Systematic Review of Variations in Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Peritoneal Metastasis from Colorectal Cancer
Abstract
:1. Introduction
2. Methods
2.1. Database Search and Source of Information
2.2. Quality Assessment
2.3. Data Extraction
2.4. Data Synthesis
2.5. Compliance with Applicable Guidelines
3. Results
3.1. Literature Search and Evaluation
3.2. Heterogeneity in HIPEC Conduct
3.3. Drugs Used for HIPEC in Peritoneal Metastasis from Colorectal Cancer
3.4. HIPEC Conduct with Mitomycin C (MMC)
3.5. HIPEC Conduct with Oxaliplatin (L-OHP)
3.6. HIPEC Protocols Describing Combined Drug Use
3.7. Uncommon HIPEC Drugs
3.8. Differences in Exposure Times to Cytotoxic Drugs
3.9. Differences in Benchmarking Applied Drug Dosages
3.10. Carrier Solutions and Diluent Quantities
3.11. HIPEC Protocols Describing the Use of an Open and Closed Technique
3.12. HIPEC Protocols Entailing Simultaneous Intravenous and Intraperitoneal (Bidirectional) Drug Administration and Early Postoperative Intraperitoneal Chemotherapy (EPIC)
3.13. Hyperthermia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| °C | degree Celsius |
| CDDP | cisplatin; (cis-diamminedichloridoplatinum(II)) |
| CRC | colorectal carcinoma |
| CRS | cytoreductive surgery |
| CS | carrier solution |
| DOX | doxorubicin |
| EPIC | early postoperative intraperitoneal chemotherapy |
| ETO | etoposide |
| HIPEC | hyperthermic intraperitoneal chemotherapy |
| HCPT | hydroxycamptothecin |
| IRI | irinotecan |
| L | liter |
| L-OHP | oxaliplatin; (trans-L-(–)-Diaminocyclohexanoxalatoplatin) |
| i.v. | intravenous |
| L-PAM | melphalan; (L-Phenylalanine Mustard) |
| m2 | square meter; refers to body surface area |
| MMC | mitomycin C |
| PM | peritoneal metastasis |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCT | randomized controlled trial |
References
- Lambert, L.A. Looking up: Recent advances in understanding and treating peritoneal carcinomatosis. CA Cancer J. Clin. 2015, 65, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Sloothen, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Surgical treatment of peritoneal carcinomatosis: 1988 Du Pont lecture. Can. J. Surg. 1989, 32, 164–170. [Google Scholar]
- Jacquet, P.; Sugarbaker, P.H. Peritoneal-plasma barrier. Cancer Treat. Res. 1996, 82, 53–63. [Google Scholar]
- Ceelen, W.P.; Pahlman, L.; Mahteme, H. Pharmacodynamic aspects of intraperitoneal cytotoxic therapy. Cancer Treat. Res. 2007, 134, 195–214. [Google Scholar]
- Esquivel, J. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer: Survival outcomes and patient selection. J. Gastrointest. Oncol. 2016, 7, 72–78. [Google Scholar] [PubMed]
- Ceelen, W. HIPEC with oxaliplatin for colorectal peritoneal metastasis: The end of the road? Eur. J. Surg. Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Kuijpers, A.M.; Aalbers, A.G.; Nienhuijs, S.W.; de Hingh, I.H.; Wiezer, M.J.; van Ramshorst, B.; van Ginkel, R.J.; Havenga, K.; Heemsbergen, W.D.; Hauptmann, M.; et al. Implementation of a standardized HIPEC protocol improves outcome for peritoneal malignancy. World J. Surg. 2015, 39, 453–460. [Google Scholar] [CrossRef]
- Lemoine, L.; Sugarbaker, P.; Van der Speeten, K. Drugs, doses, and durations of intraperitoneal chemotherapy: Standardising HIPEC and EPIC for colorectal, appendiceal, gastric, ovarian peritoneal surface malignancies and peritoneal mesothelioma. Int. J. Hyperth. 2017, 33, 582–592. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.F.; Liang, H.; Wang, H.Q.; Hao, J.H.; Zhu, Z.G.; Wan, D.S.; Qin, L.X.; Cui, S.Z.; Ji, J.F.; et al. Chinese expert consensus on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal malignancies. World J. Gastroenterol. 2016, 22, 6906–6916. [Google Scholar] [CrossRef] [PubMed]
- Turaga, K.; Levine, E.; Barone, R.; Sticca, R.; Petrelli, N.; Lambert, L.; Nash, G.; Morse, M.; Adbel-Misih, R.; Alexander, H.R.; et al. Consensus guidelines from The American Society of Peritoneal Surface Malignancies on standardizing the delivery of hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer patients in the United States. Ann. Surg. Oncol. 2014, 21, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing Vienna, Austria. 2018. Available online: http://www.R-project.org/ (accessed on 2 November 2018).
- Wickham, H.; Françoise, R.; Henry, L.; Müller, K. dplyr (R-package). The Comprehensive R Archive Network. 2018. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 2 November 2018).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Gentleman, R., Hornik, K., Parmigiani, G., Eds.; Springer: New York, NY, USA, 2009; Available online: https://CRAN.R-project.org/package=ggplot2 (accessed on 2 November 2018).
- Wickham, H. tidyverse (R-package). The Comprehensive R Archive Network. 2018. Available online: https://CRAN.R-project.org/package=tidyverse (accessed on 2 November 2018).
- Wickham, H. The Split-Apply-Combine Strategy for Data Analysis. J. Stat. Softw. 2011, 40, 29. Available online: https://CRAN.R-project.org/package=plyr (accessed on 2 November 2018). [CrossRef]
- Kassambara, A. ggpubr (R-package). The Comprehensive R Archive Network. 2018. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 2 November 2018).
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Witkamp, A.J.; de Bree, E.; Kaag, M.M.; Boot, H.; Beijnen, J.H.; van Slooten, G.W.; van Coevorden, F.; Zoetmulder, F.A. Extensive cytoreductive surgery followed by intra-operative hyperthermic intraperitoneal chemotherapy with mitomycin-C in patients with peritoneal carcinomatosis of colorectal origin. Eur. J. Cancer 2001, 37, 979–984. [Google Scholar] [CrossRef]
- Beaujard, A.C.; Glehen, O.; Caillot, J.L.; Francois, Y.; Bienvenu, J.; Panteix, G.; Garbit, F.; Grandclément, E.; Vignal, J.; Gilly, F.N. Intraperitoneal chemohyperthermia with mitomycin C for digestive tract cancer patients with peritoneal carcinomatosis. Cancer 2000, 88, 2512–2519. [Google Scholar] [CrossRef] [Green Version]
- Benizri, E.I.; Bernard, J.L.; Rahili, A.; Benchimol, D.; Bereder, J.M. Small bowel involvement is a prognostic factor in colorectal carcinomatosis treated with complete cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy. World J. Surg. Oncol. 2012, 10, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bijelic, L.; Yan, T.D.; Sugarbaker, P.H. Failure analysis of recurrent disease following complete cytoreduction and perioperative intraperitoneal chemotherapy in patients with peritoneal carcinomatosis from colorectal cancer. Ann. Surg. Oncol. 2007, 14, 2281–2288. [Google Scholar] [CrossRef]
- Bijelic, L.; Yan, T.D.; Sugarbaker, P.H. Treatment failure following complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from colorectal or appendiceal mucinous neoplasms. J. Surg. Oncol. 2008, 98, 295–299. [Google Scholar] [CrossRef]
- Boutros, C.; Somasundar, P.; Espat, N.J. Early results on the use of biomaterials as adjuvant to abdominal wall closure following cytoreduction and hyperthermic intraperitoneal chemotherapy. World J. Surg. Oncol. 2010, 8, 72. [Google Scholar] [CrossRef] [Green Version]
- Braam, H.J.; Boerma, D.; Wiezer, M.J.; van Ramshorst, B. Hyperthermic intraperitoneal chemotherapy during primary tumour resection limits extent of bowel resection compared to two-stage treatment. Eur. J. Surg. Oncol. 2013, 39, 988–993. [Google Scholar] [CrossRef]
- Bretcha-Boix, P.; Farre-Alegre, J.; Sureda, M.; Dussan, C.; Perez Ruixo, J.J.; Brugarolas Masllorens, A. Cytoreductive surgery and perioperative intraperitoneal chemotherapy in patients with peritoneal carcinomatosis of colonic origin: Outcomes after 7 years’ experience of a new centre for peritoneal surface malignancies. Clin. Transl. Oncol. 2010, 12, 437–442. [Google Scholar] [CrossRef]
- Cao, C.Q.; Yan, T.D.; Liauw, W.; Morris, D.L. Comparison of optimally resected hepatectomy and peritonectomy patients with colorectal cancer metastasis. J. Surg. Oncol. 2009, 100, 529–533. [Google Scholar] [CrossRef]
- Carmignani, C.P.; Ortega-Perez, G.; Sugarbaker, P.H. The management of synchronous peritoneal carcinomatosis and hematogenous metastasis from colorectal cancer. Eur. J. Surg. Oncol. 2004, 30, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Cashin, P.H.; Dranichnikov, F.; Mahteme, H. Cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy treatment of colorectal peritoneal metastases: Cohort analysis of high volume disease and cure rate. J. Surg. Oncol. 2014, 110, 203–206. [Google Scholar] [CrossRef]
- Cashin, P.H.; Graf, W.; Nygren, P.; Mahteme, H. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis: Prognosis and treatment of recurrences in a cohort study. Eur. J. Surg. Oncol. 2012, 38, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Cashin, P.H.; Graf, W.; Nygren, P.; Mahteme, H. Intraoperative hyperthermic versus postoperative normothermic intraperitoneal chemotherapy for colonic peritoneal carcinomatosis: A case-control study. Ann. Oncol. 2012, 23, 647–652. [Google Scholar] [CrossRef]
- Ceelen, W.; Van Nieuwenhove, Y.; Putte, D.V.; Pattyn, P. Neoadjuvant chemotherapy with bevacizumab may improve outcome after cytoreduction and hyperthermic intraperitoneal chemoperfusion (HIPEC) for colorectal carcinomatosis. Ann. Surg. Oncol. 2014, 21, 3023–3028. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.C.; Liauw, W.; Zhao, J.; Morris, D.L. Comparative analysis of perioperative intraperitoneal chemotherapy regimen in appendiceal and colorectal peritoneal carcinomatosis. Int. J. Clin. Oncol. 2013, 18, 439–446. [Google Scholar] [CrossRef]
- Chua, T.C.; Morris, D.L.; Esquivel, J. Impact of the peritoneal surface disease severity score on survival in patients with colorectal cancer peritoneal carcinomatosis undergoing complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2010, 17, 1330–1336. [Google Scholar] [CrossRef]
- Chua, T.C.; Pelz, J.O.; Kerscher, A.; Morris, D.L.; Esquivel, J. Critical analysis of 33 patients with peritoneal carcinomatosis secondary to colorectal and appendiceal signet ring cell carcinoma. Ann. Surg. Oncol. 2009, 16, 2765–2770. [Google Scholar] [CrossRef]
- Chua, T.C.; Yan, T.D.; Ng, K.M.; Zhao, J.; Morris, D.L. Significance of lymph node metastasis in patients with colorectal cancer peritoneal carcinomatosis. World J. Surg. 2009, 33, 1488–1494. [Google Scholar] [CrossRef]
- Chua, T.C.; Yan, T.D.; Zhao, J.; Morris, D.L. Peritoneal carcinomatosis and liver metastases from colorectal cancer treated with cytoreductive surgery perioperative intraperitoneal chemotherapy and liver resection. Eur. J. Surg. Oncol. 2009, 35, 1299–1305. [Google Scholar] [CrossRef]
- Da Silva, R.G.; Sugarbaker, P.H. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J. Am. Coll. Surg. 2006, 203, 878–886. [Google Scholar] [CrossRef]
- De Bree, E.; Koops, W.; Kroger, R.; van Ruth, S.; Verwaal, V.J.; Zoetmulder, F.A. Preoperative computed tomography and selection of patients with colorectal peritoneal carcinomatosis for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2006, 32, 65–71. [Google Scholar] [CrossRef]
- Elias, D.; Antoun, S.; Goharin, A.; Otmany, A.E.; Puizillout, J.M.; Lasser, P. Research on the best chemohyperthermia technique of treatment of peritoneal carcinomatosis after complete resection. Int. J. Surg. Investig. 2000, 1, 431–439. [Google Scholar]
- Elias, D.; Blot, F.; El Otmany, A.; Antoun, S.; Lasser, P.; Boige, V.; Rougier, P.; Ducreux, M. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer 2001, 92, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Franko, J.; Gusani, N.J.; Holtzman, M.P.; Ahrendt, S.A.; Jones, H.L.; Zeh, H.J., 3rd; Bartlett, D.L. Multivisceral resection does not affect morbidity and survival after cytoreductive surgery and chemoperfusion for carcinomatosis from colorectal cancer. Ann. Surg. Oncol. 2008, 15, 3065–3072. [Google Scholar] [CrossRef]
- Franko, J.; Ibrahim, Z.; Gusani, N.J.; Holtzman, M.P.; Bartlett, D.L.; Zeh, H.J., 3rd. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer 2010, 116, 3756–3762. [Google Scholar] [CrossRef] [Green Version]
- Frøysnes, I.S.; Larsen, S.G.; Spasojevic, M.; Dueland, S.; Flatmark, K. Complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastasis in Norway: Prognostic factors and oncologic outcome in a national patient cohort. J. Surg. Oncol. 2016, 114, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Gilly, F.N.; Carry, P.Y.; Sayag, A.C.; Brachet, A.; Panteix, G.; Salle, B.; Bienvenu, J.; Burgard, G.; Guibert, B.; Banssillon, V. Regional chemotherapy (with mitomycin C) and intra-operative hyperthermia for digestive cancers with peritoneal carcinomatosis. Hepato-Gastroenterol. 1994, 41, 124–129. [Google Scholar]
- Glehen, O.; Cotte, E.; Schreiber, V.; Sayag-Beaujard, A.C.; Vignal, J.; Gilly, F.N. Intraperitoneal chemohyperthermia and attempted cytoreductive surgery in patients with peritoneal carcinomatosis of colorectal origin. Br. J. Surg. 2004, 91, 747–754. [Google Scholar] [CrossRef]
- Glehen, O.; Mithieux, F.; Osinsky, D.; Beaujard, A.C.; Freyer, G.; Guertsch, P.; Francois, Y.; Peyrat, P.; Panteix, G.; Vignal, J.; et al. Surgery combined with peritonectomy procedures and intraperitoneal chemohyperthermia in abdominal cancers with peritoneal carcinomatosis: A phase II study. J. Clin. Oncol. 2003, 21, 799–806. [Google Scholar] [CrossRef]
- Gomes da Silva, R.; Cabanas, J.; Sugarbaker, P.H. Limited survival in the treatment of carcinomatosis from rectal cancer. Dis. Colon Rectum 2005, 48, 2258–2263. [Google Scholar] [CrossRef]
- Gusani, N.J.; Cho, S.W.; Colovos, C.; Seo, S.; Franko, J.; Richard, S.D.; Edwards, R.P.; Brown, C.K.; Holtzman, M.P.; Zeh, H.J.; et al. Aggressive surgical management of peritoneal carcinomatosis with low mortality in a high-volume tertiary cancer center. Ann. Surg. Oncol. 2008, 15, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Hadi, R.; Saunders, V.; Utkina, O.; Clingan, P.; Kam, P.; Links, M.; Morris, D.L. Review of patients with peritoneal malignancy treated with peritonectomy and heated intraperitoneal chemotherapy. ANZ J. Surg. 2006, 76, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Hagendoorn, J.; van Lammeren, G.; Boerma, D.; van der Beek, E.; Wiezer, M.J.; van Ramshorst, B. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal and gastrointestinal origin shows acceptable morbidity and high survival. Eur. J. Surg. Oncol. 2009, 35, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Hompes, D.; D’Hoore, A.; Wolthuis, A.; Fieuws, S.; Mirck, B.; Bruin, S.; Verwaal, V. The use of Oxaliplatin or Mitomycin C in HIPEC treatment for peritoneal carcinomatosis from colorectal cancer: A comparative study. J. Surg. Oncol. 2014, 109, 527–532. [Google Scholar] [CrossRef]
- Huang, Y.; Alzahrani, N.A.; Chua, T.C.; Liauw, W.; Morris, D.L. Impacts of low peritoneal cancer index on the survival outcomes of patient with peritoneal carcinomatosis of colorectal origin. Int. J. Surg. 2015, 23, 181–185. [Google Scholar] [CrossRef]
- Iversen, L.H.; Rasmussen, P.C.; Hagemann-Madsen, R.; Laurberg, S. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis: The Danish experience. Colorectal Dis. 2013, 15, e365–e372. [Google Scholar] [CrossRef]
- Jacquet, P.; Averbach, A.; Stephens, A.D.; Stuart, O.A.; Chang, D.; Sugarbaker, P.H. Heated intraoperative intraperitoneal mitomycin C and early postoperative intraperitoneal 5-fluorouracil: Pharmacokinetic studies. Oncology 1998, 55, 130–138. [Google Scholar] [CrossRef]
- Jacquet, P.; Stephens, A.D.; Averbach, A.M.; Chang, D.; Ettinghausen, S.E.; Dalton, R.R.; Steves, M.A.; Sugarbaker, P.H. Analysis of morbidity and mortality in 60 patients with peritoneal carcinomatosis treated by cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy. Cancer 1996, 77, 2622–2629. [Google Scholar] [CrossRef] [Green Version]
- Kecmanovic, D.M.; Pavlov, M.J.; Ceranic, M.S.; Sepetkovski, A.V.; Kovacevic, P.A.; Stamenkovic, A.B. Treatment of peritoneal carcinomatosis from colorectal cancer by cytoreductive surgery and hyperthermic perioperative intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2005, 31, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Klaver, Y.L.; Chua, T.C.; de Hingh, I.H.; Morris, D.L. Outcomes of elderly patients undergoing cytoreductive surgery and perioperative intraperitoneal chemotherapy for colorectal cancer peritoneal carcinomatosis. J. Surg. Oncol. 2012, 105, 113–118. [Google Scholar] [CrossRef]
- Klaver, Y.L.; Lemmens, V.E.; Nienhuijs, S.W.; Nieuwenhuijzen, G.A.; Rutten, H.J.; de Hingh, I.H. Intraoperative radiotherapy and cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Five consecutive case reports of locally advanced rectal cancer with synchronous peritoneal carcinomatosis. Strahlentherapie und Onkologie 2013, 189, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Königsrainer, I.; Zieker, D.; Glatzle, J.; Lauk, O.; Klimek, J.; Symons, S.; Brücher, B.; Beckert, S.; Königsrainer, A. Experience after 100 patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J. Gastroenterol. 2012, 18, 2061–2066. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, A.M.; Mehta, A.M.; Boot, H.; van Leerdam, M.E.; Hauptmann, M.; Aalbers, A.G.; Verwaal, V.J. Perioperative systemic chemotherapy in peritoneal carcinomatosis of lymph node positive colorectal cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann. Oncol. 2014, 25, 864–869. [Google Scholar] [CrossRef] [Green Version]
- Kuijpers, A.M.; Mirck, B.; Aalbers, A.G.; Nienhuijs, S.W.; de Hingh, I.H.; Wiezer, M.J.; van Ramshorst, B.; van Ginkel, R.J.; Havenga, K.; Bremers, A.J.; et al. Cytoreduction and HIPEC in the Netherlands: Nationwide long-term outcome following the Dutch protocol. Ann. Surg. Oncol. 2013, 20, 4224–4230. [Google Scholar] [CrossRef]
- Lam, J.Y.; McConnell, Y.J.; Rivard, J.D.; Temple, W.J.; Mack, L.A. Hyperthermic intraperitoneal chemotherapy + early postoperative intraperitoneal chemotherapy versus hyperthermic intraperitoneal chemotherapy alone: Assessment of survival outcomes for colorectal and high-grade appendiceal peritoneal carcinomatosis. Am. J. Surg. 2015, 210, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Leung, V.; Huo, Y.R.; Liauw, W.; Morris, D.L. Oxaliplatin versus Mitomycin C for HIPEC in colorectal cancer peritoneal carcinomatosis. Eur. J. Surg. Oncol. 2017, 43, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Levine, E.A.; Stewart, J.H., IV; Shen, P.; Russell, G.B.; Loggie, B.L.; Votanopoulos, K.I. Intraperitoneal chemotherapy for peritoneal surface malignancy: Experience with 1,000 patients. J. Am. Coll. Surg. 2014, 218, 573–585. [Google Scholar] [CrossRef]
- Massalou, D.; Benizri, E.; Chevallier, A.; Duranton-Tanneur, V.; Pedeutour, F.; Benchimol, D.; Béréder, J.M. Peritoneal carcinomatosis of colorectal cancer: Novel clinical and molecular outcomes. Am. J. Surg. 2017, 213, 377–387. [Google Scholar] [CrossRef] [PubMed]
- McConnell, Y.J.; Mack, L.A.; Francis, W.P.; Ho, T.; Temple, W.J. HIPEC + EPIC versus HIPEC-alone: Differences in major complications following cytoreduction surgery for peritoneal malignancy. J. Surg. Oncol. 2013, 107, 591–596. [Google Scholar] [CrossRef] [PubMed]
- McQuellon, R.P.; Loggie, B.W.; Fleming, R.A.; Russell, G.B.; Lehman, A.B.; Rambo, T.D. Quality of life after intraperitoneal hyperthermic chemotherapy (IPHC) for peritoneal carcinomatosis. Eur. J. Surg. Oncol. 2001, 27, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Moran, B.; Cecil, T.; Chandrakumaran, K.; Arnold, S.; Mohamed, F.; Venkatasubramaniam, A. The results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1200 patients with peritoneal malignancy. Colorectal Dis. 2015, 17, 772–778. [Google Scholar] [CrossRef]
- Navez, J.; Remue, C.; Leonard, D.; Bachmann, R.; Kartheuser, A.; Hubert, C.; Coubeau, L.; Komuta, M.; Van den Eynde, M.; Zech, F.; et al. Surgical Treatment of Colorectal Cancer with Peritoneal and Liver Metastases Using Combined Liver and Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Report from a Single-Centre Experience. Ann. Surg. Oncol. 2016, 23, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Pelz, J.O.; Stojadinovic, A.; Nissan, A.; Hohenberger, W.; Esquivel, J. Evaluation of a peritoneal surface disease severity score in patients with colon cancer with peritoneal carcinomatosis. J. Surg. Oncol. 2009, 99, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Pestieau, S.R.; Sugarbaker, P.H. Treatment of primary colon cancer with peritoneal carcinomatosis: Comparison of concomitant vs. delayed management. Dis. Colon Rectum 2000, 43, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Prada-Villaverde, A.; Esquivel, J.; Lowy, A.M.; Markman, M.; Chua, T.; Pelz, J.; Baratti, D.; Baumgartner, J.M.; Berri, R.; Bretcha-Boix, P.; et al. The American Society of Peritoneal Surface Malignancies evaluation of HIPEC with Mitomycin C versus Oxaliplatin in 539 patients with colon cancer undergoing a complete cytoreductive surgery. J. Surg. Oncol. 2014, 110, 779–785. [Google Scholar] [CrossRef]
- Rouers, A.; Laurent, S.; Detroz, B.; Meurisse, M. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis: Higher complication rate for oxaliplatin compared to Mitomycin, C. Acta Chirurgica Belgica 2006, 106, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Yan, T.D.; Morris, D.L. A critical evaluation of risk factors for complications after cytoreductive surgery and perioperative intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis. World J. Surg. 2010, 34, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Schneebaum, S.; Arnold, M.W.; Staubus, A.; Young, D.C.; Dumond, D.; Martin, E.W., Jr. Intraperitoneal hyperthermic perfusion with mitomycin C for colorectal cancer with peritoneal metastases. Ann. Surg. Oncol. 1996, 3, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Hawksworth, J.; Lovato, J.; Loggie, B.W.; Geisinger, K.R.; Fleming, R.A.; Levine, E.A. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Ann. Surg. Oncol. 2004, 11, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Levine, E.A.; Hall, J.; Case, D.; Russell, G.; Fleming, R.; McQuellon, R.; Geisinger, K.R.; Loggie, B.W. Factors predicting survival after intraperitoneal hyperthermic chemotherapy with mitomycin C after cytoreductive surgery for patients with peritoneal carcinomatosis. Arch. Surg. 2003, 138, 26–33. [Google Scholar] [CrossRef]
- Simkens, G.A.; van Oudheusden, T.R.; Braam, H.J.; Wiezer, M.J.; Nienhuijs, S.W.; Rutten, H.J.; van Ramshorst, B.; de Hingh, I.H. Cytoreductive surgery and HIPEC offers similar outcomes in patients with rectal peritoneal metastases compared to colon cancer patients: A matched case control study. J. Surg. Oncol. 2016, 113, 548–553. [Google Scholar] [CrossRef]
- Simkens, G.A.; van Oudheusden, T.R.; Nieboer, D.; Steyerberg, E.W.; Rutten, H.J.; Luyer, M.D.; Nienhuijs, S.W.; de Hingh, I.H. Development of a Prognostic Nomogram for Patients with Peritoneally Metastasized Colorectal Cancer Treated with Cytoreductive Surgery and HIPEC. Ann. Surg. Oncol. 2016, 23, 4214–4221. [Google Scholar] [CrossRef] [PubMed]
- Spiliotis, J.; Tentes, A.A.; Vaxevanidou, A.; Korakianitis, O.S.; Rogdakis, A.; Mirelis, C.G.; Datsis, A.C.; Kekelos, S. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal carcinomatosis. Preliminary results and cost from two centers in Greece. J. BUON 2008, 13, 205–210. [Google Scholar] [PubMed]
- Tabrizian, P.; Jibara, G.; Shrager, B.; Franssen, B.; Yang, M.J.; Sarpel, U.; Hiotis, S.; Labow, D. Outcomes for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the elderly. Surg. Oncol. 2013, 22, 184–189. [Google Scholar] [CrossRef]
- Tabrizian, P.; Shrager, B.; Jibara, G.; Yang, M.J.; Romanoff, A.; Hiotis, S.; Sarpel, U.; Labow, D.M. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis: Outcomes from a single tertiary institution. J. Gastrointest. Surg. 2014, 18, 1024–1031. [Google Scholar] [CrossRef]
- Tan, G.; Chia, C.; Kumar, M.; Choo, S.P.; Chia, J.; Tham, C.K.; Chua, C.; Soo, K.C.; Teo, M. 201 consecutive cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) procedures in a single Asian tertiary centre. Int. J. Hyperth. 2016, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Teo, M.C.; Ching Tan, G.H.; Lim, C.; Chia, C.S.; Tham, C.K.; Soo, K.C. Colorectal peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: The experience of a tertiary Asian center. Asian J. Surg. 2015, 38, 65–73. [Google Scholar] [CrossRef]
- Teo, M.C.; Tan, G.H.; Tham, C.K.; Lim, C.; Soo, K.C. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in Asian patients: 100 consecutive patients in a single institution. Ann. Surg. Oncol. 2013, 20, 2968–2974. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, T.M.; Zhang, Y.; Greeno, E.; Knutsen, A. Toxicity and quality of life after cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2006, 13, 1627–1632. [Google Scholar] [CrossRef]
- Ung, L.; Chua, T.C.; Morris, D.L. Peritoneal metastases of lower gastrointestinal tract origin: A comparative study of patient outcomes following cytoreduction and intraperitoneal chemotherapy. J. Cancer Res. Clin. Oncol. 2013, 139, 1899–1908. [Google Scholar] [CrossRef]
- Vaira, M.; Cioppa, T.; D’Amico, S.; de Marco, G.; D’Alessandro, M.; Fiorentini, G.; De Simone, M. Treatment of peritoneal carcinomatosis from colonic cancer by cytoreduction, peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC). Experience of ten years. In Vivo 2010, 24, 79–84. [Google Scholar] [PubMed]
- Van Oudheusden, T.R.; Braam, H.J.; Nienhuijs, S.W.; Wiezer, M.J.; van Ramshorst, B.; Luyer, M.D.; Lemmens, V.E.; de Hingh, I.H. Cytoreduction and hyperthermic intraperitoneal chemotherapy: A feasible and effective option for colorectal cancer patients after emergency surgery in the presence of peritoneal carcinomatosis. Ann. Surg. Oncol. 2014, 21, 2621–2626. [Google Scholar] [CrossRef] [PubMed]
- Van Oudheusden, T.R.; Braam, H.J.; Nienhuijs, S.W.; Wiezer, M.J.; van Ramshorst, B.; Luyer, P.; de Hingh, I.H. Poor outcome after cytoreductive surgery and HIPEC for colorectal peritoneal carcinomatosis with signet ring cell histology. J. Surg. Oncol. 2015, 111, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Van Ruth, S.; Mathot, R.A.; Sparidans, R.W.; Beijnen, J.H.; Verwaal, V.J.; Zoetmulder, F.A. Population pharmacokinetics and pharmacodynamics of mitomycin during intraoperative hyperthermic intraperitoneal chemotherapy. Clin. Pharmacokinet. 2004, 43, 131–143. [Google Scholar] [CrossRef]
- Van Sweringen, H.L.; Hanseman, D.J.; Ahmad, S.A.; Edwards, M.J.; Sussman, J.J. Predictors of survival in patients with high-grade peritoneal metastases undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Surgery 2012, 152, 617–625. [Google Scholar] [CrossRef]
- van Vugt, J.L.; Braam, H.J.; van Oudheusden, T.R.; Vestering, A.; Bollen, T.L.; Wiezer, M.J.; de Hingh, I.H.; van Ramshorst, B.; Boerma, D. Skeletal Muscle Depletion is Associated with Severe Postoperative Complications in Patients Undergoing Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis of Colorectal Cancer. Ann. Surg. Oncol. 2015, 22, 3625–3631. [Google Scholar] [CrossRef] [PubMed]
- Varban, O.; Levine, E.A.; Stewart, J.H.; McCoy, T.P.; Shen, P. Outcomes associated with cytoreductive surgery and intraperitoneal hyperthermic chemotherapy in colorectal cancer patients with peritoneal surface disease and hepatic metastases. Cancer 2009, 115, 3427–3436. [Google Scholar] [PubMed] [Green Version]
- Verwaal, V.J.; Bruin, S.; Boot, H.; van Slooten, G.; van Tinteren, H. 8-year follow-up of randomized trial: Cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann. Surg. Oncol. 2008, 15, 2426–2432. [Google Scholar] [CrossRef] [PubMed]
- Verwaal, V.J.; van Tinteren, H.; van Ruth, S.; Zoetmulder, F.A. Predicting the survival of patients with peritoneal carcinomatosis of colorectal origin treated by aggressive cytoreduction and hyperthermic intraperitoneal chemotherapy. Br. J. Surg. 2004, 91, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.; Zenati, M.; Ramalingam, L.; Jones, H.; Zureikat, A.; Holtzman, M.; Lee, K.; Ahrendt, S.; Pingpank, J.; Zeh, H.J.; et al. Impact of aggressive histology and location of primary tumor on the efficacy of surgical therapy for peritoneal carcinomatosis of colorectal origin. Ann. Surg. Oncol. 2014, 21, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.D.; Chu, F.; Links, M.; Kam, P.C.; Glenn, D.; Morris, D.L. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma: Non-mucinous tumour associated with an improved survival. Eur. J. Surg. Oncol. 2006, 32, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.D.; Morris, D.L. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for isolated colorectal peritoneal carcinomatosis: Experimental therapy or standard of care? Ann. Surg. 2008, 248, 829–835. [Google Scholar] [PubMed]
- Zanon, C.; Bortolini, M.; Chiappino, I.; Simone, P.; Bruno, F.; Gaglia, P.; Airoldi, M.; Deriu, L.; Mashiah, A. Cytoreductive surgery combined with intraperitoneal chemohyperthermia for the treatment of advanced colon cancer. World J. Surg. 2006, 30, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Asero, S.; Caruso, M.; Vallone, N.; Luciani, A.G.; Lombardo, V.; Terranova, G.; Ettore, G.; Giannone, G. Cytoreductive surgery (cs) and hyperthermic intraperitoneal chemotherapy (hipec) in treatment of peritoneal surface malignances: Report of a phase II clinical study. In Vivo 2009, 23, 645–647. [Google Scholar] [PubMed]
- Baratti, D.; Kusamura, S.; Iusco, D.; Bonomi, S.; Grassi, A.; Virzì, S.; Leo, E.; Deraco, M. Postoperative complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy affect long-term outcome of patients with peritoneal metastases from colorectal cancer: A two-center study of 101 patients. Dis. Colon Rectum 2014, 57, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Baratti, D.; Kusamura, S.; Iusco, D.; Gimondi, S.; Pietrantonio, F.; Milione, M.; Guaglio, M.; Bonomi, S.; Grassi, A.; Virzì, S. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) at the Time of Primary Curative Surgery in Patients with Colorectal Cancer at High Risk for Metachronous Peritoneal Metastases. Ann. Surg. Oncol. 2017, 24, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Bouhadjari, N.; Gabato, W.; Calabrese, D.; Msika, S.; Keita, H. Hyperthermic intraperitoneal chemotherapy with cisplatin: Amifostine prevents acute severe renal impairment. Eur. J. Surg. Oncol. 2016, 42, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, F.; De Simone, M.; Virzì, S.; Deraco, M.; Rossi, C.R.; Garofalo, A.; Di Filippo, F.; Giannarelli, D.; Vaira, M.; Valle, M.; et al. Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: Italian multicenter study S.I.T.I.L.O. Eur. J. Surg. Oncol. 2011, 37, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, F.; Di Filippo, F.; Botti, C.; Cosimelli, M.; Giannarelli, D.; Aloe, L.; Arcuri, E.; Aromatario, C.; Consolo, S.; Callopoli, A.; et al. Peritonectomy and hyperthermic antiblastic perfusion in the treatment of peritoneal carcinomatosis. Eur. J. Surg. Oncol. 2000, 26, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, F.; Perri, P.; Di Filippo, F.; Giannarelli, D.; Botti, C.; Cosimelli, M.; Tedesco, M.; Principi, F.; Laurenzi, L.; Cavaliere, R. Treatment of peritoneal carcinomatosis with intent to cure. J. Surg. Oncol. 2000, 74, 41–44. [Google Scholar] [CrossRef]
- Cavaliere, F.; Valle, M.; De Simone, M.; Deraco, M.; Rossi, C.R.; Di Filippo, F.; Verzi, S.; Giannarelli, D.; Perri, P.; Pilati, P.L.; et al. 120 peritoneal carcinomatoses from colorectal cancer treated with peritonectomy and intra-abdominal chemohyperthermia: A S.I.T.I.L.O. multicentric study. In Vivo 2006, 20, 747–750. [Google Scholar]
- Elias, D.; Gilly, F.; Boutitie, F.; Quénet, F.; Bereder, J.M.; Mansvelt, B.; Lorimier, G.; Dubè, P.; Glehen, O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: Retrospective analysis of 523 patients from a multicentric French study. J. Clin. Oncol. 2010, 28, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Q.; Yang, X.J.; Yu, Y.; Wu, H.T.; Liu, Y.; Yonemura, Y.; Li, Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for patients with peritoneal carcinomatosis from colorectal cancer: A phase II study from a Chinese center. PLoS ONE 2014, 9, e108509. [Google Scholar] [CrossRef]
- Kianmanesh, R.; Scaringi, S.; Sabate, J.M.; Castel, B.; Pons-Kerjean, N.; Coffin, B.; Hay, J.M.; Flamant, Y.; Msika, S. Iterative cytoreductive surgery associated with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis of colorectal origin with or without liver metastases. Ann. Surg. 2007, 245, 597–603. [Google Scholar] [CrossRef]
- Lin, E.K.; Hsieh, M.C.; Chen, C.H.; Lu, Y.J.; Wu, S.Y. Outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer with peritoneal metastasis. Medicine 2016, 95, e5522. [Google Scholar] [CrossRef] [PubMed]
- Lorimier, G.; Linot, B.; Paillocher, N.; Dupoiron, D.; Verri èle, V.; Wernert, R.; Hamy, A.; Capitain, O. Curative cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis and synchronous resectable liver metastases arising from colorectal cancer. Eur. J. Surg. Oncol. 2017, 43, 150–158. [Google Scholar] [CrossRef]
- Pilati, P.; Mocellin, S.; Rossi, C.R.; Foletto, M.; Campana, L.; Nitti, D.; Lise, M. Cytoreductive surgery combined with hyperthermic intraperitoneal intraoperative chemotherapy for peritoneal carcinomatosis arising from colon adenocarcinoma. Ann. Surg. Oncol. 2003, 10, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Robella, M.; Vaira, M.; Marsanic, P.; Mellano, A.; Cinquegrana, A.; Sottile, A.; De Simone, M. Treatment of peritoneal carcinomatosis from colonic cancer by cytoreduction, peritonectomy and HIPEC: Preliminary results in highly selected patients. Minerva Chir. 2013, 68, 551–558. [Google Scholar]
- Yamaguchi, A.; Tsukioka, Y.; Fushida, S.; Kurosaka, Y.; Kanno, M.; Yonemura, Y.; Miwa, K.; Miyazaki, I. Intraperitoneal hyperthermic treatment for peritoneal dissemination of colorectal cancers. Dis. Colon Rectum 1992, 35, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, Y.; Canbay, E.; Ishibashi, H. Prognostic factors of peritoneal metastases from colorectal cancer following cytoreductive surgery and perioperative chemotherapy. Sci. World J. 2013, 2013, 978394. [Google Scholar] [CrossRef]
- Majerović, M.; Milinović, D.; Oresković, S.; Matosević, P.; Mirić, M.; Kekez, T.; Kinda, E.; Augustin, G.; Silovski, H. Hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery (CS) as treatment of peritoneal carcinomatosis: Preliminary results in Croatia. Coll. Antropol. 2011, 35, 1349–1352. [Google Scholar] [PubMed]
- Melero, J.T.; Ortega, F.G.; Gonzalez, A.M.; Carmona-Saez, P.; Garcia Puche, J.L.; Sugarbaker, P.H.; Delgado, M.; Lorente, J.A.; Serrano, M.J. Prognostic factor analysis of circulating tumor cells in peripheral blood of patients with peritoneal carcinomatosis of colon cancer origin treated with cytoreductive surgery plus an intraoperative hyperthermic intraperitoneal chemotherapy procedure (CRS + HIPEC). Surgery 2016, 159, 728–735. [Google Scholar]
- Piso, P.; Dahlke, M.H.; Ghali, N.; Iesalnieks, I.; Loss, M.; Popp, F.; von Breitenbuch, P.; Agha, A.; Lang, S.A.; Kullmann, F.; et al. Multimodality treatment of peritoneal carcinomatosis from colorectal cancer: First results of a new German centre for peritoneal surface malignancies. Int. J. Colorectal Dis. 2007, 22, 1295–1300. [Google Scholar] [CrossRef]
- Sugarbaker, P.H.; Van der Speeten, K.; Anthony Stuart, O.; Chang, D. Impact of surgical and clinical factors on the pharmacology of intraperitoneal doxorubicin in 145 patients with peritoneal carcinomatosis. Eur. J. Surg. Oncol. 2011, 37, 719–726. [Google Scholar] [CrossRef]
- Shimizu, T.; Murata, S.; Sonoda, H.; Mekata, E.; Ohta, H.; Takebayashi, K.; Miyake, T.; Tani, T. Hyperthermic intraperitoneal chemotherapy with mitomycin C and 5-fluorouracil in patients at high risk of peritoneal metastasis from colorectal cancer: A preliminary clinical study. Mol. Clin. Oncol. 2014, 2, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.J.; Li, Y.; al-shammaa Hassan, A.H.; Yang, G.L.; Liu, S.Y.; Lu, Y.L.; Zhang, J.W.; Yonemura, Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival in selected patients with peritoneal carcinomatosis from abdominal and pelvic malignancies: Results of 21 cases. Ann. Surg. Oncol. 2009, 16, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, N.; Ferguson, J.S.; Valle, S.J.; Liauw, W.; Chua, T.; Morris, D.L. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: Long-term results at St George Hospital, Australia. ANZ J. Surg. 2016, 86, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Ceelen, W.P.; Peeters, M.; Houtmeyers, P.; Breusegem, C.; De Somer, F.; Pattyn, P. Safety and efficacy of hyperthermic intraperitoneal chemoperfusion with high-dose oxaliplatin in patients with peritoneal carcinomatosis. Ann. Surg. Oncol. 2008, 15, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Benizri, E.; Di Pietrantonio, D.; Menegon, P.; Malka, D.; Raynard, B. Comparison of two kinds of intraperitoneal chemotherapy following complete cytoreductive surgery of colorectal peritoneal carcinomatosis. Ann. Surg. Oncol. 2007, 14, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Benizri, E.; Pocard, M.; Ducreux, M.; Boige, V.; Lasser, P. Treatment of synchronous peritoneal carcinomatosis and liver metastases from colorectal cancer. Eur. J. Surg. Oncol. 2006, 32, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Bonnay, M.; Puizillou, J.M.; Antoun, S.; Demirdjian, S.; El, O.A.; Pignon, J.P.; Drouard-Troalen, L.; Ouellet, J.F.; Ducreux, M. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: Pharmacokinetics and tissue distribution. Ann. Oncol. 2002, 13, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; El Otmany, A.; Bonnay, M.; Paci, A.; Ducreux, M.; Antoun, S.; Lasser, P.; Laurent, S.; Bourget, P. Human pharmacokinetic study of heated intraperitoneal oxaliplatin in increasingly hypotonic solutions after complete resection of peritoneal carcinomatosis. Oncology 2002, 63, 346–352. [Google Scholar] [CrossRef]
- Elias, D.; Lefevre, J.H.; Chevalier, J.; Brouquet, A.; Marchal, F.; Classe, J.M.; Ferron, G.; Guilloit, J.M.; Meeus, P.; Goéré, D.; et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J. Clin. Oncol. 2009, 27, 681–685. [Google Scholar] [CrossRef]
- Elias, D.; Mariani, A.; Cloutier, A.S.; Blot, F.; Goéré, D.; Dumont, F.; Honoré, C.; Billard, V.; Dartigues, P.; Ducreux, M. Modified selection criteria for complete cytoreductive surgery plus HIPEC based on peritoneal cancer index and small bowel involvement for peritoneal carcinomatosis of colorectal origin. Eur. J. Surg. Oncol. 2014, 40, 1467–1473. [Google Scholar] [CrossRef]
- Elias, D.; Pocard, M.; Sideris, L.; Edè, C.; Ducreux, M.; Boige, V.; Lasser, P. Preliminary results of intraperitoneal chemohyperthermia with oxaliplatin in peritoneal carcinomatosis of colorectal origin. Br. J. Surg. 2004, 91, 455–456. [Google Scholar] [CrossRef]
- Elias, D.; Raynard, B.; Farkhondeh, F.; Goéré, D.; Rouquie, D.; Ciuchendea, R.; Pocard, M.; Ducreux, M. Peritoneal carcinomatosis of colorectal origin: Long-term results of intraperitoneal chemohyperthermia with oxaliplatin following complete cytoreductive surgery. Gastroentérologie Clinique et Biologique 2006, 30, 1200–1204. [Google Scholar] [CrossRef]
- Elias, D.; Sideris, L.; Pocard, M.; Edè, C.; Ben Hassouna, D.; Ducreux, M.; Boige, V.; Côté, J.F.; Lasser, P. Efficacy of intraperitoneal chemohyperthermia with oxaliplatin in colorectal peritoneal carcinomatosis. Preliminary results in 24 patients. Ann. Oncol. 2004, 15, 781–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gervais, M.K.; Dube, P.; McConnell, Y.; Drolet, P.; Mitchell, A.; Sideris, L. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with oxaliplatin for peritoneal carcinomatosis arising from colorectal cancer. J. Surg. Oncol. 2013, 108, 438–443. [Google Scholar] [CrossRef]
- Glockzin, G.; Gerken, M.; Lang, S.A.; Klinkhammer-Schalke, M.; Piso, P.; Schlitt, H.J. Oxaliplatin-based versus irinotecan-based hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal metastasis from appendiceal and colorectal cancer: A retrospective analysis. BMC Cancer 2014, 14, 807. [Google Scholar] [CrossRef] [PubMed]
- Goéré, D.; Malka, D.; Tzanis, D.; Gava, V.; Boige, V.; Eveno, C.; Maggiori, L.; Dumont, F.; Ducreux, M.; Elias, D. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann. Surg. 2013, 257, 1065–1071. [Google Scholar] [CrossRef]
- Hompes, D.; D’Hoore, A.; Van Cutsem, E.; Fieuws, S.; Ceelen, W.; Peeters, M.; Van der Speeten, K.; Bertrand, C.; Legendre, H.; Kerger, J. The treatment of peritoneal carcinomatosis of colorectal cancer with complete cytoreductive surgery and hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) with oxaliplatin: A Belgian multicentre prospective phase II clinical study. Ann. Surg. Oncol. 2012, 19, 2186–2194. [Google Scholar] [CrossRef]
- Mehta, A.M.; Huitema, A.D.; Burger, J.W.; Brandt-Kerkhof, A.R.; van den Heuvel, S.F.; Verwaal, V.J. Standard Clinical Protocol for Bidirectional Hyperthermic Intraperitoneal Chemotherapy (HIPEC): Systemic Leucovorin, 5-Fluorouracil, and Heated Intraperitoneal Oxaliplatin in a Chloride-Containing Carrier Solution. Ann. Surg. Oncol. 2017, 24, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, S.; Dzodic, R.; Zegarac, M.; Djurisic, I.; Gavrilovic, D.; Vojinovic, V.; Kocic, M.; Santrac, N.; Radlovic, P.; Radosavljevic, D.; et al. Survival prognostic factors in patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery and intraoperative hyperthermic intraperitoneal chemotherapy: A single institution experience. J. BUON 2014, 19, 66–74. [Google Scholar] [CrossRef]
- Quénet, F.; Goéré, D.; Mehta, S.S.; Roca, L.; Dumont, F.; Hessissen, M.; Saint-Aubert, B.; Elias, D. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann. Surg. 2011, 254, 294–301. [Google Scholar] [CrossRef]
- Somashekhar, S.P.; Prasanna, G.; Jaka, R.; Rauthan, A.; Murthy, H.S.; Karanth, S. Hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancies: A single institution Indian experience. Natl. Med. J. India 2016, 29, 262–266. [Google Scholar]
- Stewart, J.H., IV; Shen, P.; Russell, G.; Fenstermaker, J.; McWilliams, L.; Coldrun, F.M.; Levine, K.E.; Jones, B.T.; Levine, E.A. A phase I trial of oxaliplatin for intraperitoneal hyperthermic chemoperfusion for the treatment of peritoneal surface dissemination from colorectal and appendiceal cancers. Ann. Surg. Oncol. 2008, 15, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Topgül, K.; Çetinkaya, M.B.; Ciğdem Arslan, N.; Gül, M.K.; Çan, M.; Gürsel, M.F.; Erdem, D.; Malazgirt, Z. Cytoreductive surgery (SRC) and hyperthermic intraperitoneal chemotherapy (HIPEC) for treatment of peritoneal carcinomatosis: Our initial experience and technical details. Turk. J. Surg. 2015, 31, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Turrini, O.; Lambaudie, E.; Faucher, M.; Viret, F.; Blache, J.L.; Houvenaeghel, G.; Delpero, J.R. Initial experience with hyperthermic intraperitoneal chemotherapy. Arch. Surg. 2012, 147, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Chemama, S.; Bayar, M.A.; Lanoy, E.; Ammari, S.; Stoclin, A.; Goéré, D.; Elias, D.; Raynard, B.; Antoun, S. Sarcopenia is Associated with Chemotherapy Toxicity in Patients Undergoing Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis from Colorectal Cancer. Ann. Surg. Oncol. 2016, 23, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Matsuhisa, T.; Sideris, L.; Liberale, G.; Drouard-Troalen, L.; Raynard, B.; Pocard, M.; Puizillou, J.M.; Billard, V.; Bourget, P.; et al. Heated intra-operative intraperitoneal oxaliplatin plus irinotecan after complete resection of peritoneal carcinomatosis: Pharmacokinetics, tissue distribution and tolerance. Ann. Oncol. 2004, 15, 1558–1565. [Google Scholar] [CrossRef]
- Sardi, A.; Jimenez, W.; Nieroda, C.; Sittig, M.; Shankar, S.; Gushchin, V. Melphalan: A promising agent in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2014, 21, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Füzün, M.; Sökmen, S.; Terzi, C.; Canda, A.E. Cytoreductive approach to peritoneal carcinomatosis originated from colorectal cancer: Turkish experience. Acta Chir. Iugosl. 2006, 53, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Cravioto-Villanueva, A.; Cavazos, M.; Luna-Perez, P.; Martinez-Gomez, H.; Ramirez, M.L.; Solorzano, J.; Montiel, H.; Esquivel, J. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) delivered via a modified perfusion system for peritoneal carcinomatosis of colorectal origin. Surg. Today 2016, 46, 979–984. [Google Scholar] [CrossRef]
- Murata, S.; Yamamoto, H.; Naitoh, H.; Yamaguchi, T.; Kaida, S.; Shimizu, T.; Shiomi, H.; Naka, S.; Tani, T.; Tani, M. Feasibility and safety of hyperthermic intraperitoneal chemotherapy using 5-fluorouracil combined with cisplatin and mitomycin C in patients undergoing gastrectomy for advanced gastric cancer. J. Surg. Oncol. 2017, 116, 1159–1165. [Google Scholar] [CrossRef]
- Kusamura, S.; Dominique, E.; Baratti, D.; Younan, R.; Deraco, M. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J. Surg. Oncol. 2008, 98, 247–252. [Google Scholar] [CrossRef]
- Glehen, O.; Cotte, E.; Kusamura, S.; Deraco, M.; Baratti, D.; Passot, G.; Beaujard, A.C.; Noel, G.F. Hyperthermic intraperitoneal chemotherapy: Nomenclature and modalities of perfusion. J. Surg. Oncol. 2008, 98, 242–246. [Google Scholar] [CrossRef] [PubMed]
- De Simone, M.; Vaira, M.; Caponi, A.; Ciaccio, B.; Fiorentini, G.; Turrisi, G.; Ferri, L.; Buti, G. Ten years experience in the treatment of pseudomyxoma peritonei by cytoreduction, peritonectomy and semi-closed hyperthermic antiblastic peritoneal perfusion. In Vivo 2006, 20, 725–727. [Google Scholar] [PubMed]
- Benoit, L.; Cheynel, N.; Ortega-Deballon, P.; Giacomo, G.D.; Chauffert, B.; Rat, P. Closed hyperthermic intraperitoneal chemotherapy with open abdomen: A novel technique to reduce exposure of the surgical team to chemotherapy drugs. Ann. Surg. Oncol. 2008, 15, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Deballon, P.; Facy, O.; Jambet, S.; Magnin, G.; Cotte, E.; Beltramo, J.L.; Chauffert, B.; Rat, P. Which method to deliver hyperthermic intraperitoneal chemotherapy with oxaliplatin? An experimental comparison of open and closed techniques. Ann. Surg. Oncol. 2010, 17, 1957–1963. [Google Scholar] [CrossRef]
- Becouarn, Y.; Rougier, P. Clinical efficacy of oxaliplatin monotherapy: Phase II trials in advanced colorectal cancer. Semin. Oncol. 1998, 25, 23–31. [Google Scholar] [PubMed]
- Charrier, T.; Passot, G.; Peron, J.; Maurice, C.; Gocevska, S.; Quénet, F.; Eveno, C.; Pocard, M.; Goéré, D.; Elias, D.; et al. Cytoreductive Surgery Combined with Hyperthermic Intraperitoneal Chemotherapy with Oxaliplatin Increases the Risk of Postoperative Hemorrhagic Complications: Analysis of Predictive Factors. Ann. Surg. Oncol. 2016, 23, 2315–2322. [Google Scholar] [CrossRef]
- Loggie, B.W.; Thomas, P. Gastrointestinal Cancers with Peritoneal Carcinomatosis: Surgery and Hyperthermic Intraperitoneal Chemotherapy. Oncology 2015, 29, 515–521. [Google Scholar] [PubMed]
- Maciver, A.H.; Al-Sukhni, E.; Esquivel, J.; Skitzki, J.J.; Kane, J.M., 3rd; Francescutti, V.A. Current Delivery of Hyperthermic Intraperitoneal Chemotherapy with Cytoreductive Surgery (CS/HIPEC) and Perioperative Practices: An International Survey of High-Volume Surgeons. Ann. Surg. Oncol. 2017, 24, 923–930. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Sehouli, J.; Mahner, S.; Harter, P.; Van Nieuwenhuysen, E.; Gonzalez-Martin, A.; Vergote, I.; Chiva, L.; Du Bois, A. HIPEC: HOPE or HYPE in the fight against advanced ovarian cancer? Ann. Oncol. 2018, 29, 1610–1613. [Google Scholar] [CrossRef]
- Wu, M.H.; Yan, B.; Humerickhouse, R.; Dolan, M.E. Irinotecan activation by human carboxylesterases in colorectal adenocarcinoma cells. Clin. Cancer Res. 2002, 8, 2696–2700. [Google Scholar]
- Czejka, M.; Kiss, A.; Koessner, C.; Terkola, R.; Ettlinger, D.; Schueller, J. Metabolic activation of irinotecan during intra-arterial chemotherapy of metastatic colorectal cancer. Anticancer Res. 2011, 31, 3573–3578. [Google Scholar] [PubMed]
- Mehta, A.M.; Van den Hoven, J.M.; Rosing, H.; Hillebrand, M.J.; Nuijen, B.; Huitema, A.D.; Beijnen, J.H.; Verwaal, V.J. Stability of oxaliplatin in chloride-containing carrier solutions used in hyperthermic intraperitoneal chemotherapy. Int. J. Pharm. 2015, 479, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Löffler, M.W.; Schuster, H.; Zeck, A.; Quilitz, N.; Weinreich, J.; Tolios, A.; Haen, S.P.; Horvath, P.; Löb, S.; Rammensee, H.G.; et al. Pharmacodynamics of Oxaliplatin-Derived Platinum Compounds During Hyperthermic Intraperitoneal Chemotherapy (HIPEC): An Emerging Aspect Supporting the Rational Design of Treatment Protocols. Ann. Surg. Oncol. 2017, 24, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Tentes, A.A.; Spiliotis, I.D.; Korakianitis, O.S.; Vaxevanidou, A.; Kyziridis, D. Adjuvant perioperative intraperitoneal chemotherapy in locally advanced colorectal carcinoma: Preliminary results. ISRN Surg. 2011, 2011, 529876. [Google Scholar] [CrossRef] [PubMed]
- Waite, K.; Youssef, H. The Role of Neoadjuvant and Adjuvant Systemic Chemotherapy with Cytoreductive Surgery and Heated Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Systematic Review. Ann. Surg. Oncol. 2017, 24, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Mas-Fuster, M.I.; Ramon-Lopez, A.; Nalda-Molina, R. Importance of standardizing the dose in hyperthermic intraperitoneal chemotherapy (HIPEC): A pharmacodynamic point of view. Cancer Chemother. Pharmacol. 2013, 72, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, L.; van der Kuip, H.; Zopf, W.; Winter, S.; Münch, M.; Mürdter, T.E.; Thon, K.P.; Steurer, W.; Aulitzky, W.E.; Ulmer, C. A Temperature of 40 degrees C Appears to be a Critical Threshold for Potentiating Cytotoxic Chemotherapy In Vitro and in Peritoneal Carcinomatosis Patients Undergoing HIPEC. Ann. Surg. Oncol. 2015, 22 (Suppl. S3), S758–S765. [Google Scholar] [CrossRef]
- Kyang, L.S.; Alzahrani, N.A.; Zhao, J.; Morris, D.L. Gastric perforation following cytoreductive surgery and perioperative intraperitoneal chemotherapy: A case series of six. World J. Surg. Oncol. 2017, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Zappa, L.; Savady, R.; Sugarbaker, P.H. Gastric perforation following cytoreductive surgery with perioperative intraperitoneal chemotherapy. J. Surg. Oncol. 2010, 101, 634–636. [Google Scholar] [CrossRef]
- De Somer, F.; Ceelen, W.; Delanghe, J.; De Smet, D.; Vanackere, M.; Pattyn, P.; Mortier, E. Severe hyponatremia, hyperglycemia, and hyperlactatemia are associated with intraoperative hyperthermic intraperitoneal chemoperfusion with oxaliplatin. Perit. Dial. Int. 2008, 28, 61–66. [Google Scholar]
- Tan, G.H.C.; Shannon, N.B.; Chia, C.S.; Soo, K.C.; Teo, M.C.C. Platinum agents and mitomycin C-specific complications in cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Int. J. Hyperth. 2018, 34, 595–600. [Google Scholar] [CrossRef] [PubMed]



 (amount of diluent);
(amount of diluent);  (HIPEC duration);
(HIPEC duration);  (male) and
(male) and  (female).
(female).
 (amount of diluent);
(amount of diluent);  (HIPEC duration);
(HIPEC duration);  (male) and
(male) and  (female).
(female).

 (amount of diluent);
(amount of diluent);  (duration of HIPEC); * (bidirectional protocol with 5-FU/Leucovorin); and L-OHP (oxaliplatin).
(duration of HIPEC); * (bidirectional protocol with 5-FU/Leucovorin); and L-OHP (oxaliplatin).
 (amount of diluent);
(amount of diluent);  (duration of HIPEC); * (bidirectional protocol with 5-FU/Leucovorin); and L-OHP (oxaliplatin).
(duration of HIPEC); * (bidirectional protocol with 5-FU/Leucovorin); and L-OHP (oxaliplatin).
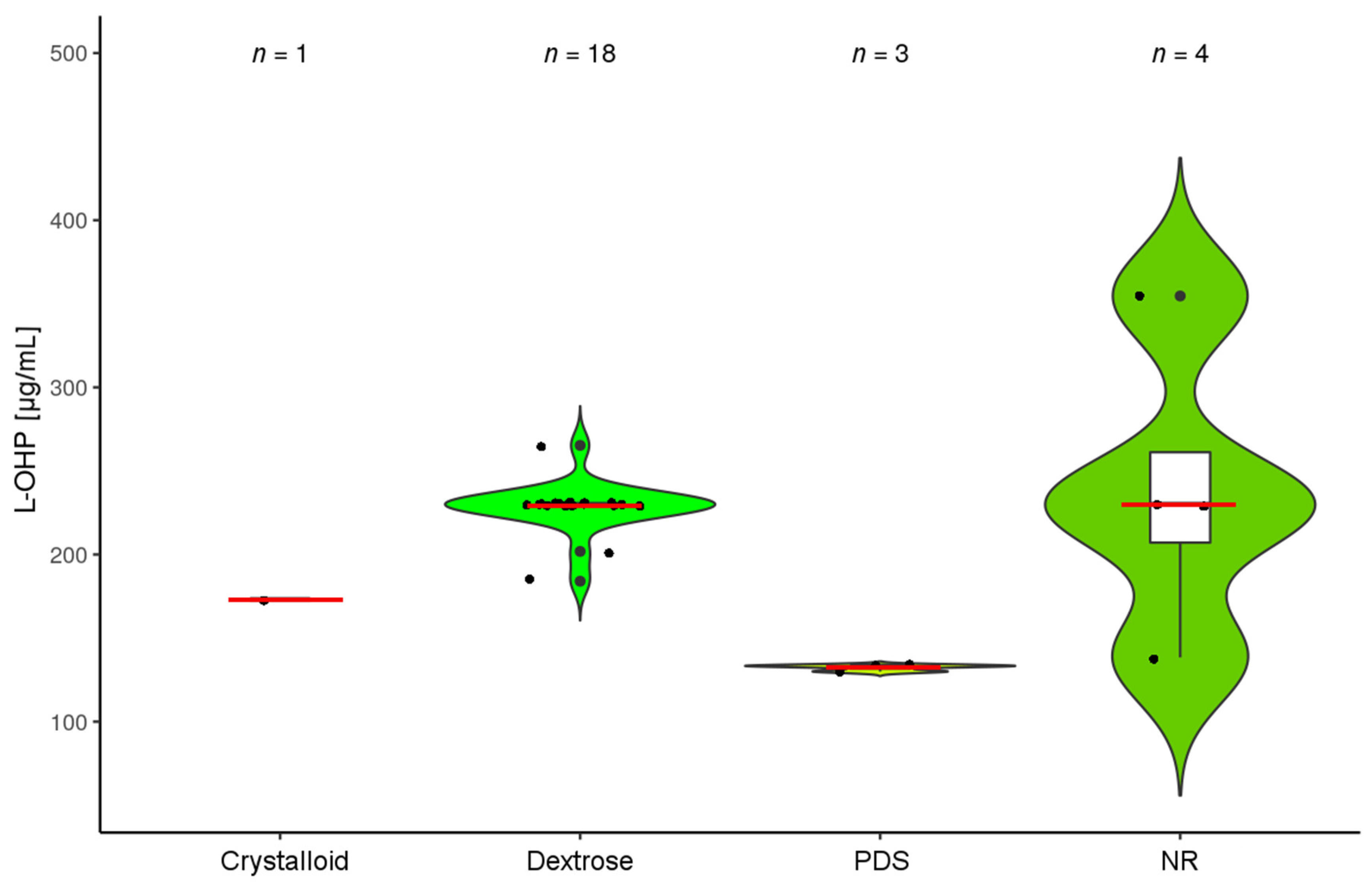
 (amount of diluent); and
(amount of diluent); and  (HIPEC duration).
(HIPEC duration).
 (amount of diluent); and
(amount of diluent); and  (HIPEC duration).
(HIPEC duration).





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurttas, C.; Hoffmann, G.; Tolios, A.; Haen, S.P.; Schwab, M.; Königsrainer, I.; Königsrainer, A.; Beckert, S.; Löffler, M.W. Systematic Review of Variations in Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Peritoneal Metastasis from Colorectal Cancer. J. Clin. Med. 2018, 7, 567. https://doi.org/10.3390/jcm7120567
Yurttas C, Hoffmann G, Tolios A, Haen SP, Schwab M, Königsrainer I, Königsrainer A, Beckert S, Löffler MW. Systematic Review of Variations in Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Peritoneal Metastasis from Colorectal Cancer. Journal of Clinical Medicine. 2018; 7(12):567. https://doi.org/10.3390/jcm7120567
Chicago/Turabian StyleYurttas, Can, Giulia Hoffmann, Alexander Tolios, Sebastian P. Haen, Matthias Schwab, Ingmar Königsrainer, Alfred Königsrainer, Stefan Beckert, and Markus W. Löffler. 2018. "Systematic Review of Variations in Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Peritoneal Metastasis from Colorectal Cancer" Journal of Clinical Medicine 7, no. 12: 567. https://doi.org/10.3390/jcm7120567
APA StyleYurttas, C., Hoffmann, G., Tolios, A., Haen, S. P., Schwab, M., Königsrainer, I., Königsrainer, A., Beckert, S., & Löffler, M. W. (2018). Systematic Review of Variations in Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Peritoneal Metastasis from Colorectal Cancer. Journal of Clinical Medicine, 7(12), 567. https://doi.org/10.3390/jcm7120567





