The Association Between Central Venous Pressure and Acute Kidney Injury Development in Patients with Septic Shock †
Abstract
:1. Introduction
2. Materials and Methods
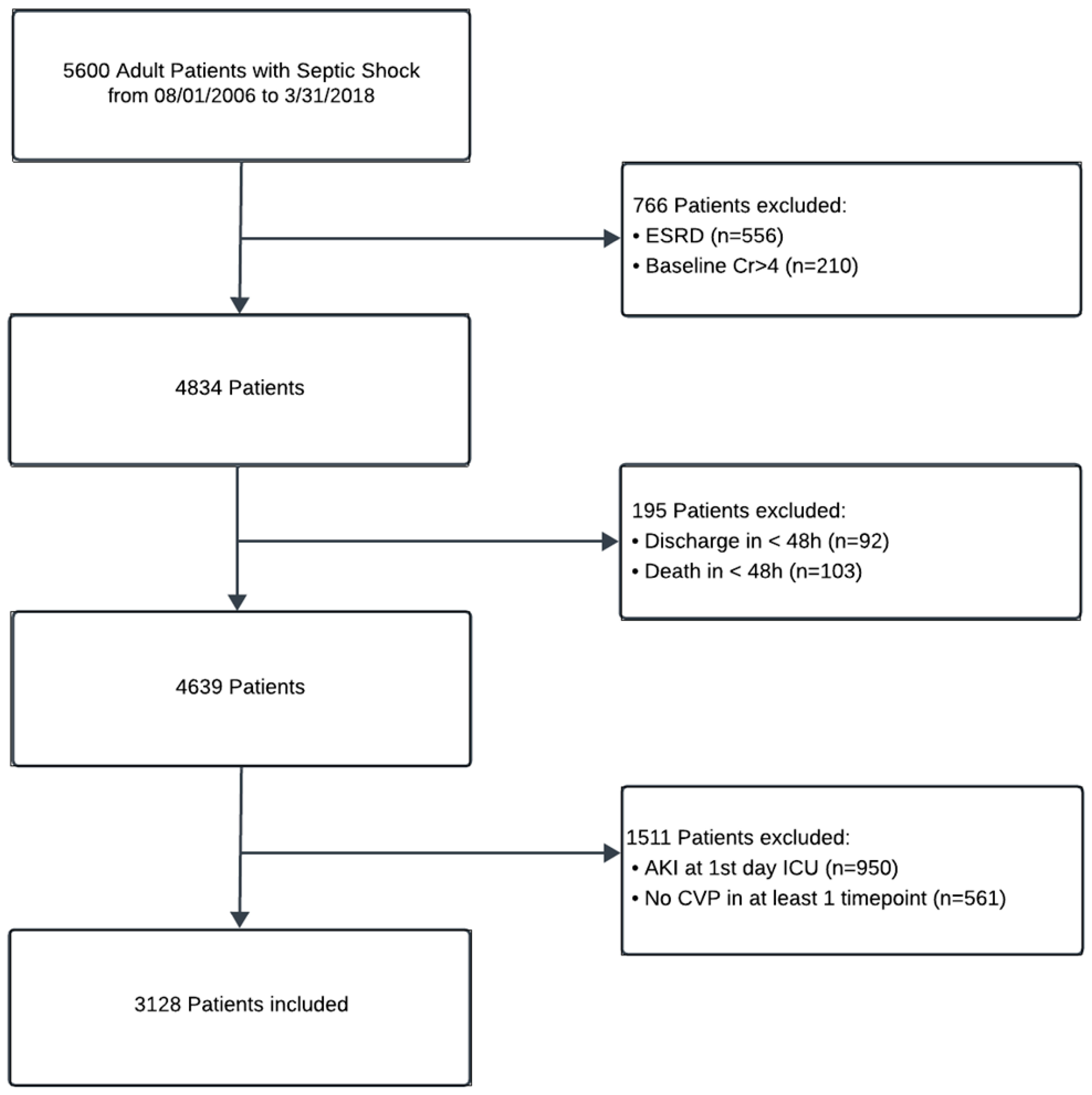
2.1. Study Setting and Population
2.2. Data Collection and Variables
2.3. Definitions and Outcomes
2.4. Statistical Analysis
3. Result
3.1. Patient Characteristics
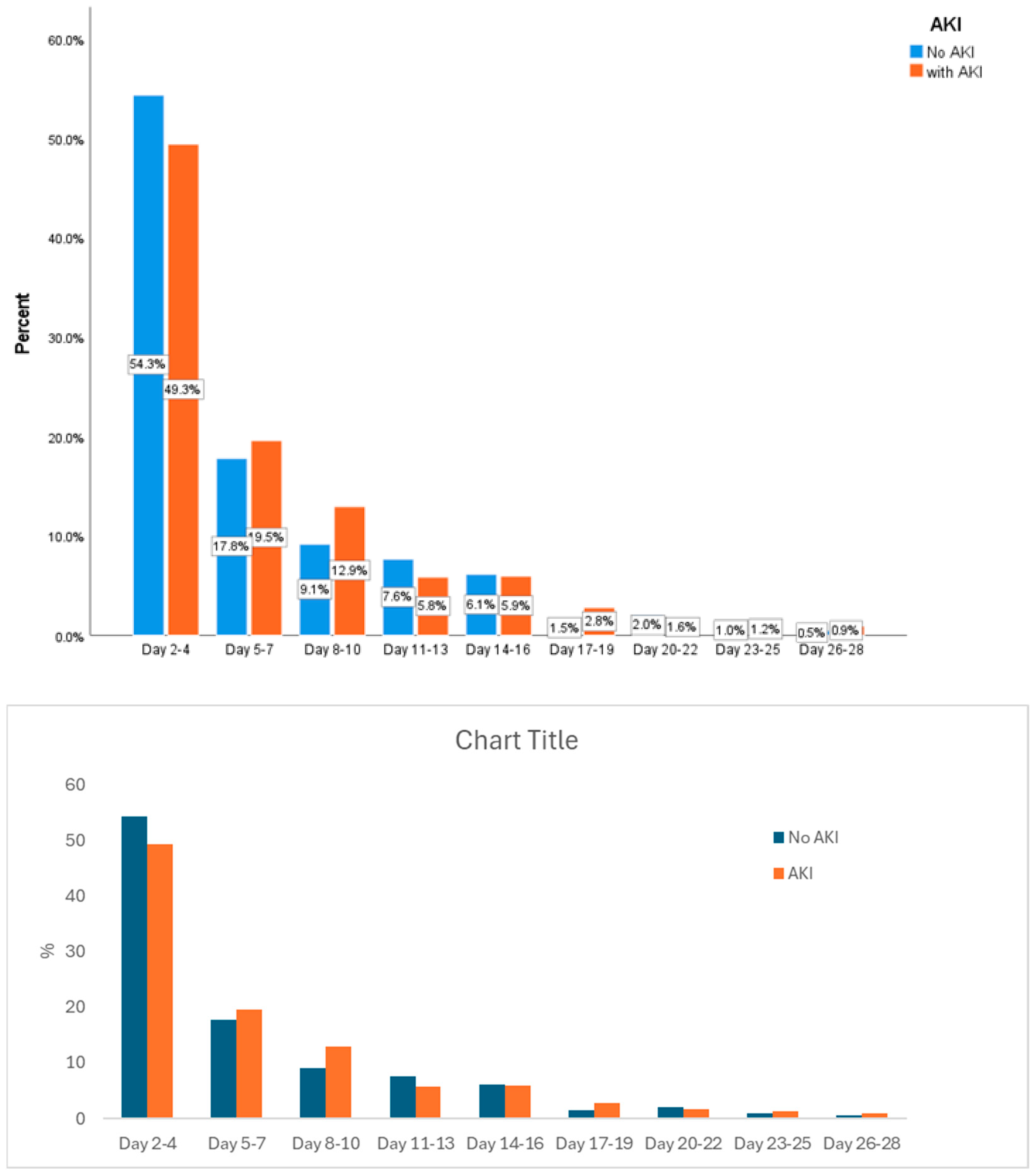
3.2. CVP at Different Time Points and AKI
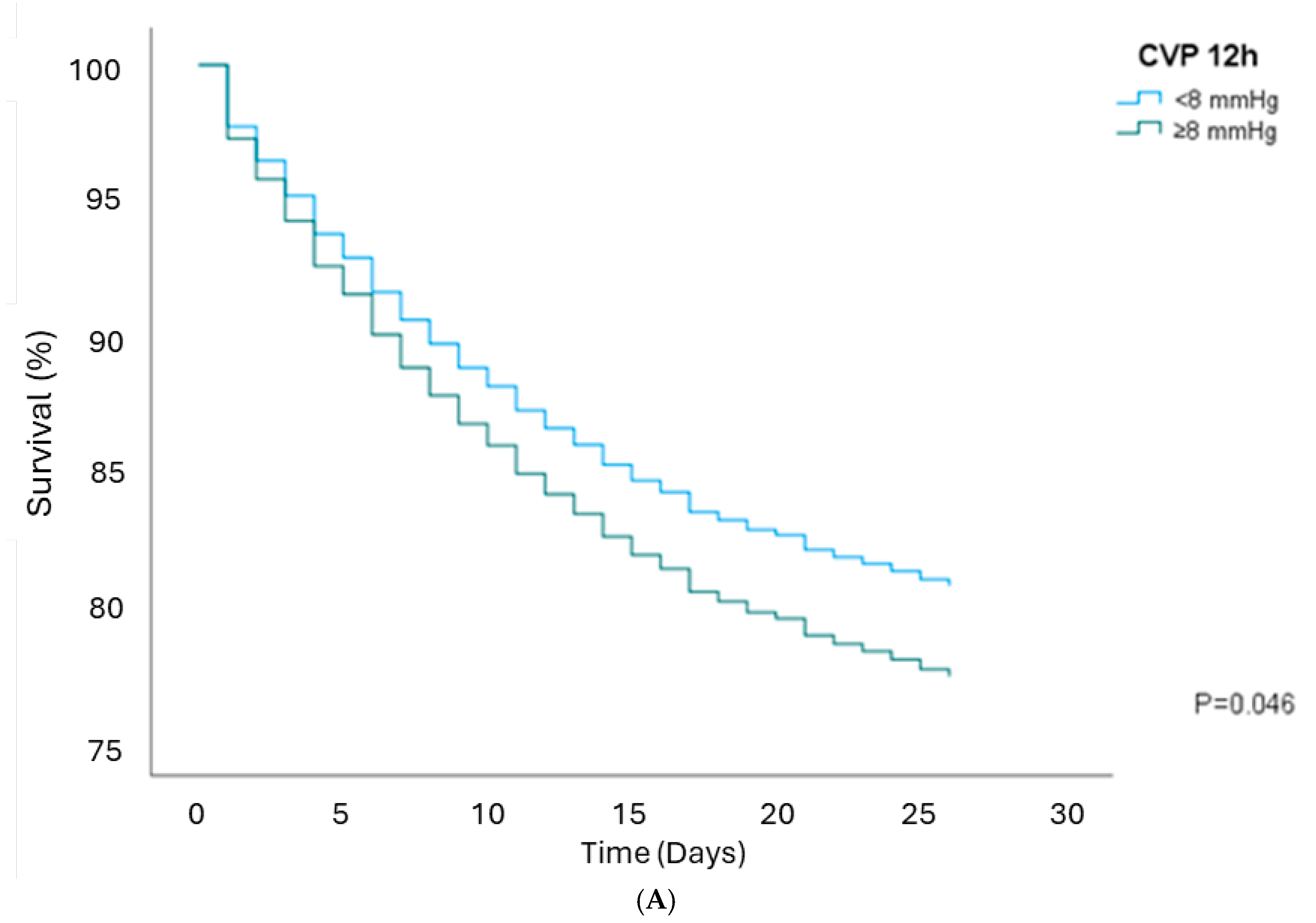
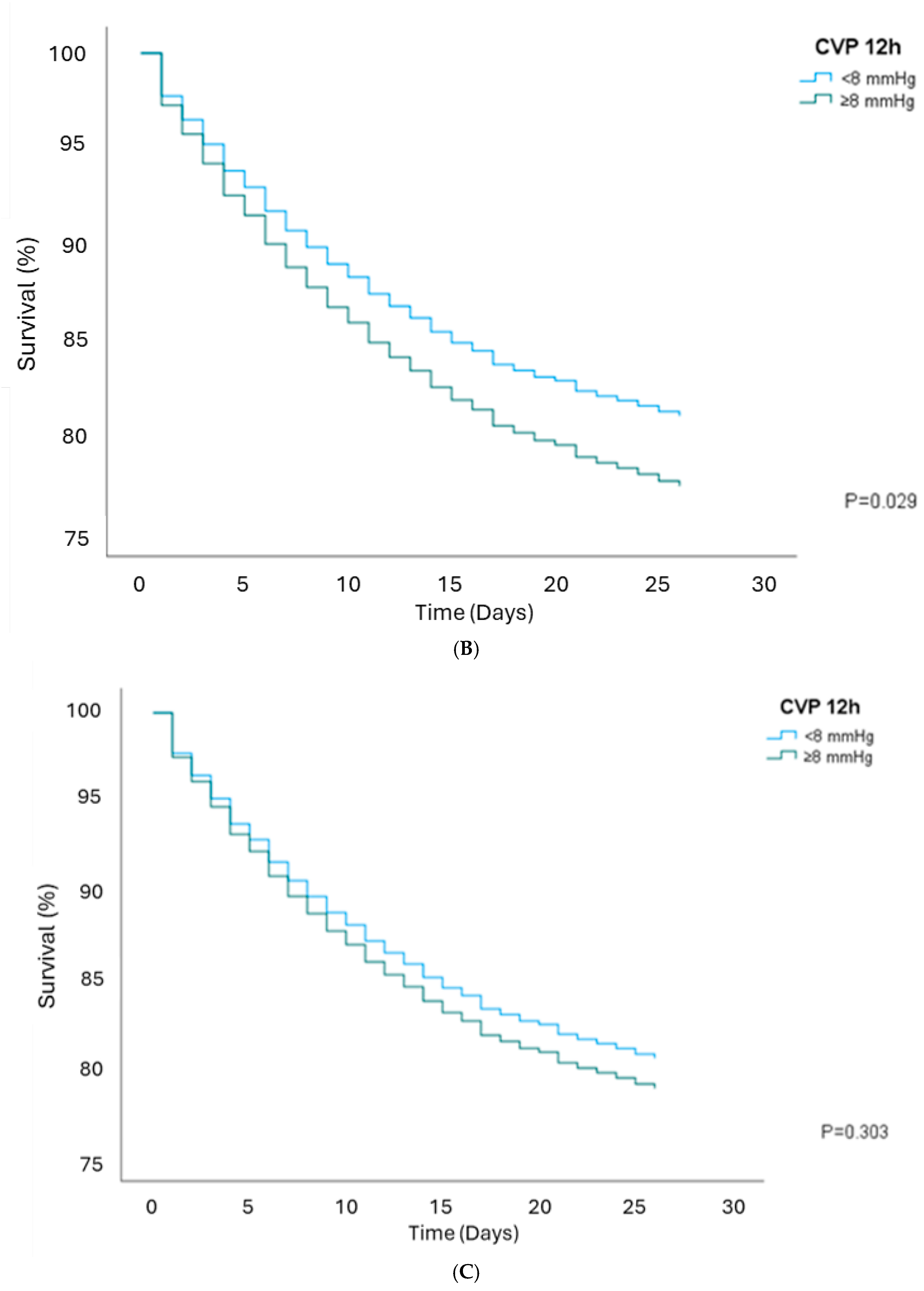
3.3. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a systematic review and meta-analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef] [PubMed]
- Kaukonen, K.M.; Bailey, M.; Suzuki, S.; Pilcher, D.; Bellomo, R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA 2014, 311, 1308–1316. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Lapinsky, S.; Dial, S.; Arabi, Y.; Dodek, P.; Wood, G.; Ellis, P.; Guzman, J.; Marshall, J.; Parrillo, J.E.; et al. Acute kidney injury in septic shock: Clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 2009, 35, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, S.M.; Uchino, S.; Bellomo, R.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; Gibney, N.; et al. Septic acute kidney injury in critically ill patients: Clinical characteristics and outcomes. Clin. J. Am. Soc. Nephrol. 2007, 2, 431–439. [Google Scholar] [CrossRef]
- Poston, J.T.; Koyner, J.L. Sepsis associated acute kidney injury. BMJ 2019, 364, k4891. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Carlet, J.M.; Masur, H.; Gerlach, H.; Calandra, T.; Cohen, J.; Gea-Banacloche, J.; Keh, D.; Marshall, J.C.; Parker, M.M.; et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit. Care Med. 2004, 32, 858–873. [Google Scholar] [CrossRef]
- Marik, P.E.; Baram, M.; Vahid, B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest 2008, 134, 172–178. [Google Scholar] [CrossRef]
- Rivers, E.; Nguyen, B.; Havstad, S.; Ressler, J.; Muzzin, A.; Knoblich, B.; Peterson, E.; Tomlanovich, M.; Early Goal-Directed Therapy Collaborative, G. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N. Engl. J. Med. 2001, 345, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Investigators, A.; Group, A.C.T.; Peake, S.L.; Delaney, A.; Bailey, M.; Bellomo, R.; Cameron, P.A.; Cooper, D.J.; Higgins, A.M.; Holdgate, A.; et al. Goal-directed resuscitation for patients with early septic shock. N. Engl. J. Med. 2014, 371, 1496–1506. [Google Scholar] [CrossRef]
- Pro, C.I.; Yealy, D.M.; Kellum, J.A.; Huang, D.T.; Barnato, A.E.; Weissfeld, L.A.; Pike, F.; Terndrup, T.; Wang, H.E.; Hou, P.C.; et al. A randomized trial of protocol-based care for early septic shock. N. Engl. J. Med. 2014, 370, 1683–1693. [Google Scholar] [CrossRef]
- Mouncey, P.R.; Osborn, T.M.; Power, G.S.; Harrison, D.A.; Sadique, M.Z.; Grieve, R.D.; Jahan, R.; Harvey, S.E.; Bell, D.; Bion, J.F.; et al. Trial of early, goal-directed resuscitation for septic shock. N. Engl. J. Med. 2015, 372, 1301–1311. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Hamzaoui, O.; Teboul, J.L. Central venous pressure (CVP). Intensive Care Med. 2022, 48, 1498–1500. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.P.; Cavender, S.; Lee, J.; Feng, M.; Mark, R.G.; Celi, L.A.; Mukamal, K.J.; Danziger, J. Peripheral Edema, Central Venous Pressure, and Risk of AKI in Critical Illness. Clin. J. Am. Soc. Nephrol. 2016, 11, 602–608. [Google Scholar] [CrossRef]
- Sun, R.; Guo, Q.; Wang, J.; Zou, Y.; Chen, Z.; Wang, J.; Zhang, Y. Central venous pressure and acute kidney injury in critically ill patients with multiple comorbidities: A large retrospective cohort study. BMC Nephrol. 2022, 23, 83. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; van Deursen, V.M.; Navis, G.; Voors, A.A.; van Veldhuisen, D.J.; Hillege, H.L. Increased Central Venous Pressure Is Associated With Impaired Renal Function and Mortality in a Broad Spectrum of Patients With Cardiovascular Disease. J. Am. Coll. Cardiol. 2009, 53, 582–588. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Vandenberghe, W.; Bové, T.; De Somer, F.; Herck, I.; François, K.; Peperstraete, H.; Dhondt, A.; Martens, T.; Schaubroeck, H.; Philipsen, T.; et al. Impact of mean perfusion pressure and vasoactive drugs on occurrence and reversal of cardiac surgery-associate acute kidney injury: A cohort study. J. Crit. Care 2022, 71, 154101. [Google Scholar] [CrossRef]
- Ostermann, M.; Hall, A.; Crichton, S. Low mean perfusion pressure is a risk factor for progression of acute kidney injury in critically ill patients—A retrospective analysis. BMC Nephrol. 2017, 18, 151. [Google Scholar] [CrossRef] [PubMed]
- Panwar, R.; Lanyon, N.; Davies, A.R.; Bailey, M.; Pilcher, D.; Bellomo, R. Mean perfusion pressure deficit during the initial management of shock--an observational cohort study. J. Crit. Care 2013, 28, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wu, B.; Xing, C.; Mao, H. Severe fluctuation in mean perfusion pressure is associated with increased risk of in-hospital mortality in critically ill patients with central venous pressure monitoring: A retrospective observational study. PLoS ONE 2023, 18, e0287046. [Google Scholar] [CrossRef]
- Xiao, W.; Liu, W.; Zhang, J.; Huang, L.; Liu, Y.; Hu, J.; Hua, T.; Yang, M. Early persistent exposure to high CVP is associated with increased mortality and AKI in septic shock: A retrospective study. Am. J. Emerg. Med. 2023, 74, 146–151. [Google Scholar] [CrossRef]
- Yegenaga, I.; Hoste, E.; Van Biesen, W.; Vanholder, R.; Benoit, D.; Kantarci, G.; Dhondt, A.; Colardyn, F.; Lameire, N. Clinical characteristics of patients developing ARF due to sepsis/systemic inflammatory response syndrome: Results of a prospective study. Am. J. Kidney Dis. 2004, 43, 817–824. [Google Scholar] [CrossRef]
- Legrand, M.; Dupuis, C.; Simon, C.; Gayat, E.; Mateo, J.; Lukaszewicz, A.C.; Payen, D. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: A retrospective observational study. Crit. Care 2013, 17, R278. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.H.; Forbes, J.; Nakada, T.A.; Walley, K.R.; Russell, J.A. Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Critical Care Med. 2011, 39, 259–265. [Google Scholar] [CrossRef]
- Zheng, F.D.; Wang, L.H.; Pang, Y.X.; Chen, Z.G.; Lu, Y.T.; Yang, Y.D.; Wu, J.F. ShockSurv: A machine learning model to accurately predict 28-day mortality for septic shock patients in the intensive care unit. Biomed. Signal Process. Control. 2023, 86, 105146. [Google Scholar] [CrossRef]
- Wang, M.; Shi, Y.; Pan, X.; Wang, B.; Lu, B.; Ouyang, J. An Individualized Nomogram for Predicting Mortality Risk of Septic Shock Patients During Hospitalization: A ten Years Retrospective Analysis. Infect Drug Resist 2023, 16, 6247–6257. [Google Scholar] [CrossRef]
- Speiser, J.L.; Karvellas, C.J.; Shumilak, G.; Sligl, W.I.; Mirzanejad, Y.; Gurka, D.; Kumar, A.; Kumar, A.; Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Predicting in-hospital mortality in pneumonia-associated septic shock patients using a classification and regression tree: A nested cohort study. J. Intensive Care 2018, 6, 66. [Google Scholar] [CrossRef]
- Huang, A.; Liao, L.; Pan, L.; Pinhu, L. Association Between the Central Venous Pressure and All-Cause Mortality in Critically Ill Patients with Acute Kidney Injury. Int. J. Gen. Med. 2021, 14, 8019–8027. [Google Scholar] [CrossRef] [PubMed]
- Leligdowicz, A.; Dodek, P.M.; Norena, M.; Wong, H.; Kumar, A.; Kumar, A.; Co-operative Antimicrobial Therapy of Septic Shock Database Research Group. Association between source of infection and hospital mortality in patients who have septic shock. Am. J. Respir. Crit. Care Med. 2014, 189, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C.; Maier, R.V.; Jimenez, M.; Dellinger, E.P. Source control in the management of severe sepsis and septic shock: An evidence-based review. Crit. Care Med. 2004, 32, S513–S526. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, J.T.; Liu, J.C. The association between mortality and door-to-antibiotic time: A systematic review and meta-analysis. Postgrad. Med. J. 2023, 99, 1000–1007. [Google Scholar] [CrossRef]
- Xiang, M.J.; Chen, G.L. Impact of cancer on mortality rates in patients with sepsis: A meta-analysis and meta-regression of current studies. World J. Clin. Cases 2022, 10, 7386–7396. [Google Scholar] [CrossRef]
| Total (3128) | AKI (1098) | No AKI (2030) | p-Value | |
|---|---|---|---|---|
| Sex (Male %) | 1681 (53.74) | 679 (61.83) | 1002 (49.35) | <0.001 |
| Age (Median [IQR]) | 65.54 [54.63–75.52] | 65.24 [54.97–74.76] | 65.63 [54.49–75.91] | 0.313 |
| BMI (Median [IQR]) | 27.52 [23.45–32.64] | 28.73 [24.39–34.42] | 26.94 [22.89–31.68] | <0.001 |
| Hypertension (%) | 2180 (69.69) | 791 (72.04) | 1389 (68.42) | 0.036 |
| Diabetes Mellitus (%) | 795 (25.41) | 300 (27.32) | 495 (24.38) | 0.072 |
| Myocardial Infarction (%) | 340 (10.87) | 106 (9.65) | 234 (11.52) | 0.108 |
| Congestive Heart Failure (%) | 369 (11.79) | 144 (13.11) | 225 (11.08) | 0.093 |
| Diastolic Heart Failure (%) | 319 (10.19) | 132 (12.02) | 187 (9.21) | 0.013 |
| Pulmonary Hypertension (%) | 504 (16.11) | 180 (16.39) | 324 (15.96) | 0.753 |
| Cerebrovascular accidents (%) | 266 (8.50) | 66 (6.01) | 200 (9.85) | <0.001 |
| Chronic Kidney Disease (%) | 461 (14.73) | 176 (16.02) | 285 (14.04) | 0.134 |
| Baseline Cr (Median [IQR]) | 0.90 [0.75–1.10] | 0.90 [0.74–1.06] | 0.93 [0.75–1.10] | <0.001 |
| Baseline eGFR (Median [IQR]) | 72.93 [53.86–99.24] | 81.34 [57.57–102.05] | 70.57 [52.45–97.17] | <0.001 |
| Cr Closest to ICU Admission (Median [IQR]) | 1.00 [0.70–1.40] | 1.40 [1.00–2.02] | 0.80 [0.60–1.10] | <0.001 |
| Max Cr before AKI (Median [IQR]) | 1.60 [1.30–2.10] | 1.60 [1.30–2.20] | 1.30 [1.10–1.80] | <0.001 |
| APACHE III Score SAS 1 h (Median [IQR]) | 51 [39–65] | 53 [40–69] | 50 [38–64] | <0.001 |
| APACHE III Score SAS 24 h (Median [IQR]) | 80 [65–97] | 90 [74–106] | 75 [61–91] | <0.001 |
| Dobutamine 24 h (%) | 146 (4.66) | 60 (5.46) | 86 (4.23) | 0.120 |
| Dopamine 24 h (%) | 126 (4.03) | 46 (4.20) | 80 (3.94) | 0.736 |
| Epinephrine 24 h (%) | 180 (5.75) | 98 (8.92) | 82 (4.04) | <0.001 |
| Norepinephrine 24 h (%) | 1594 (51.00) | 608 (55.37) | 986 (48.57) | <0.001 |
| Phenylephrine 24 h (%) | 486 (15.54) | 188 (17.12) | 298 (14.70) | 0.072 |
| Vasopressin 24 h (%) | 909 (29.06) | 380 (34.61) | 529 (26.06) | <0.001 |
| WBC closest to ICU admission | 10.70 [6.40–16.20] | 10.45 [5.77–16.72] | 10.80 [6.60–16.10] | 0.198 |
| Max WBC closest to AKI (Median [IQR]) | 12.70 [7.50–18.90] | 12.60 [7.30–18.80] | 13.40 [8.70–19.10] | 0.253 |
| Lactate closest to ICU admission (Median [IQR]) | 1.80 [1.10–3.10] | 2.00 [1.30–3.60] | 1.70 [1.10–2.80] | <0.001 |
| Net Fluid Balance 24 h (Median [IQR]) | 4547.64 [2139.03–7345.28] | 4833.82 [2214.10–7838.27] | 4382.44 [2063.83–7101.68] | 0.002 |
| Mechanical ventilation (%) | 2324 (74.3) | 849 (77.3) | 1475 (72.7) | 0.005 |
| Average PEEP (Median [IQR]) | 6.86 [5.03–9.15] | 7.24 [5.37–9.47] | 6.61 [5.00–8.96] | <0.001 |
| In-ICU Dialysis (%) | 360 (11.5) | 315 (28.7) | 45 (2.2) | <0.001 |
| In-Hospital Dialysis (%) | 416 (13.3) | 347 (31.6) | 69 (3.4) | <0.001 |
| Length of Hospital Stay | 16.24 [9.66–28.36] | 20.60 [12.89–35.15] | 14.22 [8.73–24.55] | <0.001 |
| 28-day mortality (%) | 684 (21.90) | 345 (31.42) | 339 (16.70) | <0.001 |
| 90-day mortality (%) | 977 (31.23) | 454 (41.35) | 523 (25.80) | <0.001 |
| Total (3128) | AKI (1098) | No AKI (2030) | p-Value | |
|---|---|---|---|---|
| After 6 h | ||||
| CVP 6 h (Median [IQR]) | 9 [5–13] | 10 [6–15] | 8 [4–12] | <0.001 |
| MPP 6 h (Median [IQR]) | 62 [54–72] | 60 [52–68] | 63 [56–74] | <0.001 |
| CVP 6 h ≥ 8 (%) | 1189 (38.01) | 445 (40.52) | 744 (36.65) | <0.001 |
| MAP 6 h (Median [IQR]) | 71 [63–80] | 70 [63–79] | 71 [64–81] | 0.034 |
| MAP 6 h ≥ 65 (%) | 2208 (70.58) | 756 (68.85) | 1452 (71.53) | 0.111 |
| After 12 h | ||||
| CVP 12 h (Median [IQR]) | 8 [4–13] | 10 [6–14] | 7 [4–12] | <0.001 |
| MPP12 h (Median [IQR]) | 63 [55–73] | 60 [52–70] | 65 [57–74] | <0.001 |
| CVP 12 h ≥ 8 (%) | 1290 (41.24) | 511 (46.54) | 779 (39.36) | <0.001 |
| MAP 12 h (Median [IQR]) | 72 [65–81] | 70 [63–79] | 72 [65–82] | <0.001 |
| MAP 12 h ≥ 65 (%) | 2346 (75.00) | 777 (70.76) | 1569 (77.29) | <0.001 |
| After 24 h | ||||
| CVP 24 h (Median [IQR]) | 9 [5–13] | 10 [6–15] | 8 [4–12.75] | <0.001 |
| MPP 24 h (Median [IQR]) | 64 [56–74] | 61 [53–71] | 66 [57–75] | <0.001 |
| CVP 24 h ≥ 8 (%) | 1543 (49.33) | 616 (56.25) | 927 (45.66) | <0.001 |
| MAP 24 h (Median [IQR]) | 73 [65–83] | 72 [64–81] | 74 [65–84] | <0.001 |
| MAP 24 h ≥ 65 (%) | 2443 (78.10) | 817 (74.41) | 1626 (80.10) | <0.001 |
| After 48 h | ||||
| CVP 48 h (Median [IQR]) | 9 [5–14] | 10 [6–15] | 8 [4–13] | <0.001 |
| MPP 48 h (Median [IQR]) | 67 [58–79] | 64 [55–74] | 68 [60–80] | <0.001 |
| CVP 48 h ≥ 8 (%) | 1660 (53.70) | 684 (62.30) | 976 (48.08) | <0.001 |
| MAP 48 h (Median [IQR]) | 76 [68–87] | 74 [67–85] | 77 [68–88] | <0.001 |
| MAP 48 h ≥ 65 (%) | 2625 (83.92) | 888 (80.87) | 1737 (85.56) | <0.001 |
| CVP and AKI | ||||||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | p Value | Adjusted OR (95% CI) [Model 1] | p Value | Adjusted OR (95% CI) [Model 2] | p Value | |
| CVP 6 h ≥ 8 | 1.56 (1.30–1.89) | <0.001 | 1.61 (1.33–1.94) | <0.001 | 1.20 (0.92–1.57) | 0.173 |
| CVP 12 h ≥ 8 | 1.75 (1.47–2.09) | <0.001 | 1.81 (1.52–2.16) | <0.001 | 1.60 (1.26–2.05) | <0.001 |
| CVP 24 h ≥ 8 | 1.87 (1.58–2.21) | <0.001 | 1.89 (1.60–2.24) | <0.001 | 1.25 (0.99–1.57) | 0.056 |
| CVP 48 h ≥ 8 | 1.78 (1.52–2.09) | <0.001 | 1.78 (1.52–2.09) | <0.001 | 1.60 (1.29–1.99) | <0.001 |
| CVP and In-Hospital (28-Day) Mortality | ||||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | p Value | Adjusted HR (95% CI) (Model 1) | p Value | Adjusted HR (95% CI) (Model 2) | p Value | Adjusted HR (95% CI) (Model 3) | p Value | |
| CVP 6 h ≥8 | 1.18 (0.97–1.44) | 0.090 | 1.21 (0.99–1.48) | 0.051 | 1.09 (0.90–1.33) | 0.346 | 1.05 (0.81–1.35) | 0.704 |
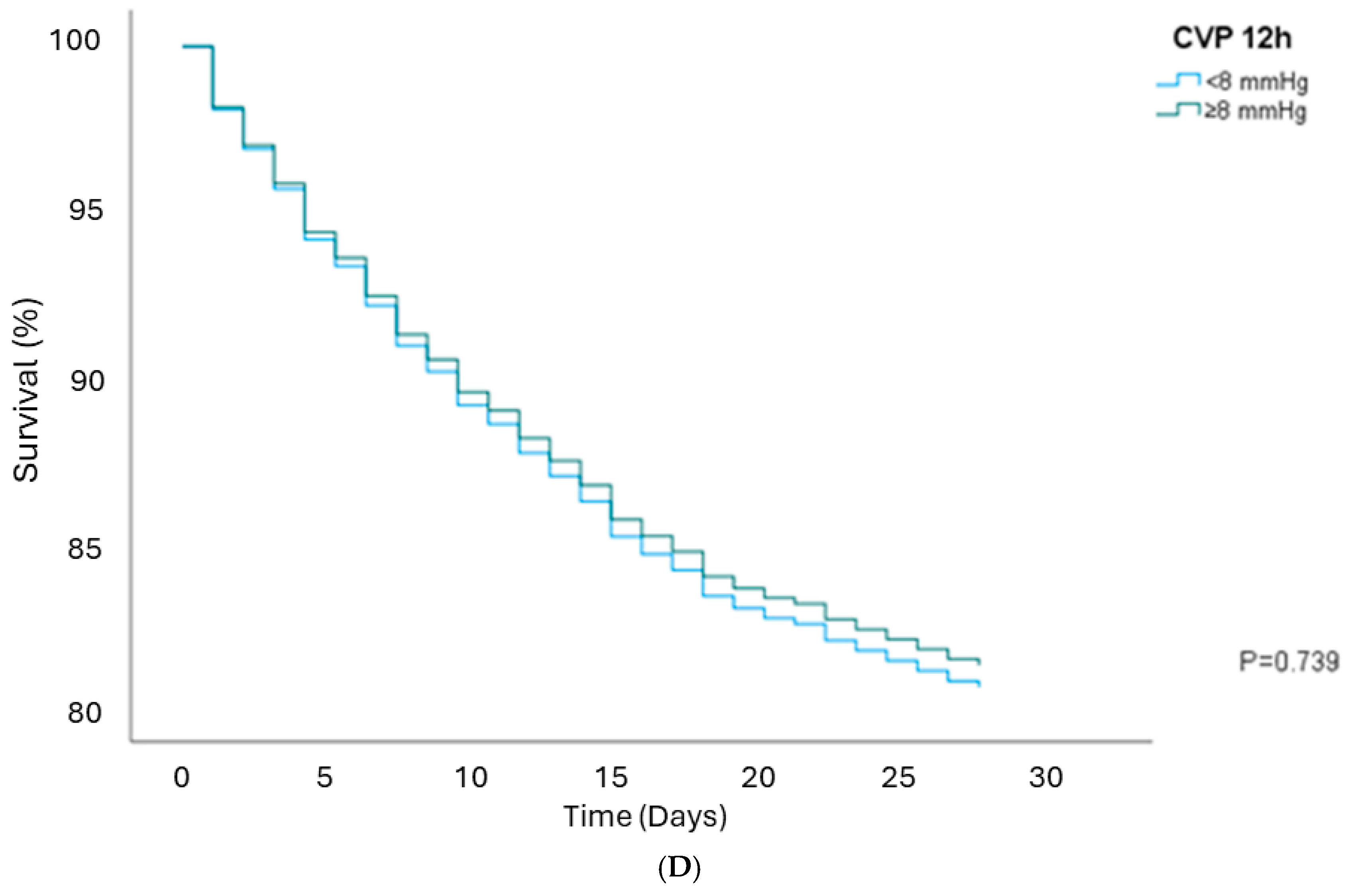
| CVP 12 h ≥8 | 1.19 (1.00–1.43) | 0.046 | 1.22 (1.02–1.46) | 0.029 | 1.09 (0.92–1.31) | 0.303 | 0.96 (0.76–1.21) | 0.739 |
| CVP 24 h ≥8 | 1.08 (0.92–1.29) | 0.325 | 1.09 (0.93–1.30) | 0.285 | 0.97 (0.82–1.16) | 0.792 | 0.82 (0.66–1.02) | 0.075 |
| CVP 48 h ≥8 | 1.13 (0.96–1.33) | 0.126 | 1.12 (0.96–1.32) | 0.147 | 1.03 (0.87–1.21) | 0.724 | 1.11 (0.91–1.35) | 0.315 |
| CVP and 90-Day Mortality | ||||||||
| CVP 6 h ≥8 | 1.17 (0.99–1.37) | 0.058 | 1.20 (1.02–1.41) | 0.031 | 1.10 (0.93–1.30) | 0.251 | 1.08 (0.87–1.34) | 0.488 |
| CVP 12 h ≥8 | 1.12 (0.97–1.30) | 0.120 | 1.14 (0.98–1.32) | 0.080 | 1.05 (0.90–1.22) | 0.521 | 0.92 (0.75–1.11) | 0.371 |
| CVP 24 h ≥8 | 0.99 (0.86–1.14) | 0.888 | 0.99 (0.87–1.14) | 0.943 | 0.91 (0.79–1.04) | 0.178 | 0.82 (0.68–0.98) | 0.028 |
| CVP 48 h ≥8 | 1.06 (0.93–1.21) | 0.354 | 1.06 (0.93–1.21) | 0.402 | 0.98 (0.86–1.12) | 0.818 | 1.06 (0.89–1.25) | 0.518 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikravangolsefid, N.; Ninan, J.; Suppadungsuk, S.; Singh, W.; Kashani, K.B. The Association Between Central Venous Pressure and Acute Kidney Injury Development in Patients with Septic Shock. J. Clin. Med. 2025, 14, 3027. https://doi.org/10.3390/jcm14093027
Nikravangolsefid N, Ninan J, Suppadungsuk S, Singh W, Kashani KB. The Association Between Central Venous Pressure and Acute Kidney Injury Development in Patients with Septic Shock. Journal of Clinical Medicine. 2025; 14(9):3027. https://doi.org/10.3390/jcm14093027
Chicago/Turabian StyleNikravangolsefid, Nasrin, Jacob Ninan, Supawadee Suppadungsuk, Waryaam Singh, and Kianoush B. Kashani. 2025. "The Association Between Central Venous Pressure and Acute Kidney Injury Development in Patients with Septic Shock" Journal of Clinical Medicine 14, no. 9: 3027. https://doi.org/10.3390/jcm14093027
APA StyleNikravangolsefid, N., Ninan, J., Suppadungsuk, S., Singh, W., & Kashani, K. B. (2025). The Association Between Central Venous Pressure and Acute Kidney Injury Development in Patients with Septic Shock. Journal of Clinical Medicine, 14(9), 3027. https://doi.org/10.3390/jcm14093027






