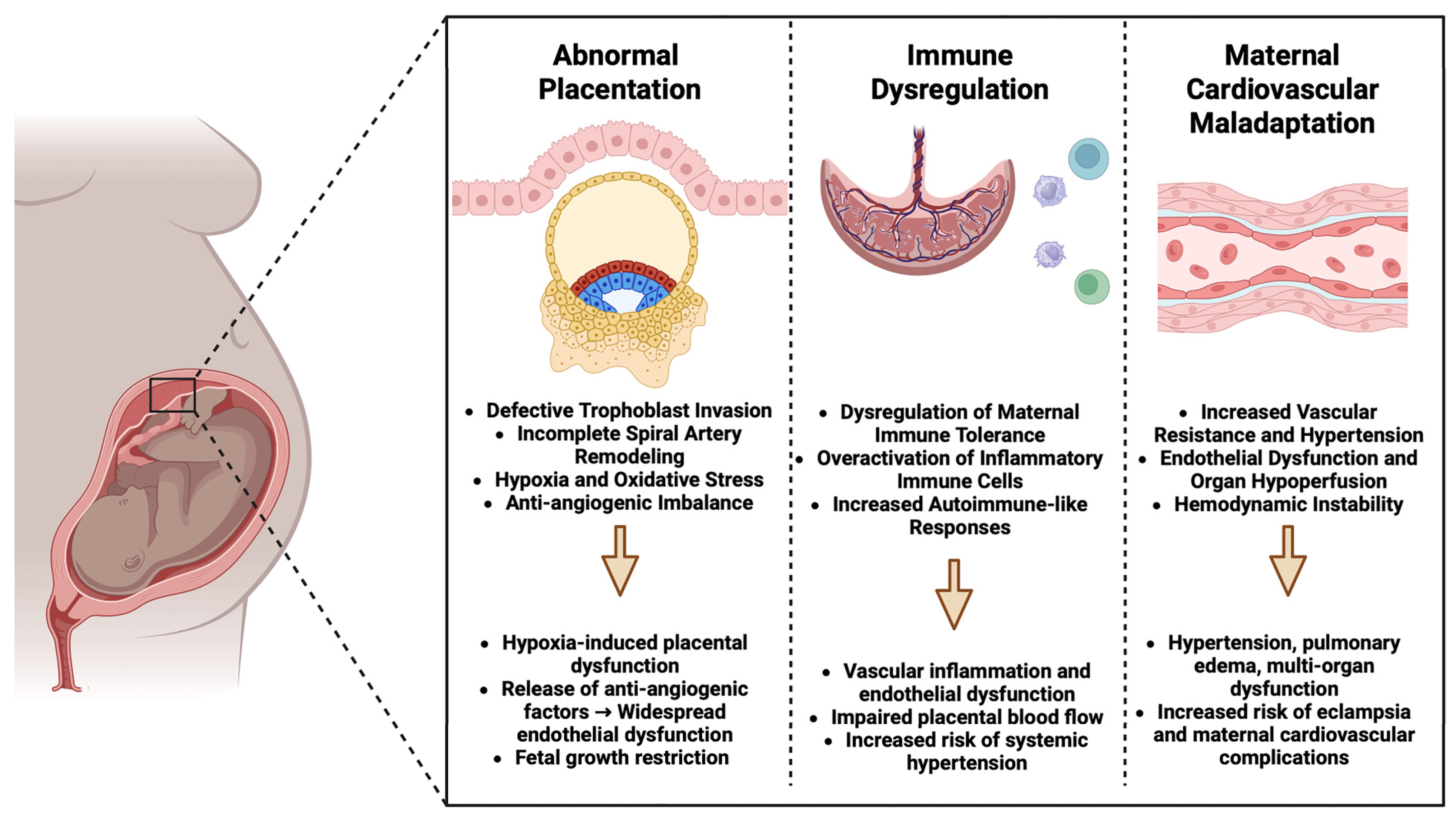
3. Pathophysiology of Pre-Eclampsia
4. Risk Factors for Pre-Eclampsia
5. Screening Methods for Early Detection of Pre-Eclampsia
5.1. Clinical Screening Tools
5.2. Biochemical Markers
5.3. Ultrasound Parameters
5.4. Combined and Machine Learning-Based Models for Risk Prediction
6. Current Strategies for the Prevention of Pre-Eclampsia
7. Innovations and Future Directions
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roberts, J.M.; Taylor, R.N.; Musci, T.J.; Rodgers, G.M.; Hubel, C.A.; McLaughlin, M.K. Preeclampsia: An endothelial cell disorder. Am. J. Obstet. Gynecol. 1989, 161, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Mol, B.W.; Roberts, C.T.; Thangaratinam, S.; Magee, L.A.; De Groot, C.J.; Hofmeyr, G.J. Pre-eclampsia. Lancet 2016, 387, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Steegers, E.A.; Von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Le Ray, I.; Zhu, J.; Zhang, J.; Hua, J.; Reilly, M. Preeclampsia prevalence, risk factors, and pregnancy outcomes in Sweden and China. JAMA Netw. Open 2021, 4, e218401. [Google Scholar] [CrossRef]
- Mou, A.D.; Barman, Z.; Hasan, M.; Miah, R.; Hafsa, J.M.; Das Trisha, A.; Ali, N. Prevalence of preeclampsia and the associated risk factors among pregnant women in Bangladesh. Sci. Rep. 2021, 11, 21339. [Google Scholar] [CrossRef]
- Hao, J.; Hassen, D.; Hao, Q.; Graham, J.; Paglia, M.J.; Brown, J.; Cooper, M.; Schlieder, V.; Snyder, S.R. Maternal and infant health care costs related to preeclampsia. Obstet. Gynecol. 2019, 134, 1227–1233. [Google Scholar] [CrossRef]
- Fox, A.; McHugh, S.; Browne, J.; Kenny, L.C.; Fitzgerald, A.; Khashan, A.S.; Dempsey, E.; Fahy, C.; O’Neill, C.; Kearney, P.M. Estimating the cost of preeclampsia in the healthcare system: Cross-sectional study using data from SCOPE study (Screening for Pregnancy End Points). Hypertension 2017, 70, 1243–1249. [Google Scholar] [CrossRef]
- Chang, K.-J.; Seow, K.-M.; Chen, K.-H. Preeclampsia: Recent Advances in Predicting, Preventing, and Managing the Maternal and Fetal Life-Threatening Condition. Int. J. Environ. Res. Public Health 2023, 20, 2994. [Google Scholar] [CrossRef]
- Kovacheva, V.P.; Venkatachalam, S.; Pfister, C.; Anwer, T. Preeclampsia and eclampsia: Enhanced detection and treatment for morbidity reduction. Best Pract. Res. Clin. Anaesthesiol. 2024, 38, 246–256. [Google Scholar] [CrossRef]
- Gari, A.; Alshanqiti, W.; Alshanqiti, F.; Alquzi, R.; Alsamli, R.; Alqahtani, R. Level of knowledge on preeclampsia symptoms, complications, and risk factors among women in Saudi Arabia: A cross sectional study. Med. Sci. 2022, 26, 1–9. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, challenges, and perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef] [PubMed]
- Morley, L.; Debant, M.; Walker, J.; Beech, D.; Simpson, N. Placental blood flow sensing and regulation in fetal growth restriction. Placenta 2021, 113, 23–28. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Feng, X.; Lash, G.E. Unraveling the mysteries of spiral artery remodeling. Placenta 2023, 141, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Gyselaers, W. Hemodynamic pathways of gestational hypertension and preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S988–S1005. [Google Scholar] [CrossRef]
- Chappell, L.C.; Cluver, C.A.; Tong, S. Pre-eclampsia. Lancet 2021, 398, 341–354. [Google Scholar] [CrossRef]
- Verlohren, S.; Dröge, L.-A. The diagnostic value of angiogenic and antiangiogenic factors in differential diagnosis of preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S1048–S1058. [Google Scholar] [CrossRef]
- Rana, S.; Burke, S.D.; Karumanchi, S.A. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am. J. Obstet. Gynecol. 2022, 226, S1019–S1034. [Google Scholar] [CrossRef]
- Liu, N.; Guo, Y.-N.; Gong, L.-K.; Wang, B.-S. Advances in biomarker development and potential application for preeclampsia based on pathogenesis. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2021, 9, 100119. [Google Scholar] [CrossRef]
- Mora-Palazuelos, C.; Bermúdez, M.; Aguilar-Medina, M.; Ramos-Payan, R.; Ayala-Ham, A.; Romero-Quintana, J.G. Cytokine-polymorphisms associated with Preeclampsia: A review. Medicine 2022, 101, e30870. [Google Scholar] [CrossRef]
- Opichka, M.A.; Rappelt, M.W.; Gutterman, D.D.; Grobe, J.L.; McIntosh, J.J. Vascular dysfunction in preeclampsia. Cells 2021, 10, 3055. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, N.; Lees, C. Preeclampsia: Maternal cardiovascular function and optimising outcomes. Early Hum. Dev. 2022, 174, 105669. [Google Scholar] [CrossRef]
- Bartal, M.F.; Lindheimer, M.D.; Sibai, B.M. Proteinuria during pregnancy: Definition, pathophysiology, methodology, and clinical significance. Am. J. Obstet. Gynecol. 2022, 226, S819–S834. [Google Scholar] [CrossRef] [PubMed]
- de Logivière, V.; Tsatsaris, V.; Lepercq, J.; Goffinet, F.; Girault, A. Evaluating the proteinuria/creatininuria ratio as a rapid prognostic tool for complications of preeclampsia: A comparison with 24-hour proteinuria. J. Gynecol. Obstet. Hum. Reprod. 2025, 54, 102873. [Google Scholar] [CrossRef]
- Artemieva, K.; Nizyaeva, N.; Baev, O.; Romanov, A.Y.; Khlestova, G.; Boltovskaya, M.; Shchegolev, A.; Kakturskiy, L. Regulation of the placental renin-angiotensin-aldosterone system in early-and late-onset preeclampsia. Dokl. Biochem. Biophys. 2022, 507, 256–263. [Google Scholar] [CrossRef]
- Leal, C.R.V.; Costa, L.B.; Ferreira, G.C.; de Melo Ferreira, A.; Reis, F.M.; e Silva, A.C.S. Renin-angiotensin system in normal pregnancy and in preeclampsia: A comprehensive review. Pregnancy Hypertens. 2022, 28, 15–20. [Google Scholar] [CrossRef]
- Wei, X.; Yang, X. The central role of natural killer cells in preeclampsia. Front. Immunol. 2023, 14, 1009867. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, M.A.; Arefnezhad, R.; Parhizkar, F.; Hejazi, M.S.; Motavalli Khiavi, F.; Mahmoodpoor, A.; Yousefi, M. T lymphocytes and preeclampsia: The potential role of T-cell subsets and related MicroRNAs in the pathogenesis of preeclampsia. Am. J. Reprod. Immunol. 2021, 86, e13475. [Google Scholar] [CrossRef] [PubMed]
- Headen, K.; Jakaite, V.; Mesaric, V.A.; Scotta, C.; Lombardi, G.; Nicolaides, K.H.; Shangaris, P. The Role of Regulatory T Cells and Their Therapeutic Potential in Hypertensive Disease of Pregnancy: A Literature Review. Int. J. Mol. Sci. 2024, 25, 4884. [Google Scholar] [CrossRef]
- Kay, V.R.; Wedel, N.; Smith, G.N. Family history of hypertension, cardiovascular disease, or diabetes and risk of developing preeclampsia: A systematic review. J. Obstet. Gynaecol. Can. 2021, 43, 227–236.e19. [Google Scholar] [CrossRef]
- Wu, C.-T.; Kuo, C.-F.; Lin, C.-P.; Huang, Y.-T.; Chen, S.-W.; Wu, H.-M.; Chu, P.-H. Association of family history with incidence and gestational hypertension outcomes of preeclampsia. Int. J. Cardiol. Hypertens. 2021, 9, 100084. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, M. The association of familial hypertension and risk of gestational hypertension and preeclampsia. Int. J. Environ. Res. Public Health 2021, 18, 7045. [Google Scholar] [CrossRef]
- Meng, Y.; Meng, Y.; Li, L.; Li, Y.; He, J.; Shan, Y. The role of DNA methylation in placental development and its implications for preeclampsia. Front. Cell Dev. Biol. 2024, 12, 1494072. [Google Scholar] [CrossRef]
- Wang, J.; Song, H.; Zhang, Y. Comprehensive analysis of gene expression and DNA methylation for preeclampsia progression. J. Chin. Med. Assoc. 2021, 84, 410–417. [Google Scholar] [CrossRef]
- Wheeler, S.M.; Myers, S.O.; Swamy, G.K.; Myers, E.R. Estimated prevalence of risk factors for preeclampsia among individuals giving birth in the US in 2019. JAMA Netw. Open 2022, 5, e2142343. [Google Scholar] [CrossRef]
- Poniedziałek-Czajkowska, E.; Mierzyński, R.; Leszczyńska-Gorzelak, B. Preeclampsia and obesity—The preventive role of exercise. Int. J. Environ. Res. Public Health 2023, 20, 1267. [Google Scholar] [CrossRef] [PubMed]
- Abramova, M.; Churnosova, M.; Efremova, O.; Aristova, I.; Reshetnikov, E.; Polonikov, A.; Churnosov, M.; Ponomarenko, I. Effects of pre-pregnancy overweight/obesity on the pattern of association of hypertension susceptibility genes with preeclampsia. Life 2022, 12, 2018. [Google Scholar] [CrossRef]
- Alanazi, A.S.; Victor, F.; Rehman, K.; Khan, Y.H.; Yunusa, I.; Alzarea, A.I.; Akash, M.S.H.; Mallhi, T.H. Pre-Existing Diabetes Mellitus, Hypertension and KidneyDisease as Risk Factors of Pre-Eclampsia: A Disease of Theories and Its Association with Genetic Polymorphism. Int. J. Environ. Res. Public Health 2022, 19, 16690. [Google Scholar] [CrossRef] [PubMed]
- Coban, U.; Takmaz, T.; Unyeli, O.D.; Ozdemir, S. Adverse outcomes of preeclampsia in previous and subsequent pregnancies and the risk of recurrence. Med. Bull. Sisli Etfal Hosp. 2021, 55, 426. [Google Scholar] [CrossRef]
- Duffy, C.R. Multifetal gestations and associated perinatal risks. Neoreviews 2021, 22, e734–e746. [Google Scholar] [CrossRef]
- Mitro, S.D.; Sundaram, R.; Qiao, Y.; Gleason, J.L.; Yeung, E.; Hinkle, S.N.; Mendola, P.; Mills, J.L.; Grandi, S.M.; Mumford, S.L. History of multifetal gestation and long-term maternal mortality. Paediatr. Perinat. Epidemiol. 2024, 38, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Rector, A.; Marić, I.; Chaichian, Y.; Chakravarty, E.; Cantu, M.; Weisman, M.H.; Shaw, G.M.; Druzin, M.L.; Simard, J.F. Hydroxychloroquine in lupus pregnancy and risk of preeclampsia. Arthritis Rheumatol. 2024, 76, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Pickel, K.; Nanda, M.; Gajic, M.; Cervar-Zivkovic, M. Preeclampsia and the antiphospholipid syndrome. Biomedicines 2023, 11, 2298. [Google Scholar] [CrossRef]
- Kornfield, M.S.; Gurley, S.B.; Vrooman, L.A. Increased Risk of Preeclampsia with Assisted Reproductive Technologies. Curr. Hypertens. Rep. 2023, 25, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.; Vidaeff, A.; Pettker, C.; Simhan, H. ACOG practice bulletin no. 202: Gestational hypertension and preeclampsia. Obs. Gynecol 2019, 133, e1–e25. [Google Scholar]
- Khan, B.; Yar, R.A.; khan Khakwani, A.; Karim, S.; Ali, H.A.; Khakwani, A.; Karim, S. Preeclampsia incidence and its maternal and neonatal outcomes with associated risk factors. Cureus 2022, 14, e31143. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zhao, Y. Inflammation in preeclampsia: Genetic biomarkers, mechanisms, and therapeutic strategies. Front. Immunol. 2022, 13, 883404. [Google Scholar] [CrossRef]
- Tyrmi, J.S.; Kaartokallio, T.; Lokki, A.I.; Jääskeläinen, T.; Kortelainen, E.; Ruotsalainen, S.; Karjalainen, J.; Ripatti, S.; Kivioja, A.; Laisk, T. Genetic risk factors associated with preeclampsia and hypertensive disorders of pregnancy. JAMA Cardiol. 2023, 8, 674–683. [Google Scholar] [CrossRef]
- Gebreyohannes, R.D.; Abdella, A.; Ayele, W.; Eke, A.C. Association of dietary calcium intake, total and ionized serum calcium levels with preeclampsia in Ethiopia. BMC Pregnancy Childbirth 2021, 21, 1–7. [Google Scholar] [CrossRef]
- Afrose, D.; Alfonso-Sánchez, S.; McClements, L. Targeting oxidative stress in preeclampsia. Hypertens. Pregnancy 2025, 44, 2445556. [Google Scholar] [CrossRef]
- Hayes, L.; McParlin, C.; Azevedo, L.B.; Jones, D.; Newham, J.; Olajide, J.; McCleman, L.; Heslehurst, N. The effectiveness of smoking cessation, alcohol reduction, diet and physical activity interventions in improving maternal and infant health outcomes: A systematic review of meta-analyses. Nutrients 2021, 13, 1036. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.; Sutrave, P.; Gascoigne, E.; Givens, M.B.; Fry, R.C.; Manuck, T.A. Exposure to toxic metals and per-and polyfluoroalkyl substances and the risk of preeclampsia and preterm birth in the United States: A review. Am. J. Obstet. Gynecol. MFM 2021, 3, 100308. [Google Scholar] [CrossRef]
- Vanderlelie, J.; Scott, R.; Shibl, R.; Lewkowicz, J.; Perkins, A.; Scuffham, P.A. First trimester multivitamin/mineral use is associated with reduced risk of pre-eclampsia among overweight and obese women. Matern. Child Nutr. 2016, 12, 339–348. [Google Scholar] [CrossRef]
- Bartsch, E.; Medcalf, K.E.; Park, A.L.; Ray, J.G. Clinical risk factors for pre-eclampsia determined in early pregnancy: Systematic review and meta-analysis of large cohort studies. BMJ 2016, 353, i1753. [Google Scholar] [CrossRef]
- National Collaborating Centre for Women’s and Children’s Health. Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy; RCOG Press: London, UK, 2010. [Google Scholar]
- Obstetricians, A. Gynecologists, Pregnancy TFoHi: Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Chaemsaithong, P.; Sahota, D.S.; Poon, L.C. First trimester preeclampsia screening and prediction. Am. J. Obstet. Gynecol. 2022, 226, S1071–S1097.e2. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive Disorders of Pregnancy. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes, A. 14. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes—2019. Diabetes Care 2018, 42, S165–S172. [Google Scholar] [CrossRef]
- Ghesquière, L.; Bujold, E.; Dubé, E.; Chaillet, N. Comparison of National Factor-Based Models for Preeclampsia Screening. Am. J. Perinatol. 2024, 41, 1930–1935. [Google Scholar] [CrossRef]
- O’Gorman, N.; Wright, D.; Poon, L.C.; Rolnik, D.L.; Syngelaki, A.; de Alvarado, M.; Carbone, I.F.; Dutemeyer, V.; Fiolna, M.; Frick, A.; et al. Multicenter screening for pre-eclampsia by maternal factors and biomarkers at 11–13 weeks’ gestation: Comparison with NICE guidelines and ACOG recommendations. Ultrasound Obstet. Gynecol. 2017, 49, 756–760. [Google Scholar] [CrossRef]
- Chaemsaithong, P.; Pooh, R.K.; Zheng, M.; Ma, R.; Chaiyasit, N.; Tokunaka, M.; Shaw, S.W.; Seshadri, S.; Choolani, M.; Wataganara, T.; et al. Prospective evaluation of screening performance of first-trimester prediction models for preterm preeclampsia in an Asian population. Am. J. Obstet. Gynecol. 2019, 221, 650.e1–650.e16. [Google Scholar] [CrossRef] [PubMed]
- Staff, A.C.; Braekke, K.; Johnsen, G.M.; Karumanchi, S.A.; Harsem, N.K. Circulating concentrations of soluble endoglin (CD105) in fetal and maternal serum and in amniotic fluid in preeclampsia. Am. J. Obstet. Gynecol. 2007, 197, 176.e1–176.e6. [Google Scholar] [CrossRef]
- Vrachnis, N.; Kalampokas, E.; Sifakis, S.; Vitoratos, N.; Kalampokas, T.; Botsis, D.; Iliodromiti, Z. Placental growth factor (PlGF): A key to optimizing fetal growth. J. Matern.-Fetal Neonatal Med. 2013, 26, 995–1002. [Google Scholar] [CrossRef]
- Helmo, F.R.; Lopes, A.M.M.; Carneiro, A.C.D.M.; Campos, C.G.; Silva, P.B.; dos Reis Monteiro, M.L.G.; Rocha, L.P.; Dos Reis, M.A.; Etchebehere, R.M.; Machado, J.R. Angiogenic and antiangiogenic factors in preeclampsia. Pathol.-Res. Pract. 2018, 214, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Pankiewicz, K.; Szczerba, E.; Fijalkowska, A.; Szamotulska, K.; Szewczyk, G.; Issat, T.; Maciejewski, T.M. The association between serum galectin-3 level and its placental production in patients with preeclampsia. J. Physiol. Pharmacol. 2020, 71, 845–856. [Google Scholar] [CrossRef]
- Azimi-Nezhad, M. Vascular endothelial growth factor from embryonic status to cardiovascular pathology. Rep. Biochem. Mol. Biol. 2014, 2, 59–69. [Google Scholar] [PubMed]
- Velegrakis, A.; Kouvidi, E.; Fragkiadaki, P.; Sifakis, S. Predictive value of the sFlt-1/PlGF ratio in women with suspected preeclampsia: An update (Review). Int. J. Mol. Med. 2023, 52, 89. [Google Scholar] [CrossRef]
- Gilbert, J.S.; Ryan, M.J.; LaMarca, B.B.; Sedeek, M.; Murphy, S.R.; Granger, J.P. Pathophysiology of hypertension during preeclampsia: Linking placental ischemia with endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H541–H550. [Google Scholar] [CrossRef]
- Nagamatsu, T.; Fujii, T.; Kusumi, M.; Zou, L.; Yamashita, T.; Osuga, Y.; Momoeda, M.; Kozuma, S.; Taketani, Y. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: An implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 2004, 145, 4838–4845. [Google Scholar] [CrossRef]
- Rajakumar, A.; Doty, K.; Daftary, A.; Harger, G.; Conrad, K.P. Impaired oxygen-dependent reduction of HIF-1alpha and -2alpha proteins in pre-eclamptic placentae. Placenta 2003, 24, 199–208. [Google Scholar] [CrossRef]
- Hastie, R.; Brownfoot, F.C.; Pritchard, N.; Hannan, N.J.; Cannon, P.; Nguyen, V.; Palmer, K.; Beard, S.; Tong, S.; Kaitu’u-Lino, T.u.J. EGFR (epidermal growth factor receptor) signaling and the mitochondria regulate sFlt-1 (soluble FMS-like tyrosine kinase-1) secretion. Hypertension 2019, 73, 659–670. [Google Scholar] [CrossRef]
- Thadhani, R.; Mutter, W.P.; Wolf, M.; Levine, R.J.; Taylor, R.N.; Sukhatme, V.P.; Ecker, J.; Karumanchi, S.A. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J. Clin. Endocrinol. Metab. 2004, 89, 770–775. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Romero, R.; Kim, Y.M.; Kim, G.J.; Kim, M.R.; Espinoza, J.; Bujold, E.; Gonçalves, L.; Gomez, R.; Edwin, S. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J. Matern.-Fetal Neonatal Med. 2005, 17, 3–18. [Google Scholar] [CrossRef]
- Buhimschi, C.S.; Norwitz, E.R.; Funai, E.; Richman, S.; Guller, S.; Lockwood, C.J.; Buhimschi, I.A. Urinary angiogenic factors cluster hypertensive disorders and identify women with severe preeclampsia. Am. J. Obstet. Gynecol. 2005, 192, 734–741. [Google Scholar] [CrossRef]
- Hirashima, C.; Ohkuchi, A.; Arai, F.; Takahashi, K.; Suzuki, H.; Watanabe, T.; Kario, K.; Matsubara, S.; Suzuki, M. Establishing reference values for both total soluble Fms-like tyrosine kinase 1 and free placental growth factor in pregnant women. Hypertens. Res. 2005, 28, 727–732. [Google Scholar] [CrossRef]
- Ohkuchi, A.; Hirashima, C.; Matsubara, S.; Suzuki, H.; Takahashi, K.; Arai, F.; Watanabe, T.; Kario, K.; Suzuki, M. Alterations in placental growth factor levels before and after the onset of preeclampsia are more pronounced in women with early onset severe preeclampsia. Hypertens. Res. 2007, 30, 151–159. [Google Scholar] [CrossRef]
- Levine, R.J.; Lam, C.; Qian, C.; Yu, K.F.; Maynard, S.E.; Sachs, B.P.; Sibai, B.M.; Epstein, F.H.; Romero, R.; Thadhani, R.; et al. Soluble Endoglin and Other Circulating Antiangiogenic Factors in Preeclampsia. N. Engl. J. Med. 2006, 355, 992–1005. [Google Scholar] [CrossRef]
- Stepan, H.; Unversucht, A.; Wessel, N.; Faber, R. Predictive value of maternal angiogenic factors in second trimester pregnancies with abnormal uterine perfusion. Hypertension 2007, 49, 818–824. [Google Scholar] [CrossRef] [PubMed]
- De Vivo, A.; Baviera, G.; Giordano, D.; Todarello, G.; Corrado, F.; D’anna, R. Endoglin, PlGF and sFlt-1 as markers for predicting pre-eclampsia. Acta Obstet. Et Gynecol. Scand. 2008, 87, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Nien, J.K.; Espinoza, J.; Todem, D.; Fu, W.; Chung, H.; Kusanovic, J.P.; Gotsch, F.; Erez, O.; Mazaki-Tovi, S. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J. Matern.-Fetal Neonatal Med. 2008, 21, 9–23. [Google Scholar] [PubMed]
- Verlohren, S.; Galindo, A.; Schlembach, D.; Zeisler, H.; Herraiz, I.; Moertl, M.G.; Pape, J.; Dudenhausen, J.W.; Denk, B.; Stepan, H. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am. J. Obstet. Gynecol. 2010, 202, 161.e1–161.e11. [Google Scholar] [CrossRef]
- Sunderji, S.; Gaziano, E.; Wothe, D.; Rogers, L.C.; Sibai, B.; Karumanchi, S.A.; Hodges-Savola, C. Automated assays for sVEGF R1 and PlGF as an aid in the diagnosis of preterm preeclampsia: A prospective clinical study. Am. J. Obstet. Gynecol. 2010, 202, 40.e1–40.e7. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Romero, R.; Savasan, Z.A.; Kusanovic, J.P.; Ogge, G.; Soto, E.; Dong, Z.; Tarca, A.; Gaurav, B.; Hassan, S.S. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J. Matern.-Fetal Neonatal Med. 2011, 24, 1187–1207. [Google Scholar] [CrossRef]
- Rana, S.; Powe, C.E.; Salahuddin, S.; Verlohren, S.; Perschel, F.H.; Levine, R.J.; Lim, K.-H.; Wenger, J.B.; Thadhani, R.; Karumanchi, S.A. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 2012, 125, 911–919. [Google Scholar] [CrossRef]
- Moore, A.G.; Young, H.; Keller, J.M.; Ojo, L.R.; Yan, J.; Simas, T.A.M.; Maynard, S.E. Angiogenic biomarkers for prediction of maternal and neonatal complications in suspected preeclampsia. J. Matern.-Fetal Neonatal Med. 2012, 25, 2651–2657. [Google Scholar] [CrossRef]
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Moertl, M.; Zeisler, H.; Calda, P.; Holzgreve, W.; Galindo, A.; Engels, T. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am. J. Obstet. Gynecol. 2012, 206, 58.e1–58.e8. [Google Scholar] [CrossRef]
- Chen, W.; Wei, Q.; Liang, Q.; Song, S.; Li, J. Diagnostic capacity of sFlt-1/PlGF ratio in fetal growth restriction: A systematic review and meta-analysis. Placenta 2022, 127, 37–42. [Google Scholar] [CrossRef]
- Satorres, E.; Martínez-Varea, A.; Diago-Almela, V. sFlt-1/PlGF ratio as a predictor of pregnancy outcomes in twin pregnancies: A systematic review. J. Matern.-Fetal Neonatal Med. 2023, 36, 2230514. [Google Scholar] [CrossRef]
- Corominas, A.I.; Medina, Y.; Balconi, S.; Casale, R.; Farina, M.; Martínez, N.; Damiano, A.E. Assessing the role of uric acid as a predictor of preeclampsia. Front. Physiol. 2022, 12, 785219. [Google Scholar] [CrossRef]
- Margioula-Siarkou, G.; Margioula-Siarkou, C.; Petousis, S.; Margaritis, K.; Alexandratou, M.; Dinas, K.; Sotiriadis, A.; Mavromatidis, G. Soluble endoglin concentration in maternal blood as a diagnostic biomarker of preeclampsia: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 258, 366–381. [Google Scholar] [CrossRef]
- Shu, Z.; Wang, W. Predictive value of prenatal screening markers combined with serum placental growth factor in early pregnancy for preeclampsia. Pak. J. Med. Sci. 2025, 41, 598. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, H.; Wu, B.; Ning, W.; Chen, Y.; Chen, Y. Correlation between elevated maternal serum alpha-fetoprotein and ischemic placental disease: A retrospective cohort study. Clin. Exp. Hypertens. 2023, 45, 2175848. [Google Scholar] [CrossRef]
- Khosla, K.; Espinoza, J.; Perlaza, L.; Gencay, M.; Mueller, A.L.; Harris, J.M.; Wolf, C.; Posnett, J.W.; Woelkers, D.A.; Rana, S. Cost effectiveness of the sFlt1/PlGF ratio test as an adjunct to the current practice of evaluating suspected preeclampsia in the United States. Pregnancy Hypertens. 2021, 26, 121–126. [Google Scholar] [CrossRef]
- Schlembach, D.; Hund, M.; Schroer, A.; Wolf, C. Economic assessment of the use of the sFlt-1/PlGF ratio test to predict preeclampsia in Germany. BMC Health Serv. Res. 2018, 18, 603. [Google Scholar] [CrossRef]
- Wind, M.; van den Akker-van Marle, M.E.; Ballieux, B.; Cobbaert, C.M.; Rabelink, T.J.; van Lith, J.M.M.; Teng, Y.K.O.; Sueters, M. Clinical value and cost analysis of the sFlt-1/PlGF ratio in addition to the spot urine protein/creatinine ratio in women with suspected pre-eclampsia: PREPARE cohort study. BMC Pregnancy Childbirth 2022, 22, 910. [Google Scholar] [CrossRef]
- Frusca, T.; Gervasi, M.T.; Paolini, D.; Dionisi, M.; Ferre, F.; Cetin, I. Budget impact analysis of sFlt-1/PlGF ratio as prediction test in Italian women with suspected preeclampsia. J. Matern.-Fetal Neonatal Med. 2017, 30, 2166–2173. [Google Scholar] [CrossRef]
- Hodel, M.; Blank, P.R.; Marty, P.; Lapaire, O. sFlt-1/PlGF Ratio as a Predictive Marker in Women with Suspected Preeclampsia: An Economic Evaluation from a Swiss Perspective. Dis. Markers 2019, 2019, 4096847. [Google Scholar] [CrossRef]
- Vatish, M.; Strunz-McKendry, T.; Hund, M.; Allegranza, D.; Wolf, C.; Smare, C. sFlt-1/PlGF ratio test for pre-eclampsia: An economic assessment for the UK. Ultrasound Obstet. Gynecol. 2016, 48, 765–771. [Google Scholar] [CrossRef]
- Ohkuchi, A.; Masuyama, H.; Yamamoto, T.; Kikuchi, T.; Taguchi, N.; Wolf, C.; Saito, S. Economic evaluation of the sFlt-1/PlGF ratio for the short-term prediction of preeclampsia in a Japanese cohort of the PROGNOSIS Asia study. Hypertens. Res. 2021, 44, 822–829. [Google Scholar] [CrossRef]
- Garay, O.U.; Guiñazú, G.G.; Basualdo, N.; Di Marco, I.; Zilberman, J.; Voto, L. Economic Impact Analysis of Incorporation of Elecsys sFlt-1/PlGF Ratio Into Routine Practice for the Diagnosis and Follow-Up of Pregnant Women With Suspected Preeclampsia in Argentina. Value Health Reg. Issues 2023, 34, 1–8. [Google Scholar] [CrossRef]
- Figueira, S.F.; Wolf, C.; D’Innocenzo, M.; de Carvalho, J.P.V.; Barbosa, M.G.; Zlotnik, E.; Cordioli, E. Economic evaluation of sFlt-1/PlGF ratio test in pre-eclampsia prediction and diagnosis in two Brazilian hospitals. Pregnancy Hypertens. 2018, 13, 30–36. [Google Scholar] [CrossRef]
- Duva, A.S.; Rosim, R.P.; Ballalai Ferraz, A.F.; Cachoeira, C.; Mojica, I.L. Economic Benefits of SFLT-1/PLGF Testing for Preeclampsia in Colombia: A Fiver Year Budget Impact Analysis. Value Health 2017, 20, A864. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z. PMD21 ECONOMIC EVALUATION OF THE SFLT-1/PLGF RATIO TEST TO GUIDE THE MANAGEMENT OF CHINESE SUSPECTED PRE-ECLAMPSIA WOMEN. Value Health 2019, 22, S673. [Google Scholar] [CrossRef]
- Khatri, R.; Jain, B.; Mhapankar, S.; Kumar, S. A study of Doppler velocimetry in pre-eclampsia patients, and their perinatal outcome. Obstet. Gynecol. Res. 2021, 4, 90–100. [Google Scholar] [CrossRef]
- Abonyi, E.O.; Idigo, F.U.; Anakwue, A.-M.C.; Agbo, J.A. Sensitivity of uterine artery Doppler pulsatility index in screening for adverse pregnancy outcome in first and second trimesters. J. Ultrasound 2023, 26, 517–523. [Google Scholar]
- Liu, Y.; Xie, Z.; Huang, Y.; Lu, X.; Yin, F. Uterine arteries pulsatility index by Doppler ultrasound in the prediction of preeclampsia: An updated systematic review and meta-analysis. Arch. Gynecol. Obstet. 2024, 309, 427–437. [Google Scholar] [CrossRef]
- Schneider, E.; Kinzler, W.L. Placental Abruption: Pathophysiology, Diagnosis, and Management. Clin. Obstet. Gynecol. 2025, 68, 98–104. [Google Scholar] [CrossRef]
- Schiffer, V.; van Haren, A.; De Cubber, L.; Bons, J.; Coumans, A.; van Kuijk, S.M.; Spaanderman, M.; Al-Nasiry, S. Ultrasound evaluation of the placenta in healthy and placental syndrome pregnancies: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 262, 45–56. [Google Scholar]
- Zhou, P.; Sun, Y.; Tan, Y.; An, Y.; Wang, X.; Wang, L. Fetal and neonatal middle cerebral artery hemodynamic changes and significance under ultrasound detection in hypertensive disorder complicating pregnancy patients with different severities. Comput. Math. Methods Med. 2022, 2022, 6110228. [Google Scholar] [CrossRef]
- Rose, S.; Farooq, S.M.Y.; Fatima, M.; Gilani, S.A.; Shams Rana, A.; Ramzan, I. Correlation Between Fetal Umbilical Artery and Middle Cerebral Artery Doppler Indices in Preeclamptic and Normotensive Pregnancies. J. Diagn. Med. Sonogr. 2024, 40, 352–359. [Google Scholar] [CrossRef]
- Zhao, X.; Shen, Y. The value of ultrasound spectra of middle cerebral artery and umbilical artery blood flow in adverse pregnancy outcomes. J. Perinat. Med. 2024, 53, 234–241. [Google Scholar]
- Lakshmy, S.; Ziyaulla, T.; Rose, N. The need for implementation of first trimester screening for preeclampsia and fetal growth restriction in low resource settings. J. Matern.-Fetal Neonatal Med. 2021, 34, 4082–4089. [Google Scholar] [CrossRef]
- Özgen, G.; Cakmak, B.D.; Özgen, L.; Uguz, S.; Sager, H. The role of oligohydramnios and fetal growth restriction in adverse pregnancy outcomes in preeclamptic patients. Ginekol. Pol. 2022, 93, 235–241. [Google Scholar]
- Jayson, J.; Mandrelle, K.; Dhar, T.; Singla, S. First Trimester Uterine Artery Doppler Screening in the Prediction of Adverse Pregnancy Outcomes. Int. J. Reprod. Contracept. Obstet. Gynecol. 2021, 10, 3934. [Google Scholar]
- Poon, L.C.; Galindo, A.; Surbek, D.; Chantraine, F.; Stepan, H.; Hyett, J.; Tan, K.H.; Verlohren, S. From first-trimester screening to risk stratification of evolving pre-eclampsia in second and third trimesters of pregnancy: Comprehensive approach. Ultrasound Obstet. Gynecol. 2020, 55, 5–12. [Google Scholar] [CrossRef]
- Porter, T.F.; Gyamfi-Bannerman, C.; Manuck, T. Low-dose aspirin use during pregnancy. Obstet. Gynecol. 2018, 132, E44–E52. [Google Scholar]
- Tan, M.; Wright, D.; Syngelaki, A.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; Greco, E.; Wright, A.; Maclagan, K. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: Results of SPREE. Ultrasound Obstet. Gynecol. 2018, 51, 743–750. [Google Scholar] [CrossRef]
- Moreira, M.W.; Rodrigues, J.J.; Oliveira, A.M.; Ramos, R.F.; Saleem, K. A preeclampsia diagnosis approach using Bayesian networks. In Proceedings of the 2016 IEEE International Conference on Communications (ICC), Kuala Lumpur, Malaysia, 22–27 May 2016; pp. 1–5. [Google Scholar]
- Zeng, L.; Liao, C. Multivariate logistic regression analysis of preeclampsia in patients with pregnancy induced hypertension and the risk predictive value of monitoring platelet, coagulation function and thyroid hormone in pregnant women. Am. J. Transl. Res. 2022, 14, 6805. [Google Scholar]
- Tiruneh, S.A.; Vu, T.T.T.; Rolnik, D.L.; Teede, H.J.; Enticott, J. Machine Learning Algorithms Versus Classical Regression Models in Pre-Eclampsia Prediction: A Systematic Review. Curr. Hypertens. Rep. 2024, 26, 309–323. [Google Scholar] [CrossRef]
- Marić, I.; Tsur, A.; Aghaeepour, N.; Montanari, A.; Stevenson, D.K.; Shaw, G.M.; Winn, V.D. Early prediction of preeclampsia via machine learning. Am. J. Obstet. Gynecol. MFM 2020, 2, 100100. [Google Scholar]
- Jhee, J.H.; Lee, S.; Park, Y.; Lee, S.E.; Kim, Y.A.; Kang, S.-W.; Kwon, J.-Y.; Park, J.T. Prediction model development of late-onset preeclampsia using machine learning-based methods. PLoS ONE 2019, 14, e0221202. [Google Scholar] [CrossRef]
- Li, Y.-x.; Shen, X.-p.; Yang, C.; Cao, Z.-z.; Du, R.; Yu, M.-d.; Wang, J.-p.; Wang, M. Novel electronic health records applied for prediction of pre-eclampsia: Machine-learning algorithms. Pregnancy Hypertens. 2021, 26, 102–109. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Vieira, L.A.; Zheutlin, A.B.; Ru, B.; Schadt, E.; Wang, P.; Copperman, A.B.; Stone, J.L.; Gross, S.J.; et al. Improving preeclampsia risk prediction by modeling pregnancy trajectories from routinely collected electronic medical record data. npj Digit. Med. 2022, 5, 68. [Google Scholar] [CrossRef]
- Yang, X.; Ballard, H.K.; Mahadevan, A.D.; Xu, K.; Garmire, D.G.; Langen, E.S.; Lemas, D.J.; Garmire, L.X. Deep learning-based prognosis models accurately predict the time to delivery among preeclampsia patients using health records at the time of diagnosis. medRxiv 2024. [Google Scholar] [CrossRef]
- Vázquez-Ingelmo, A.; Alonso-Sánchez, J.; García-Holgado, A.; García Peñalvo, F.J.; Sampedro-Gómez, J.; Sánchez-Puente, A.; Vicente-Palacios, V.; Dorado-Díaz, P.I.; Sanchez, P.L. Bringing machine learning closer to non-experts: Proposal of a user-friendly machine learning tool in the healthcare domain. In Proceedings of the Ninth International Conference on Technological Ecosystems for Enhancing Multiculturality (TEEM’21), Barcelona, Spain, 26–29 October 2021; pp. 324–329. [Google Scholar]
- Ranjbar, A.; Montazeri, F.; Ghamsari, S.R.; Mehrnoush, V.; Roozbeh, N.; Darsareh, F. Machine learning models for predicting preeclampsia: A systematic review. BMC Pregnancy Childbirth 2024, 24, 6. [Google Scholar] [CrossRef]
- Kurjak, A.; Stanojević, M.; Dudenhausen, J. Why maternal mortality in the world remains tragedy in low-income countries and shame for high-income ones: Will sustainable development goals (SDG) help? J. Perinat. Med. 2023, 51, 170–181. [Google Scholar] [CrossRef]
- Von Dadelszen, P.; Vidler, M.; Tsigas, E.; Magee, L.A. Management of preeclampsia in low-and middle-income countries: Lessons to date, and questions arising, from the PRE-EMPT and related initiatives. Matern.-Fetal Med. 2021, 3, 136–150. [Google Scholar] [CrossRef]
- Woldemariam, M.T.; Jimma, W. Adoption of electronic health record systems to enhance the quality of healthcare in low-income countries: A systematic review. BMJ Health Care Inform. 2023, 30, e100704. [Google Scholar] [CrossRef]
- Saeed, S.A.; Masters, R.M. Disparities in health care and the digital divide. Curr. Psychiatry Rep. 2021, 23, 61. [Google Scholar] [CrossRef]
- Montgomery-Csobán, T.; Kavanagh, K.; Murray, P.; Robertson, C.; Barry, S.J.; Ukah, U.V.; Payne, B.A.; Nicolaides, K.H.; Syngelaki, A.; Ionescu, O. Machine learning-enabled maternal risk assessment for women with pre-eclampsia (the PIERS-ML model): A modelling study. Lancet Digit. Health 2024, 6, e238–e250. [Google Scholar] [CrossRef]
- Gómez-Jemes, L.; Oprescu, A.M.; Chimenea-Toscano, Á.; García-Díaz, L.; Romero-Ternero, M.d.C. Machine learning to predict pre-eclampsia and intrauterine growth restriction in pregnant women. Electronics 2022, 11, 3240. [Google Scholar] [CrossRef]
- Benigni, A.; Gregorini, G.; Frusca, T.; Chiabrando, C.; Ballerini, S.; Valcamonico, A.; Orisio, S.; Piccinelli, A.; Pinciroli, V.; Fanelli, R.; et al. Effect of low-dose aspirin on fetal and maternal generation of thromboxane by platelets in women at risk for pregnancy-induced hypertension. N. Engl. J. Med. 1989, 321, 357–362. [Google Scholar] [CrossRef]
- Wallenburg, H.C.; Dekker, G.A.; Makovitz, J.W.; Rotmans, P. Low-dose aspirin prevents pregnancy-induced hypertension and pre-eclampsia in angiotensin-sensitive primigravidae. Lancet 1986, 1, 1–3. [Google Scholar] [CrossRef]
- Dekker, G.A.; Sibai, B.M. Low-dose aspirin in the prevention of preeclampsia and fetal growth retardation: Rationale, mechanisms, and clinical trials. Am. J. Obstet. Gynecol. 1993, 168, 214–227. [Google Scholar] [CrossRef]
- Clarke, R.J.; Mayo, G.; Price, P.; FitzGerald, G.A. Suppression of thromboxane A2 but not of systemic prostacyclin by controlled-release aspirin. N. Engl. J. Med. 1991, 325, 1137–1141. [Google Scholar] [CrossRef]
- Roberts, J.M.; King, T.L.; Barton, J.R.; Beck, S.; Bernstein, I.M.; Buck, T.E.; Forgues-Lackie, M.A.; Facco, F.L.; Gernand, A.D.; Graves, C.R.; et al. Care plan for individuals at risk for preeclampsia: Shared approach to education, strategies for prevention, surveillance, and follow-up. Am. J. Obstet. Gynecol. 2023, 229, 193–213. [Google Scholar] [CrossRef]
- Duley, L.; Meher, S.; Hunter, K.E.; Seidler, A.L.; Askie, L.M. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst. Rev. 2019, 2019, CD004659. [Google Scholar] [CrossRef]
- Henderson, J.T.; Vesco, K.K.; Senger, C.A.; Thomas, R.G.; Redmond, N. Aspirin Use to Prevent Preeclampsia and Related Morbidity and Mortality: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2021, 326, 1192–1206. [Google Scholar] [CrossRef]
- Roberge, S.; Bujold, E.; Nicolaides, K.H. Aspirin for the prevention of preterm and term preeclampsia: Systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2018, 218, 287–293.e1. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Wright, D.; Poon, L.C.; O’Gorman, N.; Syngelaki, A.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N. Engl. J. Med. 2017, 377, 613–622. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H. Effect of low-dose aspirin intervention on pre-eclampsia prevention in high-risk pregnant women and its impact on postpartum hemorrhage. Front. Med. 2024, 11, 1414697. [Google Scholar] [CrossRef]
- Di Girolamo, R.; Alameddine, S.; Khalil, A.; Santilli, F.; Rizzo, G.; Maruotti, G.M.; Liberati, M.; D’Antonio, F. Clinical practice guidelines on the use of aspirin in pregnancy: Systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 282, 64–71. [Google Scholar] [CrossRef]
- Espinoza, J. Low-dose aspirin for the prevention of preeclampsia. JAMA 2021, 326, 1153–1155. [Google Scholar] [CrossRef]
- Cormick, G.; Belizán, J.M. Calcium intake and health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Catov, J.M.; Simhan, H.N.; Holick, M.F.; Powers, R.W.; Roberts, J.M. Maternal vitamin D deficiency increases the risk of preeclampsia. J. Clin. Endocrinol. Metab. 2007, 92, 3517–3522. [Google Scholar] [CrossRef]
- Cormick, G.; Betrán, A.P.; Harbron, J.; Seuc, A.; White, C.; Roberts, J.M.; Belizán, J.M.; Hofmeyr, G.J. The effect of calcium supplementation on body weight before and during pregnancy in women enrolled in the WHO calcium and preeclampsia trial. Food Nutr. Bull. 2020, 41, 332–342. [Google Scholar] [CrossRef]
- Woo Kinshella, M.L.; Sarr, C.; Sandhu, A.; Bone, J.N.; Vidler, M.; Moore, S.E.; Elango, R.; Cormick, G.; Belizan, J.M.; Hofmeyr, G.J.; et al. Calcium for pre-eclampsia prevention: A systematic review and network meta-analysis to guide personalised antenatal care. Bjog 2022, 129, 1833–1843. [Google Scholar] [CrossRef]
- Dwarkanath, P.; Muhihi, A.; Sudfeld, C.R.; Wylie, B.J.; Wang, M.; Perumal, N.; Thomas, T.; Kinyogoli, S.M.; Bakari, M.; Fernandez, R.; et al. Two Randomized Trials of Low-Dose Calcium Supplementation in Pregnancy. N. Engl. J. Med. 2024, 390, 143–153. [Google Scholar] [CrossRef]
- Mostello, D.; Jen Chang, J.; Allen, J.; Luehr, L.; Shyken, J.; Leet, T. Recurrent preeclampsia: The effect of weight change between pregnancies. Obstet. Gynecol. 2010, 116, 667–672. [Google Scholar] [CrossRef]
- Maggard, M.A.; Yermilov, I.; Li, Z.; Maglione, M.; Newberry, S.; Suttorp, M.; Hilton, L.; Santry, H.P.; Morton, J.M.; Livingston, E.H.; et al. Pregnancy and fertility following bariatric surgery: A systematic review. JAMA 2008, 300, 2286–2296. [Google Scholar] [CrossRef]
- Davenport, M.H.; Ruchat, S.M.; Poitras, V.J.; Jaramillo Garcia, A.; Gray, C.E.; Barrowman, N.; Skow, R.J.; Meah, V.L.; Riske, L.; Sobierajski, F.; et al. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Danielli, M.; Gillies, C.; Thomas, R.C.; Melford, S.E.; Baker, P.N.; Yates, T.; Khunti, K.; Tan, B.K. Effects of Supervised Exercise on the Development of Hypertensive Disorders of Pregnancy: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 793. [Google Scholar] [CrossRef] [PubMed]
- Layton, A.T. Artificial Intelligence and Machine Learning in Preeclampsia. Arterioscler. Thromb. Vasc. Biol. 2025, 45, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Luo, Y. Preeclampsia and its prediction: Traditional versus contemporary predictive methods. J. Matern.-Fetal Neonatal Med. 2024, 37, 2388171. [Google Scholar] [CrossRef]
- Hedley, P.L.; Hagen, C.M.; Wilstrup, C.; Christiansen, M. The use of artificial intelligence and machine learning methods in early pregnancy pre-eclampsia screening: A systematic review protocol. PLoS ONE 2023, 18, e0272465. [Google Scholar] [CrossRef]
- Desriva, N.; Sansuwito, T.B.; Dioso, R.I. Preeclampsia Screening Using The Smartphone Method: Literature Review. Int. J. Health Sci. 2023, 1, 873–883. [Google Scholar] [CrossRef]
- Shahil, A. Mobile Phone-Based Telemonitoring for Pregnant Women at High Risk for Pre-Eclampsia in Karachi, Pakistan. Ph.D. Thesis, The University of Toronto, Toronto, ON, Canada, 2024. [Google Scholar]
| Professional Organization | High-Risk Factors | Moderate Risk Factors | Indication for Aspirin Use |
|---|---|---|---|
| NICE, 2019 (United Kingdom), updated in 2023 | Previous pregnancy with PE Chronic hypertension Autoimmune disease T1DM/T2DM Chronic kidney disease Antiphospholipid syndrome | Nulliparity Age, ≥40 y Interpregnancy interval, >10 y BMI at first visit, ≥35 kg/m2 Family history of PE Multifetal pregnancy | 1 or more high-risk factors 2 or more moderate-risk factors Dose: 75 to 150 mg/d from 12 weeks until delivery |
| ACOG, 2018 (USA), updated in 2020 | Previous pregnancy with PE Chronic hypertension Autoimmune disease (systemic lupus erythematosus, the antiphospholipid syndrome) T1DM/T2DM Renal disease Multifetal gestation | Nulliparity Age, ≥35 y Interpregnancy interval, >10 y Obesity (BMI, >30 kg/m2) Family history of PE (mother or sister) History of SGA or adverse outcome Sociodemographic characteristics (African American race or low socioeconomic status) | 1 or more high-risk factors 2 or more moderate-risk factors Dose: 81 mg/d initiated between 12 and 28 weeks (better before 16 weeks), until delivery |
| ISSHP, 2018 | Prior PE Chronic hypertension Pregestational diabetes mellitus BMI, >30 kg/m2 Chronic kidney disease Antiphospholipid syndrome | Advanced maternal age, >35 y Family history of PE Short duration of sexual relationship (<6 mo) before the pregnancy Primiparity Primipaternity Connective tissue disorder | 1 or more high-risk factors 2 or more moderate risk factors Dose: 100 to 150 mg/d start before 16 weeks until 37 weeks |
| SOGC, 2014, updated in 2022 | Previous 3y Chronic hypertension Renal disease T1DM/T2DM Autoimmune disease (systemic lupus erythematosus, antiphospholipid syndrome) Chronic vascular disease Multifetal gestation Obesity (BMI ≥ 30 kg/m2) | Age ≥ 40 years Nulliparity Family history of PE (mother or sister) Interval of more than 10 years since the last pregnancy First-trimester BMI ≥ 25 kg/m2 Interpregnancy interval ≤ 2 years History of preterm birth IVF pregnancy | 1 or more high-risk factors 2 or more moderate risk factors Dose: 81 to 162 mg/d start from before 16 weeks until delivery |
| Country | Study | Key Findings | Estimated Cost Savings per Patient |
|---|---|---|---|
| United States | Khosla et al., 2021 [94] | Implementation of the sFlt-1/PlGF ratio test reduced hospital admissions by 34–49% | USD 1050 |
| Germany | Schlembach et al., 2018 [95] | Use of the sFlt-1/PlGF ratio test decreased hospitalizations from 44.6% to 24.0% | EUR361 |
| Netherlands | Wind et al., 2022 [96] | Combining sFlt-1/PlGF ratio testing with telemonitoring reduced hospital admissions by 41% and outpatient visits by 36% | EUR46 |
| Italy | Frusca et al., 2017 [97] | Introduction of the sFlt-1/PlGF ratio test reduced management costs from EUR 2384 to EUR 1714 per patient | EUR 670 |
| Switzerland | Hodel et al., 2019 [98] | The test helped stratify patients, potentially reducing unnecessary hospitalizations and associated costs. | EUR 345 |
| United Kingdom | Vatish et al., 2016 [99] | The economic analysis suggests that introduction of the test could reduce the number of women hospitalized by more than half (56%), from 36% to 16% | GBP 344 |
| Japan | Ohkuchi et al., 2021 [100] | Introduction of the sFlt-1/PlGF ratio test using a cutoff value of 38 resulted in a reduced hospitalization rate compared with the rate in the no-test scenario (14.4% versus 8.7%) | JPY 16 373 |
| Argentina | Garay et al., 2022 [101] | Nationwide implementation of the sFlt-1/PlGF test could save approximately ARS 6987 million annually, reducing costs by 39.1% through better patient triage. | ARS 80 504 |
| Brazil | Figueira et al., 2018 [102] | The sFlt-1/PlGF test reduced unnecessary hospitalizations and resulted in cost savings in both settings: public hospitals and private hospitals. | BRL 185.06 (public hospital) BRL 635.84 (private hospital) |
| Colombia | Duva et al., 2017 [103] | In Colombia, a five-year budget impact analysis projected that implementing the sFlt-1/PlGF test could save the public healthcare system 47 billion Colombian pesos. This equates to significant cost savings, primarily driven by a reduction in hospitalizations—from 36% in the standard care scenario to 16% with the use of the sFlt-1/PlGF test. | COP 182 841 |
| China | Chen et al., 2019 [104] | The pre-eclampsia cost ‘no-test’ group about EUR 1482 per patient and it cost ‘test’ group EUR 1134 per patient. | EUR 348 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alipova, G.; Ablakimova, N.; Tussupkaliyeva, K.; Bermagambetova, S.; Kosmuratova, S.; Karimsakova, B.; Gaiday, A.; Gaiday, A.; Dinets, A.; Tussupkaliyev, A. Prevention of Pre-Eclampsia: Modern Strategies and the Role of Early Screening. J. Clin. Med. 2025, 14, 2970. https://doi.org/10.3390/jcm14092970
Alipova G, Ablakimova N, Tussupkaliyeva K, Bermagambetova S, Kosmuratova S, Karimsakova B, Gaiday A, Gaiday A, Dinets A, Tussupkaliyev A. Prevention of Pre-Eclampsia: Modern Strategies and the Role of Early Screening. Journal of Clinical Medicine. 2025; 14(9):2970. https://doi.org/10.3390/jcm14092970
Chicago/Turabian StyleAlipova, Gulzhaina, Nurgul Ablakimova, Kymbat Tussupkaliyeva, Saule Bermagambetova, Sholpan Kosmuratova, Bibigul Karimsakova, Andrey Gaiday, Assel Gaiday, Andrii Dinets, and Akylbek Tussupkaliyev. 2025. "Prevention of Pre-Eclampsia: Modern Strategies and the Role of Early Screening" Journal of Clinical Medicine 14, no. 9: 2970. https://doi.org/10.3390/jcm14092970
APA StyleAlipova, G., Ablakimova, N., Tussupkaliyeva, K., Bermagambetova, S., Kosmuratova, S., Karimsakova, B., Gaiday, A., Gaiday, A., Dinets, A., & Tussupkaliyev, A. (2025). Prevention of Pre-Eclampsia: Modern Strategies and the Role of Early Screening. Journal of Clinical Medicine, 14(9), 2970. https://doi.org/10.3390/jcm14092970





