A Novel Effect of Microaneurysms and Retinal Cysts on Capillary Perfusion in Diabetic Macular Edema: A Multimodal Imaging Study
Abstract
:1. Introduction
2. Methods
2.1. Patients and Data Collection
2.2. Inclusion and Exclusion Criteria
2.3. Registration of OCT and Early-Phase FA Images
2.4. Types of Non-Perfusion
2.5. MA
2.6. Morphologic Image Analysis
3. Results
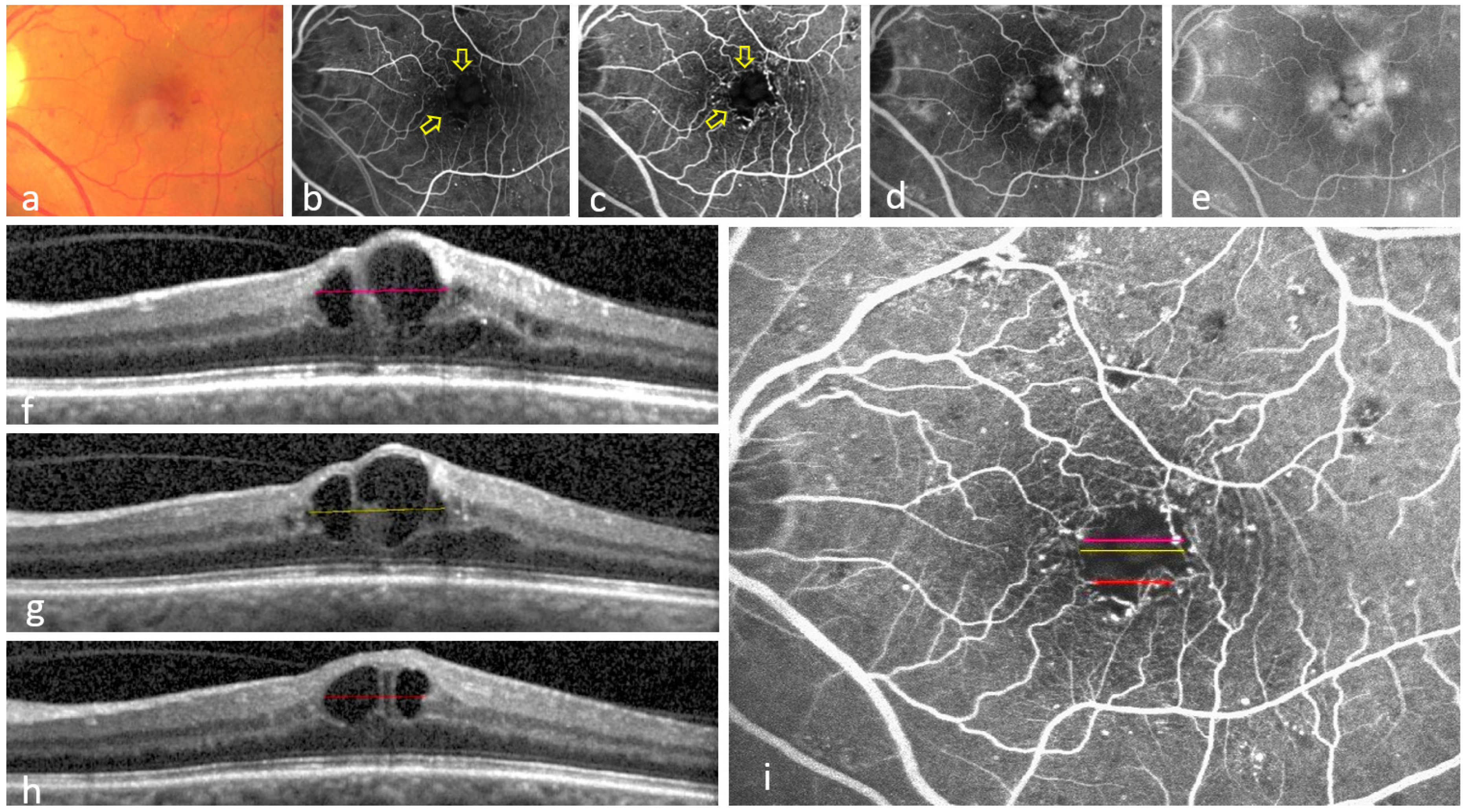
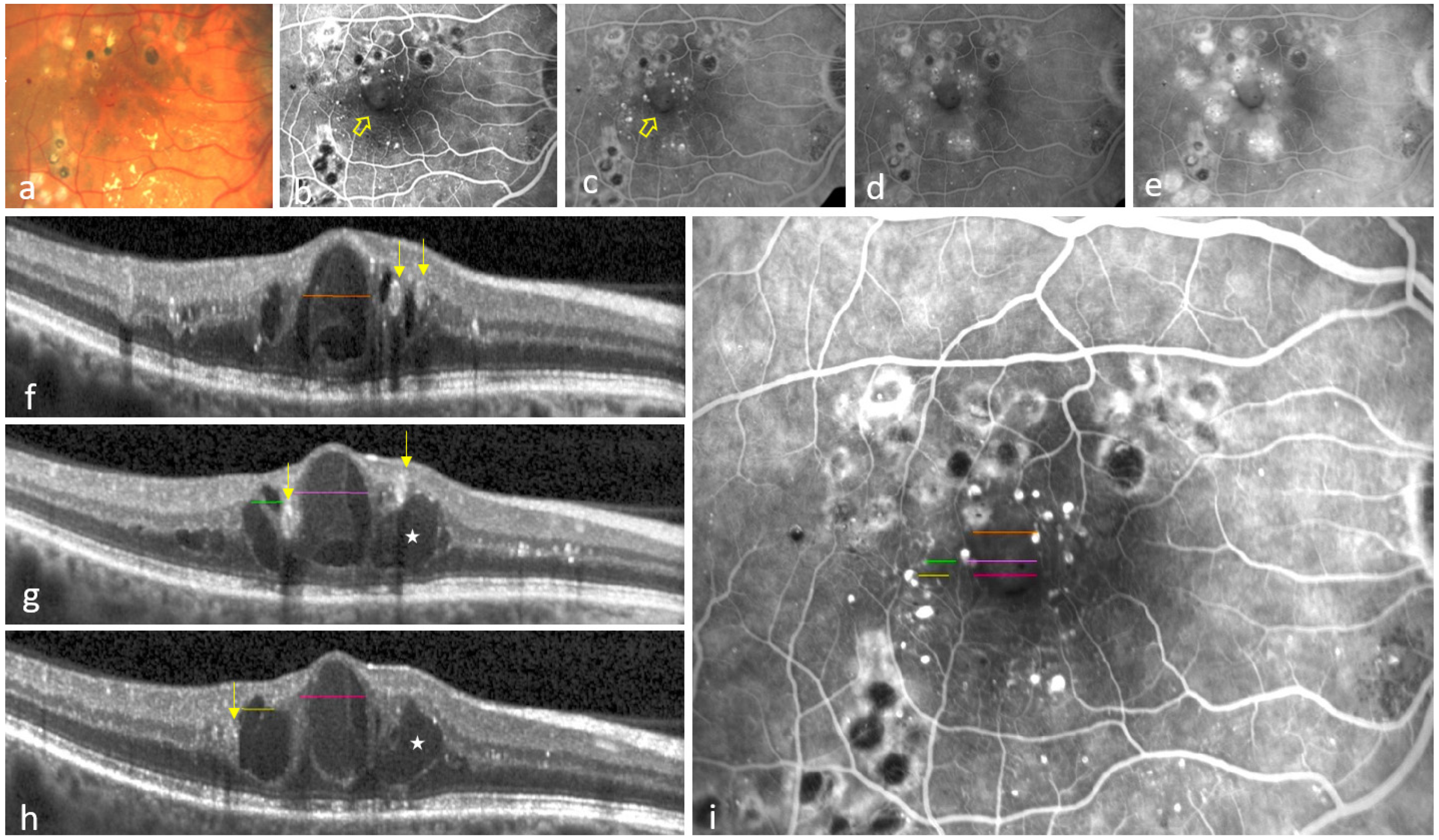
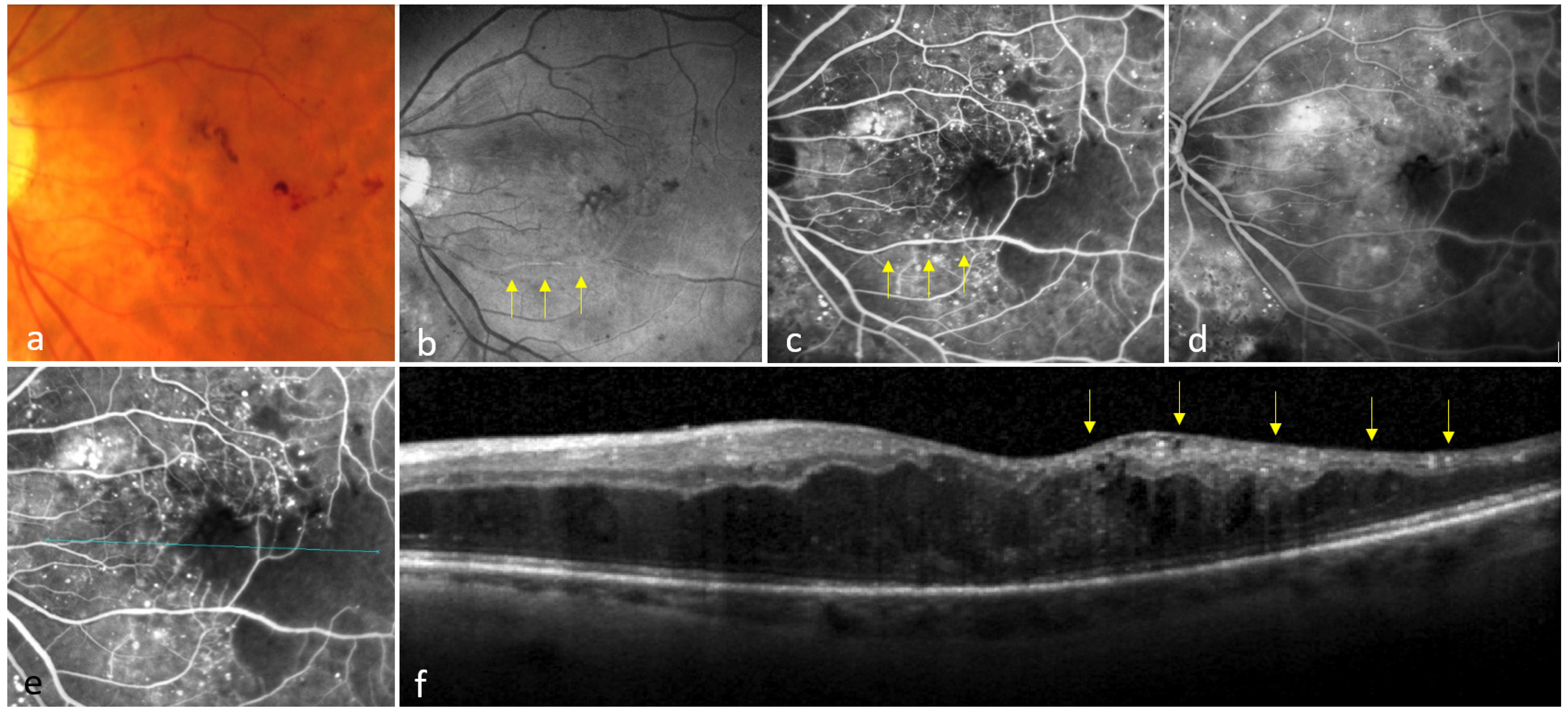
3.1. Eyes Showing at Least One Colocalization of INL-Occupying Cysts and Non-Perfusion Areas
3.2. Eyes Showing No Colocalization Between INL-Occupying Cysts and Non-Perfusion Areas
3.3. Distribution of MA
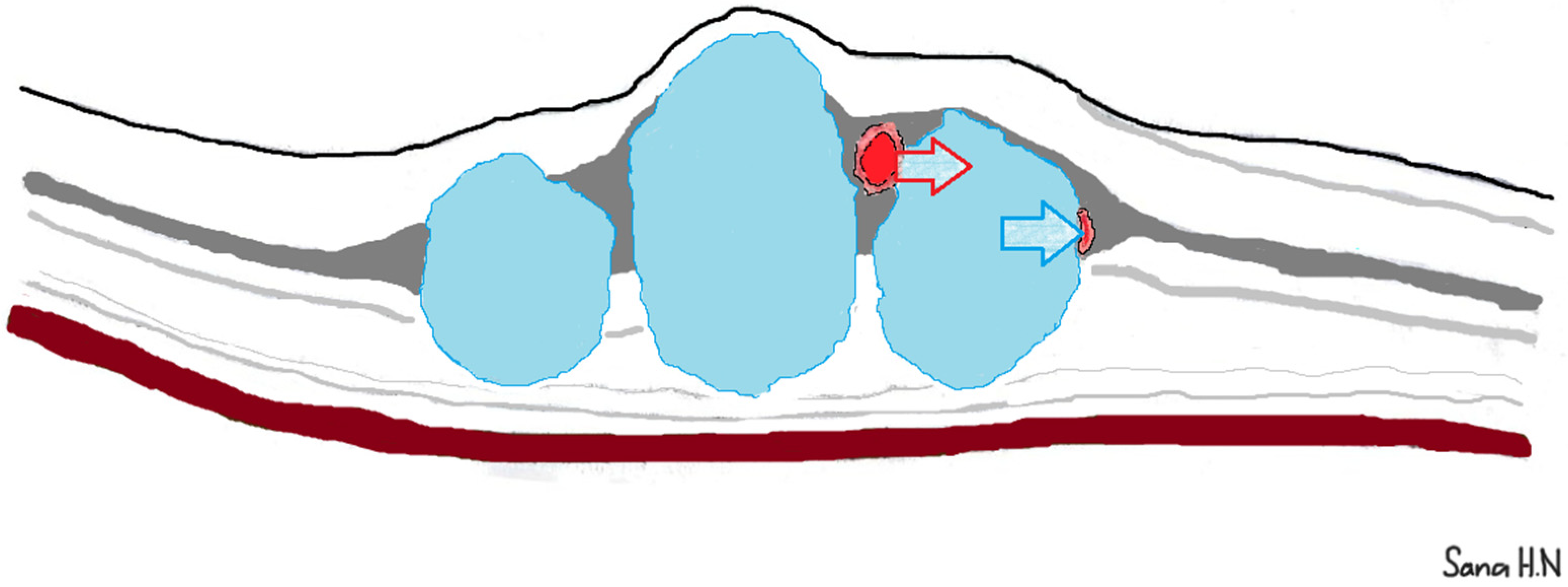
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apple, D.J.; Rabb, M.F. Ocular Pathology: Clinical Applications and Self-Assessment, 4th ed.; Mosby: St. Louis, MO, USA, 1991; ISBN 143988109X. [Google Scholar]
- de Carlo, T.E.; Chin, A.T.; Bonini Filho, M.A.; Adhi, M.; Branchini, L.; Salz, D.A.; Baumal, C.R.; Crawford, C.; Reichel, E.; Witkin, A.J.; et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina 2015, 35, 2364–2370. [Google Scholar] [CrossRef]
- Brar, M.; Yuson, R.; Kozak, I.; Mojana, F.; Cheng, L.; Bartsch, D.-U.; Oster, S.F.; Freeman, W.R. Correlation between morphologic features on spectral-domain optical coherence tomography and angiographic leakage patterns in macular edema. Retina 2010, 30, 383–389. [Google Scholar] [CrossRef]
- Haj Najeeb, B.; Simader, C.; Deak, G.; Vass, C.; Gamper, J.; Montuoro, A.; Gerendas, B.S.; Schmidt-Erfurth, U. The Distribution of Leakage on Fluorescein Angiography in Diabetic Macular Edema: A New Approach to Its Etiology. Investig. Opthalmol. Vis. Sci. 2017, 58, 3986. [Google Scholar] [CrossRef]
- Mané, V.; Dupas, B.; Gaudric, A.; Bonnin, S.; Pedinielli, A.; Bousquet, E.; Erginay, A.; Tadayoni, R.; Couturier, A. Correlation between cystoid spaces in chronic diabetic macular edema and capillary nonperfusion detected by optical coherence tomography angiography. Retina 2016, 36, S102–S110. [Google Scholar] [CrossRef]
- Kim, D.Y.; Fingler, J.; Zawadzki, R.J.; Park, S.S.; Morse, L.S.; Schwartz, D.M.; Fraser, S.E.; Werner, J.S. Noninvasive Imaging of the Foveal Avascular Zone with High-Speed, Phase-Variance Optical Coherence Tomography. Investig. Opthalmol. Vis. Sci. 2012, 53, 85–92. [Google Scholar] [CrossRef]
- Wang, Q.; Kocaoglu, O.P.; Cense, B.; Bruestle, J.; Jonnal, R.S.; Gao, W.; Miller, D.T. Imaging Retinal Capillaries Using Ultrahigh-Resolution Optical Coherence Tomography and Adaptive Optics. Investig. Opthalmol. Vis. Sci. 2011, 52, 6292–6299. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Inomata, H. Relation Between Superficial Capillaries and Foveal Structures in the Human Retina. Investig. Opthalmol. Vis. Sci. 1986, 27, 1698–1705. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Classification of Diabetic Retinopathy from Fluorescein Angiograms. Ophthalmology 1991, 98, 807–822. [Google Scholar] [CrossRef]
- John, D.; Kuriakose, T.; Devasahayam, S.; Braganza, A. Dimensions of the Foveal Avascular Zone Using the Heidelberg Retinal Angiogram-2 in Normal Eyes. Indian J. Ophthalmol. 2010, 59, 9–11. [Google Scholar] [CrossRef]
- Antcliff, R.J.; Hussain, A.A.; Marshall, J. Hydraulic Conductivity of Fixed Retinal Tissue After Sequential Excimer Laser Ablation: Barriers Limiting Fluid Distribution and Implications for Cystoid Macular Edema. Arch. Ophthalmol. 2001, 119, 539–544. [Google Scholar] [CrossRef]
- Govetto, A.; Mazzotta, F.; Al-Sheikh, M.; Mauro, A.; Romano, M.R. Macular Capillary Displacement in Exudative and Tractional Macular Oedema: A Multimodal Imaging Study and Pathophysiological Hypothesis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 3675–3685. [Google Scholar] [CrossRef] [PubMed]
- Haj Najeeb, B.; Simader, C.; Schmidt-Erfurth, U. The Role of the Macular Intraretinal Cysts in Evolution of Capillary Drop out in Diabetic Macular Retinopathy. A New Mechanism. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2364. [Google Scholar]
- Murakami, T.; Nishijima, K.; Akagi, T.; Uji, A.; Horii, T.; Ueda-Arakawa, N.; Muraoka, Y.; Yoshimura, N. Optical Coherence Tomographic Reflectivity of Photoreceptors beneath Cystoid Spaces in Diabetic Macular Edema. Investig. Opthalmol. Vis. Sci. 2012, 53, 1506. [Google Scholar] [CrossRef]
- Pelosini, L.; Hull, C.C.; Boyce, J.F.; McHugh, D.; Stanford, M.R.; Marshall, J. Optical Coherence Tomography May Be Used to Predict Visual Acuity in Patients with Macular Edema. Investig. Opthalmol. Vis. Sci. 2011, 52, 2741–2748. [Google Scholar] [CrossRef]
- Mirshahi, R.; Riazi-Esfahani, H.; Khalili Pour, E.; Fadakar, K.; Yarmohamadi, P.; Alemzadeh, S.A.; Chaibakhsh, S.; Falavarjani, K.G. Differentiating Features of OCT Angiography in Diabetic Macular Edema. Sci. Rep. 2021, 11, 23398. [Google Scholar] [CrossRef]
- Brazitikos, P.D.; Stangos, N.T. Macular Hole Formation in Diabetic Retinopathy: The Role of Coexisting Macular Edema. Doc. Ophthalmol. 1999, 97, 273–278. [Google Scholar] [CrossRef]
- Hayreh, S.S. Prevalent Misconceptions About Acute Retinal Vascular Occlusive Disorders. Prog. Retin. Eye Res. 2005, 24, 493–519. [Google Scholar] [CrossRef]
- Ahn, S.J.; Park, K.H.; Ryoo, N.K.; Hong, J.H.; Jung, C.; Yoon, C.H.; Han, M.K.; Woo, S.J. No-Reflow Phenomenon in Central Retinal Artery Occlusion: Incidence, Risk Factors, and Clinical Implications. PLoS ONE 2015, 10, e0142852. [Google Scholar] [CrossRef]
- Coscas, F.; Glacet-Bernard, A.; Miere, A.; Caillaux, V.; Uzzan, J.; Lupidi, M.; Coscas, G.; Souied, E.H. Optical Coherence Tomography Angiography in Retinal Vein Occlusion: Evaluation of Superficial and Deep Capillary Plexa. Am. J. Ophthalmol. 2016, 161, 160–171.e2. [Google Scholar] [CrossRef]
- Haj Najeeb, B.; Deak, G.G.; Schmidt-Erfurth, U.; Gerendas, B.S. The RAP Study, Report 3: Discoloration of the Macular Region in Patients with Macular Neovascularization Type 3. Acta Ophthalmol. 2022, 100, E270–E277. [Google Scholar] [CrossRef]
- Haj Najeeb, B.; Gerendas, B.S.; Deak, G.G.; Leingang, O.; Bogunovic, H.; Schmidt-Erfurth, U. An Automated Comparative Analysis of the Exudative Biomarkers in Neovascular Age-Related Macular Degeneration, The RAP Study: Report 6. Am. J. Ophthalmol. 2024, 264, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Bagley, S.; Ireland, G.; McLeod, D.; Boulton, M.E. Three Dimensional Analysis of Microaneurysms in the Human Diabetic Retina. J. Anat. 1999, 194 Pt 1, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Horii, T.; Murakami, T.; Nishijima, K.; Akagi, T.; Uji, A.; Arakawa, N.; Muraoka, Y.; Yoshimura, N. Relationship Between Fluorescein Pooling and Optical Coherence Tomographic Reflectivity of Cystoid Spaces in Diabetic Macular Edema. Ophthalmology 2012, 119, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Bolz, M.; Schmidt-Erfurth, U.; Deak, G.; Mylonas, G.; Kriechbaum, K.; Scholda, C.; Diabetic Retinopathy Research Group Vienna. Optical Coherence Tomographic Hyperreflective Foci: A Morphologic Sign of Lipid Extravasation in Diabetic Macular Edema. Ophthalmology 2009, 116, 914–920. [Google Scholar] [CrossRef]
- Sjølie, A.K.; Klein, R.; Porta, M.; Orchard, T.; Fuller, J.; Parving, H.H.; Bilous, R.; Aldington, S.; Chaturvedi, N. Retinal Microaneurysm Count Predicts Progression and Regression of Diabetic Retinopathy. Post-Hoc Results from the DIRECT Programme. Diabet. Med. 2011, 28, 345–351. [Google Scholar] [CrossRef]
- Murakami, T.; Nishijima, K.; Sakamoto, A.; Ota, M.; Horii, T.; Yoshimura, N. Foveal Cystoid Spaces Are Associated with Enlarged Foveal Avascular Zone and Microaneurysms in Diabetic Macular Edema. Ophthalmology 2011, 118, 359–367. [Google Scholar] [CrossRef]
- Arend, O.; Wolf, S.; Jung, F.; Bertram, B.; Pöstgens, H.; Toonen, H.; Reim, M. Retinal Microcirculation in Patients with Diabetes Mellitus: Dynamic and Morphological Analysis of Perifoveal Capillary Network. Br. J. Ophthalmol. 1991, 75, 514–518. [Google Scholar] [CrossRef]
- Datlinger, F.; Datlinger, A.; Pollreisz, A.; Sacu, S.; Erfurth, U.S.; Datlinger, P. Intraprocedural OCT Monitoring of the Immediate Treatment Response During Indocyanine Green Angiography—Guided Laser Therapy of Teleangiectatic Capillaries in Diabetic Macular Edema. Sci. Rep. 2022, 12, 2315. [Google Scholar] [CrossRef]
- Byeon, S.H.; Chu, Y.K.; Hong, Y.T.; Kim, M.; Kang, H.M.; Kwon, O.W. New Insights into the Pathoanatomy of Diabetic Macular Edema: Angiographic Patterns and Optical Coherence Tomography. Retina 2012, 32, 1087–1099. [Google Scholar] [CrossRef]
- Hammes, H.-P. Pericytes and the Pathogenesis of Diabetic Retinopathy. Horm. Metab. Res. 2005, 37 (Suppl. 1), 39–43. [Google Scholar] [CrossRef]
- Tsuboi, K.; You, Q.S.; Guo, Y.; Wang, J.; Flaxel, C.J.; Bailey, S.T.; Huang, D.; Jia, Y.; Hwang, T.S. Association Between Fluid Volume in Inner Nuclear Layer and Visual Acuity in Diabetic Macular Edema. Am. J. Ophthalmol. 2022, 237, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Hajnajeeb, B.; Simader, C.; Deak, G.G.; Schmidt-Erfurth, U. Reperfusion of Retinal Capillaries After Anti Vascular Endothelial Growth Factor Therapy in Diabetic Macular Edema. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4691. [Google Scholar]
- Toichiro, K.; Cogan, D.G. Retinal Vascular Patterns VII. Acellular Change. Investig. Ophthalmol. Vis. Sci. 1965, 4, 1049–1058. [Google Scholar]
- Yeung, L.; Lima, V.C.; Garcia, P.; Landa, G.; Rosen, R.B. Correlation Between Spectral Domain Optical Coherence Tomography Findings and Fluorescein Angiography Patterns in Diabetic Macular Edema. Ophthalmology 2009, 116, 1158–1167. [Google Scholar] [CrossRef]
- Parrulli, S.; Corvi, F.; Cozzi, M.; Monteduro, D.; Zicarelli, F.; Staurenghi, G. Microaneurysms Visualisation Using Five Different Optical Coherence Tomography Angiography Devices Compared to Fluorescein Angiography. Br. J. Ophthalmol. 2020, 105, 526–530. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K. Image artifacts in optical coherence tomography angiography. Retina 2015, 35, 2163–2180. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical Coherence Tomography Angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haj Najeeb, B.; Gerendas, B.S.; Montuoro, A.; Simader, C.; Deák, G.G.; Schmidt-Erfurth, U.M. A Novel Effect of Microaneurysms and Retinal Cysts on Capillary Perfusion in Diabetic Macular Edema: A Multimodal Imaging Study. J. Clin. Med. 2025, 14, 2985. https://doi.org/10.3390/jcm14092985
Haj Najeeb B, Gerendas BS, Montuoro A, Simader C, Deák GG, Schmidt-Erfurth UM. A Novel Effect of Microaneurysms and Retinal Cysts on Capillary Perfusion in Diabetic Macular Edema: A Multimodal Imaging Study. Journal of Clinical Medicine. 2025; 14(9):2985. https://doi.org/10.3390/jcm14092985
Chicago/Turabian StyleHaj Najeeb, Bilal, Bianca S. Gerendas, Alessio Montuoro, Christian Simader, Gábor G. Deák, and Ursula M. Schmidt-Erfurth. 2025. "A Novel Effect of Microaneurysms and Retinal Cysts on Capillary Perfusion in Diabetic Macular Edema: A Multimodal Imaging Study" Journal of Clinical Medicine 14, no. 9: 2985. https://doi.org/10.3390/jcm14092985
APA StyleHaj Najeeb, B., Gerendas, B. S., Montuoro, A., Simader, C., Deák, G. G., & Schmidt-Erfurth, U. M. (2025). A Novel Effect of Microaneurysms and Retinal Cysts on Capillary Perfusion in Diabetic Macular Edema: A Multimodal Imaging Study. Journal of Clinical Medicine, 14(9), 2985. https://doi.org/10.3390/jcm14092985






