Association of Maternal Exposure to Fine Particulate Matter During Pregnancy with Anterior Segment Dysgenesis Risk: A Matched Case-Control Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
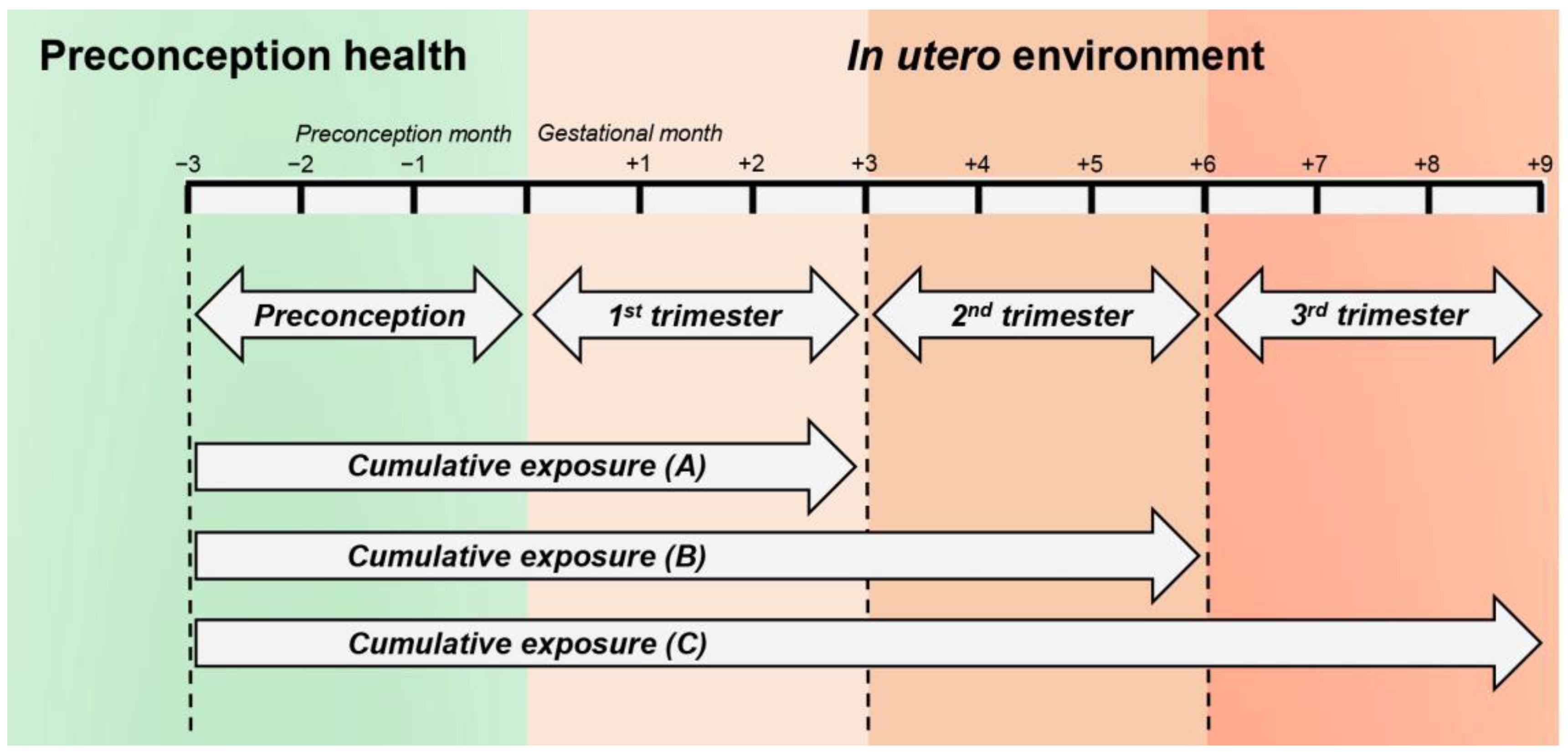
2.2. Exposure Time Windows
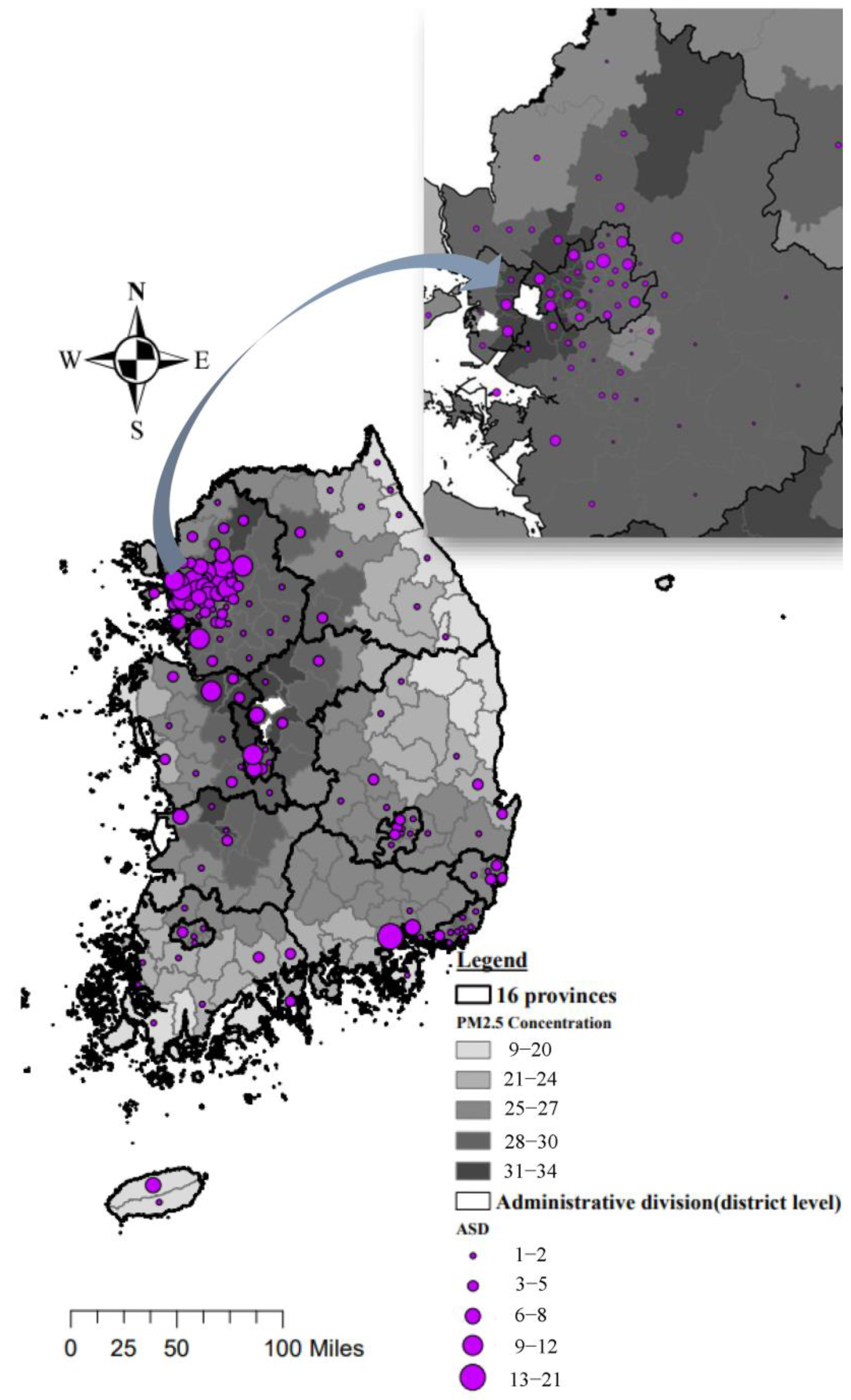
2.3. District-Specific Estimation of PM2.5 Exposure
2.4. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics of Study Population
3.2. PM2.5 Exposure in Study Subjects
3.3. Association Between Maternal PM2.5 Exposure and Risk of ASD
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASD | anterior segment dysgenesis |
| IQR | interquartile range |
| PM2.5 | fine particulate matter measuring 2.5 μm or less |
| RR | relative risk |
References
- Malley, C.S.; Kuylenstierna, J.C.; Vallack, H.W.; Henze, D.K.; Blencowe, H.; Ashmore, M.R. Preterm birth associated with maternal fine particulate matter exposure: A global, regional and national assessment. Environ. Int. 2017, 101, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.M.; Kim, K.-N.; Park, H.; Shin, H.H.; Kim, H.S.; Cho, S.J.; Kim, S.T.; Ha, E.H. Association between prenatal exposure to PM2. 5 and the increased risk of specified infant mortality in South Korea. Environ. Int. 2020, 144, 105997. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Chen, B.-Y.; Pan, S.-C.; Ho, Y.-L.; Guo, Y.L. Prenatal exposure to PM2.5 and congenital heart diseases in Taiwan. Sci. Total Environ. 2019, 655, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liang, S.; Zhao, J.; Qian, Z.; Bassig, B.A.; Yang, R.; Zhang, Y.; Hu, K.; Xu, S.; Zheng, T.; et al. Maternal exposure to air pollutant PM2.5 and PM10 during pregnancy and risk of congenital heart defects. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 422–427. [Google Scholar] [CrossRef]
- Lavigne, É.; Bélair, M.-A.; Do, M.T.; Stieb, D.M.; Hystad, P.; van Donkelaar, A.; Martin, R.V.; Crouse, D.L.; Crighton, E.; Chen, H.; et al. Maternal exposure to ambient air pollution and risk of early childhood cancers: A population-based study in Ontario, Canada. Environ. Int. 2017, 100, 139–147. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, T.-H.; Kim, S.; Song, M.; Bae, S. Association between long-term exposure to particulate matter and childhood cancer: A retrospective cohort study. Environ. Res. 2022, 205, 112418. [Google Scholar] [CrossRef]
- Lee, A.; Leon Hsu, H.H.; Mathilda Chiu, Y.H.; Bose, S.; Rosa, M.J.; Kloog, I.; Wilson, A.; Schwartz, J.; Cohen, S.; Coull, B.A.; et al. Prenatal fine particulate exposure and early childhood asthma: Effect of maternal stress and fetal sex. J. Allergy Clin. Immunol. 2018, 141, 1880–1886. [Google Scholar] [CrossRef]
- Hazlehurst, M.F.; Carroll, K.N.; Loftus, C.T.; Szpiro, A.A.; Moore, P.E.; Kaufman, J.D.; Kirwa, K.; LeWinn, K.Z.; Bush, N.R.; Sathyanarayana, S.; et al. Maternal exposure to PM2.5 during pregnancy and asthma risk in early childhood: Consideration of phases of fetal lung development. Environ. Epidemiol. 2021, 5, e130. [Google Scholar] [CrossRef]
- Kannan, S.; Misra, D.P.; Dvonch, J.T.; Krishnakumar, A. Exposures to airborne particulate matter and adverse perinatal outcomes: A biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ. Health Perspect. 2006, 114, 1636–1642. [Google Scholar] [CrossRef]
- Ma, A.; Grigg, J.; Jamieson, R. Phenotype–genotype correlations and emerging pathways in ocular anterior segment dysgenesis. Hum. Genet. 2019, 138, 899–915. [Google Scholar]
- Ito, Y.A.; Walter, M.A. Genomics and anterior segment dysgenesis: A review. Clin. Exp. Ophthalmol. 2014, 42, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Idrees, F.; Vaideanu, D.; Fraser, S.G.; Sowden, J.C.; Khaw, P.T. A review of anterior segment dysgeneses. Surv. Ophthalmol. 2006, 51, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-Y.; Luo, C.-W.; Chiang, Y.-W.; Li, K.-L.Y.Y.-C.; Ho, Y.-C.; Lee, S.-S.; Chen, W.-Y.; Chen, C.-J.; Kuan, Y.-H. Association Between PM2.5 Exposure Level and Primary Open-Angle Glaucoma in Taiwanese Adults: A Nested Case–control Study. Int. J. Environ. Res. Public Health 2021, 18, 1714. [Google Scholar] [CrossRef]
- Wang, W.; He, M.; Li, Z.; Huang, W. Epidemiological variations and trends in health burden of glaucoma worldwide. Acta Ophthalmol. 2019, 97, e349–e355. [Google Scholar] [CrossRef]
- Wolkoff, P. External eye symptoms in indoor environments. Indoor Air 2017, 27, 246–260. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Boulton, M.E. Consequences of oxidative stress in age-related macular degeneration. Mol. Asp. Med. 2012, 33, 399–417. [Google Scholar] [CrossRef]
- Shan, A.; Chen, X.; Yang, X.; Yao, B.; Liang, F.; Yang, Z.; Liu, F.; Chen, S.; Yan, X.; Huang, J.; et al. Association between long-term exposure to fine particulate matter and diabetic retinopathy among diabetic patients: A national cross-sectional study in China. Environ. Int. 2021, 154, 106568. [Google Scholar] [CrossRef]
- Chua, S.Y.L.; Khawaja, A.P.; Dick, A.D.; Morgan, J.; Dhillon, B.; Lotery, A.J.; Strouthidis, N.G.; Reisman, C.; Peto, T.; Khaw, P.T.; et al. Ambient Air Pollution Associations with Retinal Morphology in the UK Biobank. Investig. Opthalmology Vis. Sci. 2020, 61, 32. [Google Scholar] [CrossRef]
- Bergstralh, E.J.; Kosanke, J.L. Computerized Matching of Cases to Controls; Technical Report 56; Mayo Clinic: Rochester, MN, USA, 1995. [Google Scholar]
- Son, K.; You, S.; Kim, H.; Kim, B.; Kim, S. Inter-comparisons of spatially interpolated short-term and long-term PM2.5 concentrations of local authorities in South Korea 2015~2017. J. Korean Soc. Atmos. Environ. 2020, 36, 185–197. [Google Scholar] [CrossRef]
- Han, C.; Kim, S.; Lim, Y.-H.; Bae, H.-J.; Hong, Y.-C. Spatial and temporal trends of number of deaths attributable to ambient PM2.5 in the Korea. J. Korean Med. Sci. 2018, 33, 193. [Google Scholar] [CrossRef]
- Lee, K.-S.; Lim, Y.-H.; Choi, Y.-J.; Kim, S.; Bae, H.J.; Han, C.; Lee, Y.A.; Hong, Y.-C. Prenatal exposure to traffic-related air pollution and risk of congenital diseases in South Korea. Environ. Res. 2020, 191, 110060. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Shin, M.; Im, J.; Song, C.-K.; Choi, M.; Kim, J.; Lee, S.; Park, R.; Kim, J.; Lee, D.-W.; et al. Estimation of ground-level particulate matter concentrations through the synergistic use of satellite observations and process-based models over South Korea. Atmos. Chem. Phys. 2020, 20, 5987–6002. [Google Scholar] [CrossRef]
- Byun, D.; Schere, K.L. Review of the governing equations, computational algorithms, and other components of the Models-3 Community Multiscale Air Quality (CMAQ) modeling system. Appl. Mech. Rev. 2006, 59, 51–77. [Google Scholar] [CrossRef]
- Skamarock, W.C.; Klemp, J.B.; Dudhia, J.; Gill, D.O.; Barker, D.M.; Duda, M.G.; Huang, X.-Y.; Wang, W.J.; Powers, G. A Description of the Advanced Research WRF; Version 3; University Corporation for Atmospheric Research: Boulder, CO, USA, 2008; pp. 1–96. [Google Scholar]
- Lee, D.-W.; Han, C.-W.; Hong, Y.-C.; Oh, J.-M.; Bae, H.-J.; Kim, S.; Lim, Y.-H. Long-term exposure to fine particulate matter and incident asthma among elderly adults. Chemosphere 2021, 272, 129619. [Google Scholar] [CrossRef] [PubMed]
- McNutt, L.A.; Wu, C.; Xue, X.; Hafner, J.P. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am. J. Epidemiol. 2003, 157, 940–943. [Google Scholar] [CrossRef]
- Eghrari, A.O.; Riazuddin, S.A.; Gottsch, J.D. Overview of the cornea: Structure, function, and development. Prog. Mol. Biol. Transl. Sci. 2015, 134, 7–23. [Google Scholar]
- Mann, I.C. The development of the human iris. Br. J. Ophthalmol. 1925, 9, 495. [Google Scholar] [CrossRef]
- McMenamin, P.G. A quantitative study of the prenatal development of the aqueous outflow system in the human eye. Exp. Eye Res. 1991, 53, 507–517. [Google Scholar] [CrossRef]
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.J.M.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Cachon, B.F.; Firmin, S.; Verdin, A.; Ayi-Fanou, L.; Billet, S.; Cazier, F.; Martin, P.J.; Aissi, F.; Courcot, D.; Sanni, A.; et al. Proinflammatory effects and oxidative stress within human bronchial epithelial cells exposed to atmospheric particulate matter (PM2.5 and PM>2.5) collected from Cotonou, Benin. Environ. Pollut. 2014, 185, 340–351. [Google Scholar] [CrossRef]
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.; Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol. Environ. Saf. 2016, 128, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, L.; Cui, B.; Gai, Z.; Li, Q.; Wang, S.; Yan, J.; Lin, B.; Tian, L.; Liu, H.; et al. Early postnatal exposure to airborne fine particulate matter induces autism-like phenotypes in male rats. Toxicol. Sci. 2018, 162, 189–199. [Google Scholar] [CrossRef]
- Yue, H.; Ji, X.; Li, G.; Hu, M.; Sang, N. Maternal exposure to PM2.5 affects fetal lung development at sensitive windows. Environ. Sci. Technol. 2019, 54, 316–324. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Zhu, J.; Li, C.; Zhang, T.; Liu, H.; Xu, Q.; Ye, X.; Zhou, L.; Ye, L. Effect of Atmospheric PM2.5 on Expression Levels of NF-kappaB Genes and Inflammatory Cytokines Regulated by NF-kappaB in Human Macrophage. Inflammation 2018, 41, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, M.; Øvrevik, J.; Mollerup, S.; Asare, N.; Longhin, E.; Dahlman, H.-J.; Camatini, M.; Holme, J.A. Airborne urban particles (Milan winter-PM2.5) cause mitotic arrest and cell death: Effects on DNA, mitochondria, AhR binding and spindle organization. Mutat. Res. Mol. Mech. Mutagen. 2011, 713, 18–31. [Google Scholar] [CrossRef]
- Mehta, M.; Chen, L.C.; Gordon, T.; Rom, W.; Tang, M.S. Particulate matter inhibits DNA repair and enhances mutagenesis. Mutat. Res. 2008, 657, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Wang, Y.; Chen, X.; Li, J. Toxicity and mechanisms of lead on neural stem cells: A review. Environ. Res. 2022, 204, 112300. [Google Scholar] [CrossRef]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef]
- Nebert, D.W.; Dalton, T.P.; Okey, A.B.; Gonzalez, F.J. Role of aryl hydrocarbon receptor in PAH-mediated developmental toxicity. Birth Defects Res. Part A Clin. Mol. Teratol. 2004, 70, 677–684. [Google Scholar] [CrossRef]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Misra, C.; Sempf, J.; Wang, M.; Oberley, T.; Froines, J.; Nel, A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003, 111, 455–460. [Google Scholar] [CrossRef]
- Rhinehart, Z.J.; Kinnee, E.; Essien, U.R.; Saul, M.; Guhl, E.; Clougherty, J.E.; Magnani, J.W. Association of Fine Particulate Matter and Risk of Stroke in Patients with Atrial Fibrillation. JAMA Netw. Open 2020, 3, e2011760. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, L.; Drawz, P.E.; Berman, J.D. The association between fine particulate matter (PM2.5) and chronic kidney disease using electronic health record data in urban Minnesota. J. Expo. Sci. Environ. Epidemiol. 2021, 32, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Sowden, J. Molecular and developmental mechanisms of anterior segment dysgenesis. Eye 2007, 21, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
| Variable | Patients with ASD | Control Participants * | p-Value ** |
|---|---|---|---|
| Total | 582 (25.0) | 1746 (75.0) | |
| Sex | 0.85 | ||
| Boys | 322 (55.3) | 958 (54.9) | |
| Girls | 260 (44.7) | 788 (45.1) | |
| Type of insurance | 0.07 | ||
| Medical aid | 18 (3.1) | 32 (1.8) | |
| Health insurance | 564 (96.9) | 1714 (98.2) | |
| Season at conception date | 0.47 | ||
| Spring | 149 (25.6) | 490 (28.1) | |
| Summer | 169 (29.0) | 459 (26.3) | |
| Fall | 146 (25.1) | 425 (24.3) | |
| Winter | 118 (20.3) | 372 (21.3) | |
| Year of birth | 1.00 | ||
| 2007 | 43 (7.4) | 122 (7.0) | |
| 2008 | 29 (5.0) | 95 (5.4) | |
| 2009 | 33 (5.7) | 95 (5.4) | |
| 2010 | 58 (10.0) | 174 (10.0) | |
| 2011 | 47 (8.1) | 147 (8.4) | |
| 2012 | 48 (8.3) | 146 (8.4) | |
| 2013 | 56 (9.6) | 163 (9.3) | |
| 2014 | 57 (9.8) | 172 (9.9) | |
| 2015 | 51 (8.8) | 159 (9.1) | |
| 2016 | 56 (9.6) | 163 (9.3) | |
| 2017 | 31 (5.3) | 90 (5.2) | |
| 2018 | 29 (5.0) | 76 (4.4) | |
| 2019 | 31 (5.3) | 101 (5.8) | |
| 2020 | 13 (2.2) | 43 (2.5) | |
| Cities or Provinces | 1.00 | ||
| City | 281 (48.3%) | 842 (48.2%) | |
| Province | 301 (51.7%) | 904 (51.8%) |
| Period of PM2.5 Exposure (μg/m3) | Mean | SD | Median | IQR | |
|---|---|---|---|---|---|
| Patients with ASD | Preconception | 29.0 | 8.1 | 29.3 | 22.6, 34.7 |
| 1st trimester | 28.2 | 8.0 | 27.6 | 22.7, 33.3 | |
| 2nd trimester | 28.4 | 7.9 | 28.1 | 22.6, 33.8 | |
| 3rd trimester | 28.7 | 7.6 | 28.7 | 23.3, 33.4 | |
| Preconception—1st trimester | 28.6 | 6.7 | 28.3 | 24.0, 32.7 | |
| Preconception—2nd trimester | 28.5 | 5.6 | 27.9 | 24.6, 32.3 | |
| Preconception—3rd trimester | 28.6 | 5.2 | 28.3 | 25.2, 32.1 | |
| Control Participants | Preconception | 28.7 | 8.1 | 28.4 | 22.7, 34.1 |
| 1st trimester | 28.0 | 7.9 | 27.8 | 22.2, 33.4 | |
| 2nd trimester | 28.0 | 8.0 | 27.8 | 22.0, 33.2 | |
| 3rd trimester | 28.4 | 7.8 | 28.0 | 22.6, 34.0 | |
| Preconception—1st trimester | 28.3 | 6.6 | 28.2 | 24.0, 32.8 | |
| Preconception—2nd trimester | 28.2 | 5.6 | 28.0 | 24.6, 31.8 | |
| Preconception—3rd trimester | 28.3 | 5.4 | 28.0 | 24.9, 31.9 |
| Period of PM2.5 Exposure | Crude Model | Adjusted Model ** | ||
|---|---|---|---|---|
| RR (95% CI) | p-Value | RR (95% CI) | p-Value | |
| Preconception | 1.05 (0.92, 1.20) | 0.470 | 1.18 (1.03, 1.34) | 0.014 |
| 1st trimester | 1.04 (0.91, 1.18) | 0.592 | 1.15 (1.03, 1.27) | 0.009 |
| 2nd trimester | 1.09 (0.95, 1.24) | 0.208 | 1.14 (1.01, 1.29) | 0.037 |
| 3rd trimester | 1.05 (0.92, 1.20) | 0.483 | 1.10 (0.99, 1.21) | 0.061 |
| Preconception—1st trimester | 1.06 (0.93, 1.20) | 0.376 | 1.16 (1.08, 1.26) | <0.001 |
| Preconception—2nd trimester | 1.08 (0.96, 1.22) | 0.220 | 1.14 (1.07, 1.21) | <0.001 |
| Preconception—3rd trimester | 1.07 (0.95, 1.21) | 0.254 | 1.13 (1.08, 1.20) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choe, S.; Lee, K.-S.; Ha, A.; Kim, S.; Jeoung, J.-W.; Park, K.-H.; Hong, Y.-C.; Kim, Y.-K. Association of Maternal Exposure to Fine Particulate Matter During Pregnancy with Anterior Segment Dysgenesis Risk: A Matched Case-Control Study. J. Clin. Med. 2025, 14, 3003. https://doi.org/10.3390/jcm14093003
Choe S, Lee K-S, Ha A, Kim S, Jeoung J-W, Park K-H, Hong Y-C, Kim Y-K. Association of Maternal Exposure to Fine Particulate Matter During Pregnancy with Anterior Segment Dysgenesis Risk: A Matched Case-Control Study. Journal of Clinical Medicine. 2025; 14(9):3003. https://doi.org/10.3390/jcm14093003
Chicago/Turabian StyleChoe, Sooyeon, Kyung-Shin Lee, Ahnul Ha, Soontae Kim, Jin-Wook Jeoung, Ki-Ho Park, Yun-Chul Hong, and Young-Kook Kim. 2025. "Association of Maternal Exposure to Fine Particulate Matter During Pregnancy with Anterior Segment Dysgenesis Risk: A Matched Case-Control Study" Journal of Clinical Medicine 14, no. 9: 3003. https://doi.org/10.3390/jcm14093003
APA StyleChoe, S., Lee, K.-S., Ha, A., Kim, S., Jeoung, J.-W., Park, K.-H., Hong, Y.-C., & Kim, Y.-K. (2025). Association of Maternal Exposure to Fine Particulate Matter During Pregnancy with Anterior Segment Dysgenesis Risk: A Matched Case-Control Study. Journal of Clinical Medicine, 14(9), 3003. https://doi.org/10.3390/jcm14093003






