Association Between Plasma Homocysteine, Folate, Vitamin B12 Levels, and Metabolic Dysfunction Indices in Elderly with Arterial Stiffness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Definition and Criteria of Metabolic Syndrome
2.3. Body Mass Index and Anthropometrics
2.4. Biochemical Analyses
2.5. Metabolic Dysfunction Indices
LAP for females = (WC − 58) × TG (mmol/L)
LAP for males = (WC − 65) × TG (mmol/L)
CMI = (TG/HDL) × WHtR
VAI for females = WC/(36.58 + (1.89 × BMI)) × TG/0.81 × 1.52/HDL-C
VAI for males = WC/(39.68 + (1.88 × BMI)) × TG/1.03 × 1.31/HDL-C
TyG = Ln[TG(mg/dL) × fasting glucose (mg/dL)/2]
TyG-BMI = TyG × BMI
TyG-WC = TyG × WC
2.6. Measurement of CAVI
2.7. Statistical Analysis
3. Results
3.1. Comparisons Between Plasma Homocysteine Levels, the Number of Components of MetS, and Clinical, Anthropometric, and Cardiometabolic Parameters
3.2. Association Between the CAVI and Clinical, Anthropometric, and Cardiometabolic Parameters
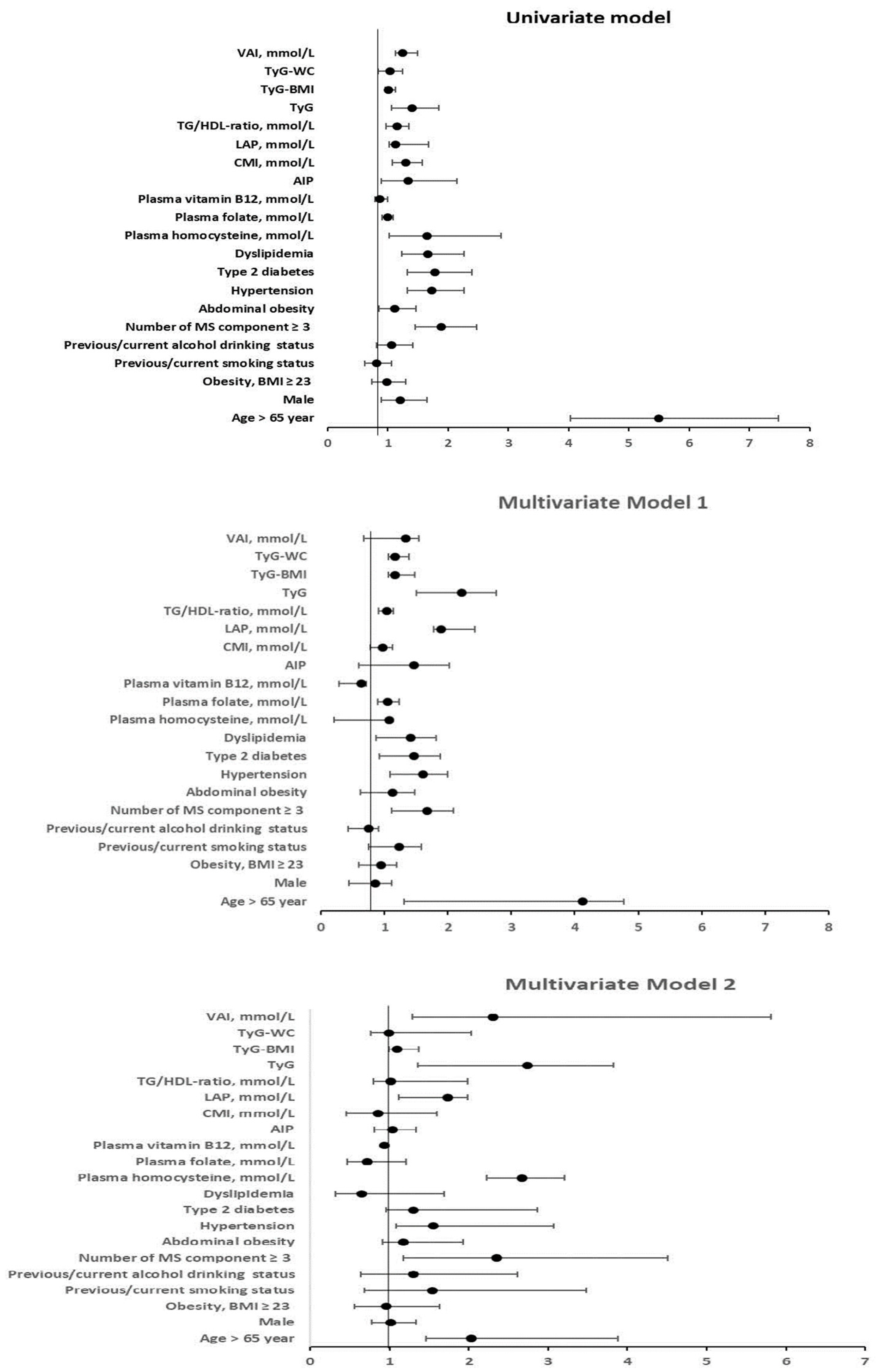
3.3. Logistic Regression Analysis Between Arterial Stiffness (with a High CAVI; ≥9) and Clinical Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shirai, K.; Utino, J.; Otsuka, K.; Takata, M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J. Atheroscler. Thromb. 2006, 13, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Ding, N.; Kim, E.D.; Budoff, M.; Chirinos, J.A.; Fernhall, B.; Hamburg, N.M.; Kario, K.; Miyoshi, T.; Tanaka, H.; et al. Cardio-ankle vascular index and cardiovascular disease: Systematic review and meta-analysis of prospective and cross-sectional studies. J. Clin. Hypertens. 2019, 21, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Boutouyrie, P. Arterial stiffness and hypertension in the elderly. Front. Cardiovasc. Med. 2020, 7, 544302. [Google Scholar] [CrossRef]
- Bäck, M.; Topouchian, J.; Labat, C.; Gautier, S.; Blacher, J.; Cwynar, M.; de la Sierra, A.; Pall, D.; Duarte, K.; Fantin, F.; et al. Cardio-ankle vascular index for predicting cardiovascular morbimortality and determinants for its progression in the prospective advanced approach to arterial stiffness (TRIPLE-A-Stiffness) study. EBioMedicine 2024, 103, 105107. [Google Scholar] [CrossRef]
- Stanek, A.; Grygiel-Górniak, B.; Brożyna-Tkaczyk, K.; Myśliński, W.; Cholewka, A.; Zolghadri, S. The influence of dietary interventions on arterial stiffness in overweight and obese subjects. Nutrients 2023, 15, 1440. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The metabolism and significance of homocysteine in nutrition and health. Nutr. Metab. 2017, 14, 78. [Google Scholar] [CrossRef]
- Wang, X.; Peng, H.; Xia, C.; Zhou, Y.; Shen, L.; Cheng, X.; Yang, C.; Yang, Y.; Long, L. Association of B vitamin intake and total homocysteine levels with all-cause and cause-specific mortality in central obesity. Nutrition 2023, 116, 112189. [Google Scholar] [CrossRef]
- Papatheodorou, L.; Weiss, N. Vascular oxidant stress and inflammation in hyperhomocysteinemia. Antioxid. Redox Signal. 2007, 9, 1941–1958. [Google Scholar] [CrossRef]
- Prauchner, G.R.K.; Ramires, O.V., Jr.; Rieder, A.S.; Wyse, A.T.S. Mild hyperhomocysteinemia alters oxidative stress profile via Nrf2, inflammation and cholinesterases in cardiovascular system of aged male rats. Chem. Biol. Interact. 2024, 396, 111028. [Google Scholar] [CrossRef]
- Tinelli, C.; Di Pino, A.; Ficulle, E.; Marcelli, S.; Feligioni, M. Hyperhomocysteinemia as a Risk Factor and Potential Nutraceutical Target for Certain Pathologies. Front. Nutr. 2019, 6, 49. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, T.; Gao, J.; Wang, Y.; Chen, Y.; Kaliannan, K.; Li, X.; Xiao, J.; Ma, T.; Zhang, L.; et al. Associations of homocysteine status and homocysteine metabolism enzyme polymorphisms with hypertension and dyslipidemia in a Chinese hypertensive population. Clin. Exp. Hypertens. 2020, 42, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yang, Q.; Wang, X.; He, X.; Zhu, W.; Lin, L.; Liu, C.; Zhu, C.; Zhang, M. Association between homocysteine levels and hyperlipidemia prevalence as well as all-cause mortality of hyperlipidemia patients in the US population: Results from NHANES database. Front. Cardiovasc. Med. 2024, 11, 1419579. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Merz, C.N.; Brewer, H.B.; Clark, L.T.; Hunninghake, D.B.; Pasternak, R.C.; Smith, S.C.; Stone, N.J. National Heart, Lung, and Blood Institute.; et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004, 110, 227–239. [Google Scholar] [CrossRef] [PubMed]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Pluta, W.; Dudzińska, W.; Lubkowska, A. Metabolic obesity in people with normal body weight (MONW)-Review of diagnostic Criteria. Int. J. Environ. Res. Public Health 2022, 19, 624. [Google Scholar] [CrossRef]
- Rodríguez-Carrillo, P.L.; Aguirre-Tostado, P.I.; Macías-Cervantes, M.H.; Alegría-Torres, J.A.; Luevano-Contreras, C. Novel adiposity and biochemical-anthropometric indices to identify cardiometabolic risk and metabolic syndrome in Mexican adults. Healthcare 2021, 9, 1561. [Google Scholar] [CrossRef]
- Tanaka, A.; Tomiyama, H.; Maruhashi, T.; Matsuzawa, Y.; Miyoshi, T.; Kabutoya, T.; Kario, K.; Sugiyama, S.; Munakata, M.; Ito, H.; et al. Physiological Diagnosis Criteria for Vascular Failure Committee. Physiological Diagnostic Criteria for Vascular Failure. Hypertension 2018, 72, 1060–1071. [Google Scholar] [CrossRef]
- Chen, L.; Wang, B.; Wang, J.; Ban, Q.; Wu, H.; Song, Y.; Zhang, J.; Cao, J.; Zhou, Z.; Liu, L.; et al. Association between serum total homocysteine and arterial stiffness in adults: A community-based study. J. Clin. Hypertens. 2018, 20, 686–693. [Google Scholar] [CrossRef]
- Wang, X.N.; Ye, P.; Cao, R.H.; Yang, X.; Xiao, W.K.; Zhang, Y.; Bai, Y.Y.; Wu, H.M. Plasma homocysteine is a predictive factor for arterial stiffness: A community-based 4.8-year prospective study. J. Clin. Hypertens. 2015, 17, 594–600. [Google Scholar] [CrossRef]
- Zou, T.; Yang, W.; Hou, Z.; Yang, J. Homocysteine enhances cell proliferation in vascular smooth muscle cells: Role of p38 MAPK and p47phox. Acta Biochim. Biophys. Sin. 2010, 42, 908–915. [Google Scholar] [CrossRef]
- McCaddon, A.; Miller, J.W. Homocysteine-a retrospective and prospective appraisal. Front. Nutr. 2023, 10, 1179807. [Google Scholar] [CrossRef]
- Harma, M.; Harma, M.; Kocyigit, A.; Yaltali, T. Intranasal 17beta-estradiol treatment and Vitamin B12, folate and homocysteine in menopause. Maturitas 2005, 50, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, G.; Pizzino, F.; Lilli, A.; De Caterina, A.R.; Esposito, A.; Dalmiani, S.; Mazzone, A.; Di Bella, G.; Berti, S.; Paradossi, U. Advanced lung cancer inflammation index as predictor of all-cause mortality in ST-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention. J. Clin. Med. 2024, 13, 6059. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.L.; Shih, C.C.; Huang, T.C.; Chen, J.Y. The relationship between elevated homocysteine and metabolic syndrome in a community-dwelling middle-aged and elderly population in Taiwan. Biomedicines 2023, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, L.; Wang, Y.; Chen, X.; Liu, J.; Peng, S.; Pi, J.; Zhang, Q.; Tomlinson, B.; Chan, P.; et al. Hyperhomocysteinemia increases risk of metabolic syndrome and cardiovascular death in an elderly Chinese community population of a 7-year follow-up study. Front. Cardiovasc. Med. 2022, 8, 811670. [Google Scholar] [CrossRef]
- Son, P.; Lewis, L. Hyperhomocysteinemia; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554408/ (accessed on 12 January 2025).
- Fardet, A.; Boirie, Y. Associations between diet-related diseases and impaired physiological mechanisms: A holistic approach based on meta-analyses to identify targets for preventive nutrition. Nutr. Rev. 2013, 71, 643–656. [Google Scholar] [CrossRef]
- Werstuck, G.H.; Lentz, S.R.; Dayal, S.; Hossain, G.S.; Sood, S.K.; Shi, Y.Y.; Zhou, J.; Maeda, N.; Krisans, S.K.; Malinow, M.R.; et al. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J. Clin. Investig. 2001, 107, 1263–1273. [Google Scholar] [CrossRef]
- Velez-Carrasco, W.; Merkel, M.; Twiss, C.O.; Smith, J.D. Dietary methionine effects on plasma homocysteine and HDL metabolism in mice. J. Nutr. Biochem. 2008, 19, 362–370. [Google Scholar] [CrossRef]
- DuPont, J.; Vorn, J.; Liu, Q.; Duffy, C.K.; Martin, G.L.; Hill, M.A. Sexually dimorphic role of smooth muscle cell estrogen receptor alpha in aging-associated arterial stiffness. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Li, S.; Cai, W.; Yongyi, B.; Wenkai, X.; Dan, F.; Ping, Y. Plasma homocysteine levels are independently associated with alterations of large artery stiffness in men but not in women. J. Geriatr. Cardiol. 2015, 12, 251–256. [Google Scholar]
- Li, H.F.; Miao, X.; Li, Y. The triglyceride glucose (TyG) index as a sensible marker for identifying insulin resistance and predicting diabetic kidney disease. Med. Sci. Monit. 2023, 29, e939482. [Google Scholar]
- Zhang, X.; Qu, Y.Y.; Liu, L.; Qiao, Y.N.; Geng, H.R.; Lin, Y.; Xu, W.; Cao, J.; Zhao, J.Y. Homocysteine inhibits pro-insulin receptor cleavage and causes insulin resistance via protein cysteine-homocysteinylation. Cell Rep. 2021, 37, 109821. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Shruthi, N.R.; Banerjee, A.; Jothimani, G.; Duttaroy, A.K.; Pathak, S. Endothelial dysfunction, platelet hyperactivity, hypertension, and the metabolic syndrome: Molecular insights and combating strategies. Front. Nutr. 2023, 10, 1221438. [Google Scholar] [CrossRef]
- Meng, S.; Ciment, S.; Jan, M.; Tran, T.; Pham, H.; Cueto, R.; Yang, X.F.; Wang, H. Homocysteine induces inflammatory transcriptional signaling in monocytes. Front. Biosci. 2013, 18, 685–695. [Google Scholar]
- Zeng, X.; Yang, Y. Molecular mechanisms underlying vascular remodeling in hypertension. Rev. Cardiovasc. Med. 2024, 25, 72. [Google Scholar] [CrossRef] [PubMed]
- Vatner, S.F.; Zhang, J.; Vyzas, C.; Mishra, K.; Graham, R.M.; Vatner, D.E. Vascular stiffness in aging and disease. Front. Physiol. 2021, 12, 762437. [Google Scholar] [CrossRef] [PubMed]
- Underwood, P.C.; Adler, G.K. The renin angiotensin aldosterone system and insulin resistance in humans. Curr. Hypertens. Rep. 2013, 15, 59–70. [Google Scholar] [CrossRef]
- Climie, R.E.; Alastruey, J.; Mayer, C.C.; Schwarz, A.; Laucyte-Cibulskiene, A.; Voicehovska, J.; Bianchini, E.; Bruno, R.-M.; Charlton, P.H.; Grillo, A.; et al. Vascular ageing: Moving from bench towards bedside. Eur. J. Prev. Cardiol. 2023, 30, 1101–1117. [Google Scholar] [CrossRef]
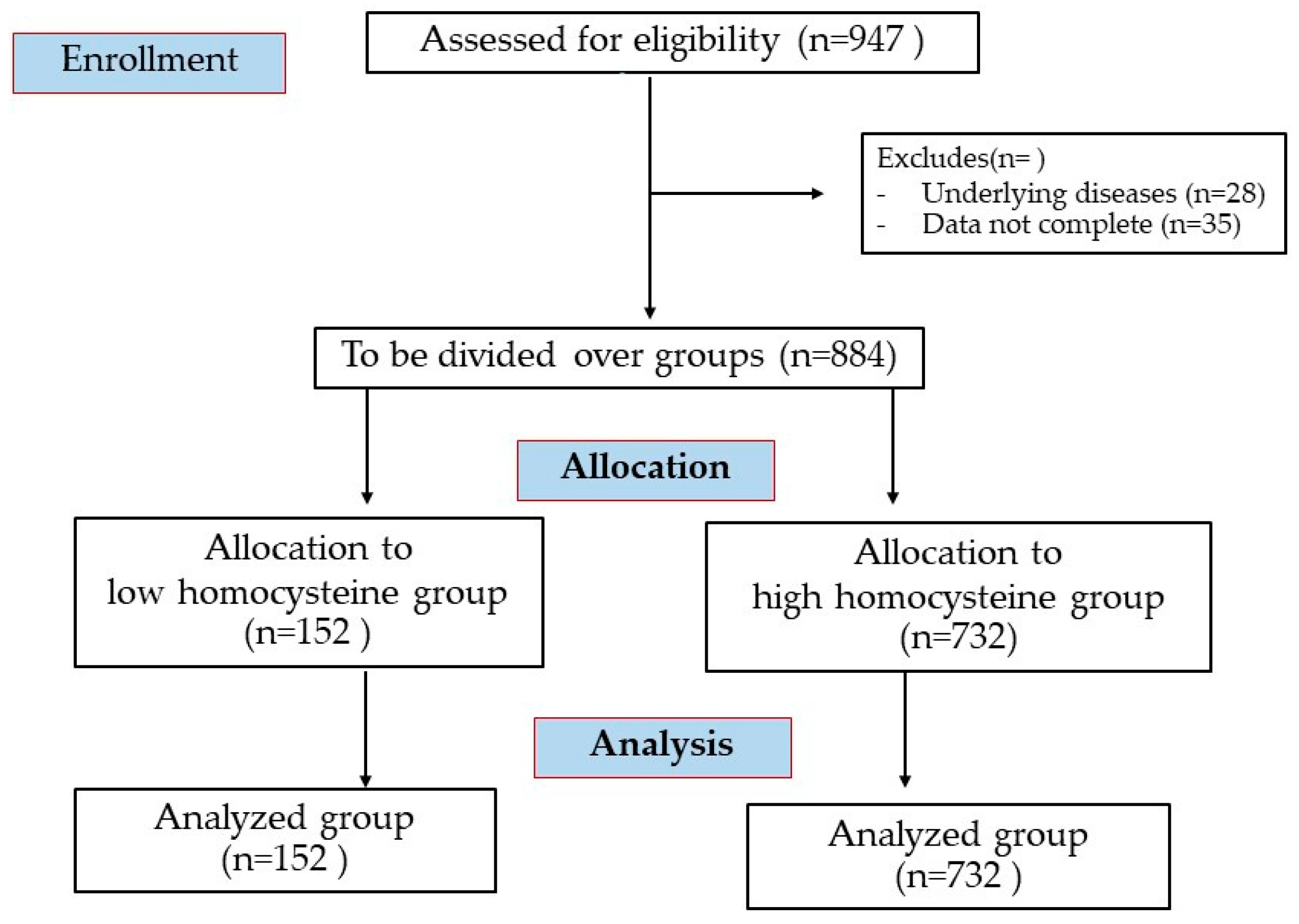

| Variables | Homocysteine Group | Number of Metabolic Syndrome Components | |||
|---|---|---|---|---|---|
| Low (<15 µmol/L) (n = 152) | High (≥15 µmol/L) (n = 732) | 3 MetS (n = 467) | 4 MetS (n = 269) | 5 MetS (n = 25) | |
| Age, years | 67.84 ± 4.06 | 68.82 ± 4.58 | 68.57 ± 4.46 | 68.95 ± 4.58 | 69.12 ± 4.21 |
| Gender, % | |||||
| Male | 60 (39.5) | 607 (82.9) | 347 (74.3) | 205 (76.2) | 21 (84.0) |
| Female | 92 (60.5) | 125 (17.1) | 120 (25.7) | 64 (23.8) | 4 (16.0) |
| Smoking status | |||||
| No smoking | 124 (81.6) | 432 (59.1) * | 300 (64.2) | 159 (59.1) | 16 (64.0) |
| Ex-smoking | 28 (18.4) | 300 (40.9) | 120 (35.8) | 110 (40.9) | 9 (36.0) |
| Alcohol drinking, % | |||||
| No drinking | 74 (48.7) | 208 (28.4) * | 154 (38.0) | 83 (30.9) | 7 (28.0) |
| Ex-drinking | 22 (14.5) | 180 (24.6) | 105 (22.5) | 62 (23.0) | 4 (16.0) |
| Occasional drinking | 46 (30.3) | 269 (36.7) | 172 (36.8) | 91 (31.8) | 11 (44.0) |
| Current drinking | 10 (6.6) | 79 (10.2) | 36 (7.1) | 33 (12.3) | 3 (12.0) |
| Type 2 diabetes, % | 17 (11.3%) | 122 (16.7%) | 79 (16.9%) | 42 (15.6%) | 5 (20%) |
| Dyslipidemia, % | 28 (18.4%) | 165 (22.6%) | 131 (28.1%) | 82 (30.5%) | 8 (32%) |
| BMI, kg/m2 | 23.27 ± 3.44 | 24.72 ± 3.37 * | 24.19 ± 3.13 | 26.29 ± 3.07 a | 26.41 ± 1.81 a,b |
| Waist circumference, cm | 85.58 ± 10.18 | 88.98 ± 10.50 * | 87.38 ± 0.42 | 94.28 ± 7.77 a | 94.80 ± 4.71 a |
| Waist/hip ratio | 0.90 ± 0.70 | 0.93 ± 0.06 * | 0.92 ± 0.06 | 0.95 ± 0.05 a | 0.98 ± 0.04 a |
| Fat mass, % | 28.52 ± 7.96 | 30.77 ± 9.63 * | 28.66 ± 8.13 | 32.26 ± 7.32 a | 31.43 ± 6.59 |
| SBP, mm Hg | 130.63 ± 19.36 | 134.44 ± 17.79 * | 133.31 ± 17.90 | 141.03 ± 16.71 a | 145.11 ± 12.54 a |
| DBP, mm Hg | 76.49 ± 10.05 | 77.63 ± 10.09 | 77.29 ± 9.78 | 80.27 ± 10.19 | 81.65 ± 6.90 a |
| TC, mmol/L | 5.32 ± 1.05 | 5.30 ± 1.16 | 5.31 ± 1.08 | 5.18 ± 1.27 | 5.43 ± 1.05 |
| TG, mmol/L | 1.29 ± 0.65 | 1.43 ± 0.75 * | 1.25 ± 0.58 | 1.77 ± 0.86 a | 2.50 ± 0.75 a,b |
| HDL-C, mmol/L | 1.71 ± 0.47 | 1.49 ± 0.39 * | 1.62 ± 0.38 | 1.27 ± 0.30 a | 1.12 ± 0.15 a |
| LDL-C, mmol/L | 3.30 ± 1.01 | 3.50 ± 1.13 * | 3.44 ± 1.04 | 3.44 ± 1.26 | 3.80 ± 0.98 |
| Fasting plasma glucose, mmol/L | 5.27 ± 0.99 | 5.47 ± 1.11 * | 5.24 ± 0.89 | 5.90 ± 1.22 a | 7.01 ± 1.90 a,b |
| HbA1C, % | 5.90 ± 0.54 | 5.99 ± 0.71 * | 5.86 ± 0.53 | 6.25 ± 0.84 a | 6.77 ± 1.16 a,b |
| Blood urea nitrogen, mmol/L | 4.94 ± 1.69 | 5.04 ± 1.47 | 5.37 ± 1.52 | 5.36 ± 1.39 | 5.14 ± 1.49 |
| Creatinine, mmol/L | 76.71 ± 47.36 | 80.36 ± 21.26 | 87.37 ± 23.25 | 91.01 ± 28.23 | 88.90 ± 29.32 |
| Homocysteine, µmol/L | 13.25 ± 1.39 | 20.29 ± 4.63 * | 18.84 ± 4.85 | 19.31 ± 5.46 | 20.98 ± 2.42 a,b |
| Plasma folate | 14.01 ± 8.51 | 9.09 ± 5.61 * | 10.22 ± 6.31 | 8.68 ± 5.56 a | 9.97 ± 5.05 |
| Plasma vitamin B12 | 921.95 ± 538.14 | 663.74 ± 377.57 * | 713.27 ± 401.72 | 669.76 ± 436.23 | 738.38 ± 549.29 |
| CAVI (m/s) | 8.75 ± 1.16 | 9.08 ± 1.11 * | 9.01 ± 1.12 | 9.20 ± 1.23 | 9.98 ± 0.94 a,b |
| Metabolic dysfunction indices: | |||||
| AIP | 0.52 ± 0.27 | 1.54 ± 0.87 * | 0.11 ± 0.08 | 0.14 ± 0.09 a | 3.08 ± 0.17 a,b |
| CMI, mmol/L | 0.79 ± 0.62 | 0.97 ± 0.89 * | 0.72 ± 0.36 | 1.40 ± 0.95 a | 2.14 ± 76 a,b |
| LAP, mmol/L | 32.18 ± 22.69 | 36.86 ± 23.09 * | 29.72 ± 15.01 | 52.30 ± 24.18 a | 75.16 ± 28.43 a,b |
| TG/HDL-ratio, mmol/L | 0.87 ± 0.60 | 1.09 ± 0.93 * | 0.82 ± 0.41 | 1.56 ± 0.76 a | 2.26 ± 0.71 a,b |
| TyG | 8.46 ± 0.49 | 8.58 ± 0.47 * | 8.42 ± 0.36 | 8.88 ± 0.44 a | 9.41 ± 0.43 a,b |
| TyG-BMI | 197.47 ± 39.21 | 212.43 ± 35.09 * | 203.58 ± 30.41 | 233.94 ± 31.38 a | 248.79 ± 21.07 a,b |
| TyG-WC | 727.06 ± 111.69 | 766.58 ± 100.64 * | 740.05 ± 83.54 | 836.27 ± 75.24 a | 894.59 ± 66.77 a |
| VAI, mmol/L | 1.39 ± 0.98 | 1.48 ± 0.16 * | 1.15 ± 0.55 | 2.15 ± 1.56 a | 3.13 ± 1.01 a,b |
| Variable | Correlation Coefficient | p-Value |
|---|---|---|
| Age, year | 0.328 | 0.000 |
| BMI, kg/m2 | 0.045 | 0.184 |
| Waist circumference, cm | 0.015 | 0.666 |
| Waist/hip ratio | 0.145 | 0.002 |
| Body fat, % | 0.039 | 0.278 |
| SBP, mm Hg | 0.170 | 0.000 |
| DBP, mm Hg | 0.042 | 0.209 |
| TC, mmol/L | 0.131 | 0.017 |
| TG, mmol/L | 0.124 | 0.005 |
| HDL-C, mmol/L | −0.047 | 0.165 |
| LDL-C, mmol/L | 0.131 | 0.000 |
| Fasting plasma glucose, mmol/L | 0.182 | 0.000 |
| HbA1C, % | 0.158 | 0.000 |
| BUN, mmol/L | 0.097 | 0.068 |
| Cr, mmol/L | 0.098 | 0.108 |
| Homocysteine, µmol/L | 0.545 | 0.000 |
| Plasma folate, mmol/L | −0.032 | 0.568 |
| Plasma vitamin B12, mmol/L | 0.029 | 0.396 |
| Metabolic dysfunction indices: | ||
| AIP | 0.136 | 0.000 |
| CMI, mmol/L | 0.032 | 0.339 |
| LAP, mmol/L | 0.163 | 0.001 |
| TG/HDL-ratio, mmol/L | 0.065 | 0.055 |
| TyG | 0.139 | 0.027 |
| TyG-BMI | 0.055 | 0.105 |
| TyG-WC | 0.018 | 0.598 |
| VAI, mmol/L | 0.149 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirivarasai, J.; Shantavasinkul, P.C.; Thitiwiwatkul, M.; Monsuwan, W.; Panpunuan, P.; Sritara, P. Association Between Plasma Homocysteine, Folate, Vitamin B12 Levels, and Metabolic Dysfunction Indices in Elderly with Arterial Stiffness. J. Clin. Med. 2025, 14, 2998. https://doi.org/10.3390/jcm14092998
Sirivarasai J, Shantavasinkul PC, Thitiwiwatkul M, Monsuwan W, Panpunuan P, Sritara P. Association Between Plasma Homocysteine, Folate, Vitamin B12 Levels, and Metabolic Dysfunction Indices in Elderly with Arterial Stiffness. Journal of Clinical Medicine. 2025; 14(9):2998. https://doi.org/10.3390/jcm14092998
Chicago/Turabian StyleSirivarasai, Jintana, Prapimporn Chattranukulchai Shantavasinkul, Manasid Thitiwiwatkul, Wutarak Monsuwan, Pachara Panpunuan, and Piyamitr Sritara. 2025. "Association Between Plasma Homocysteine, Folate, Vitamin B12 Levels, and Metabolic Dysfunction Indices in Elderly with Arterial Stiffness" Journal of Clinical Medicine 14, no. 9: 2998. https://doi.org/10.3390/jcm14092998
APA StyleSirivarasai, J., Shantavasinkul, P. C., Thitiwiwatkul, M., Monsuwan, W., Panpunuan, P., & Sritara, P. (2025). Association Between Plasma Homocysteine, Folate, Vitamin B12 Levels, and Metabolic Dysfunction Indices in Elderly with Arterial Stiffness. Journal of Clinical Medicine, 14(9), 2998. https://doi.org/10.3390/jcm14092998







