Growth Differentiation Factor-15 (GDF-15) Is a Biomarker of Muscle Wasting and Renal Dysfunction in Preoperative Cardiovascular Surgery Patients
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Enzyme Linked Immunosorbent Assay (ELISA)
2.3. Measurement of Gait Speed, Hand-Grip Strength, and Voluntary Isometric Contraction
2.4. Measurements with the Bioelectrical Impedance Analyzer (BIA)
2.5. Measurement of Muscle Thickness by Ultrasound
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
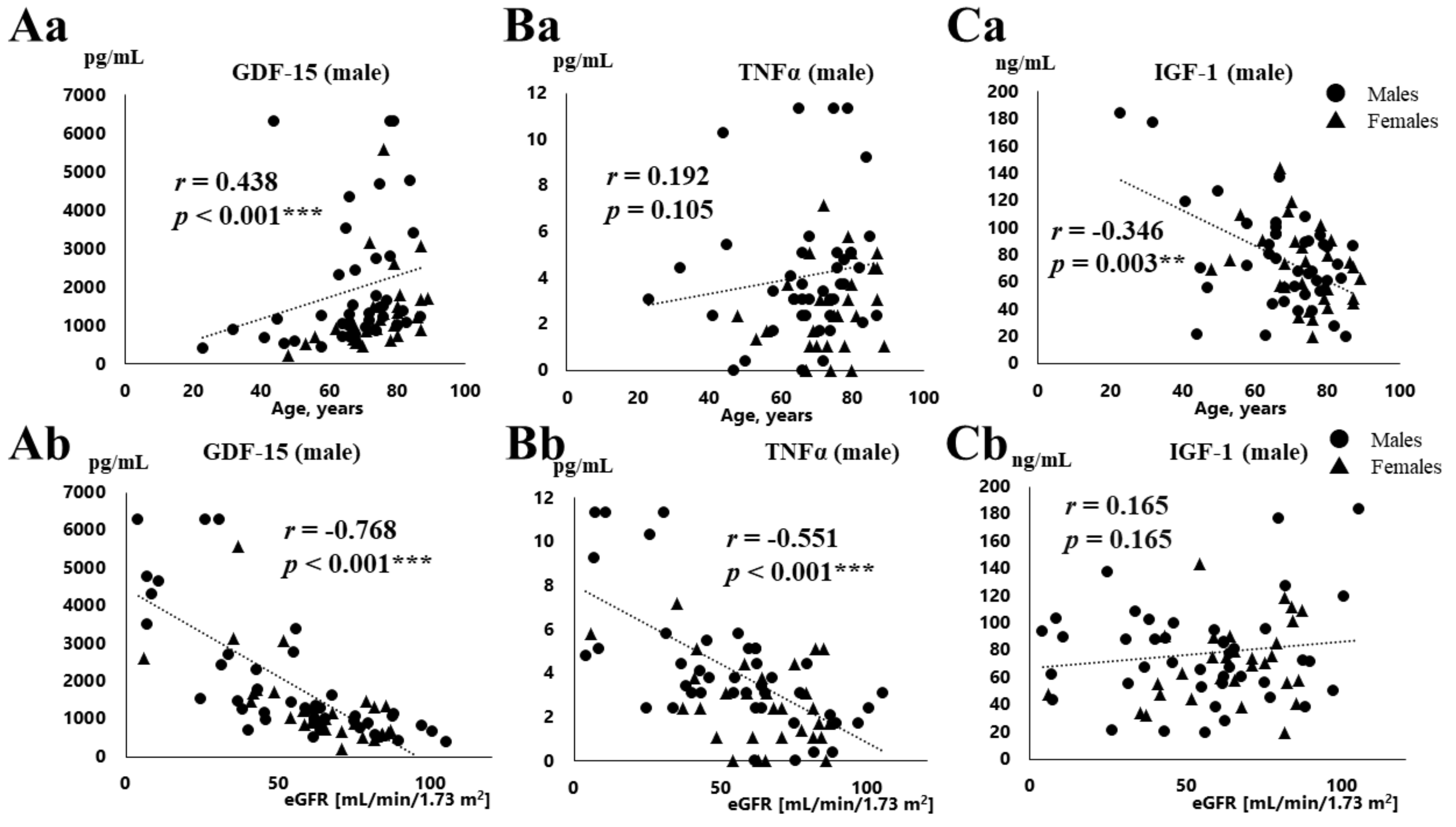
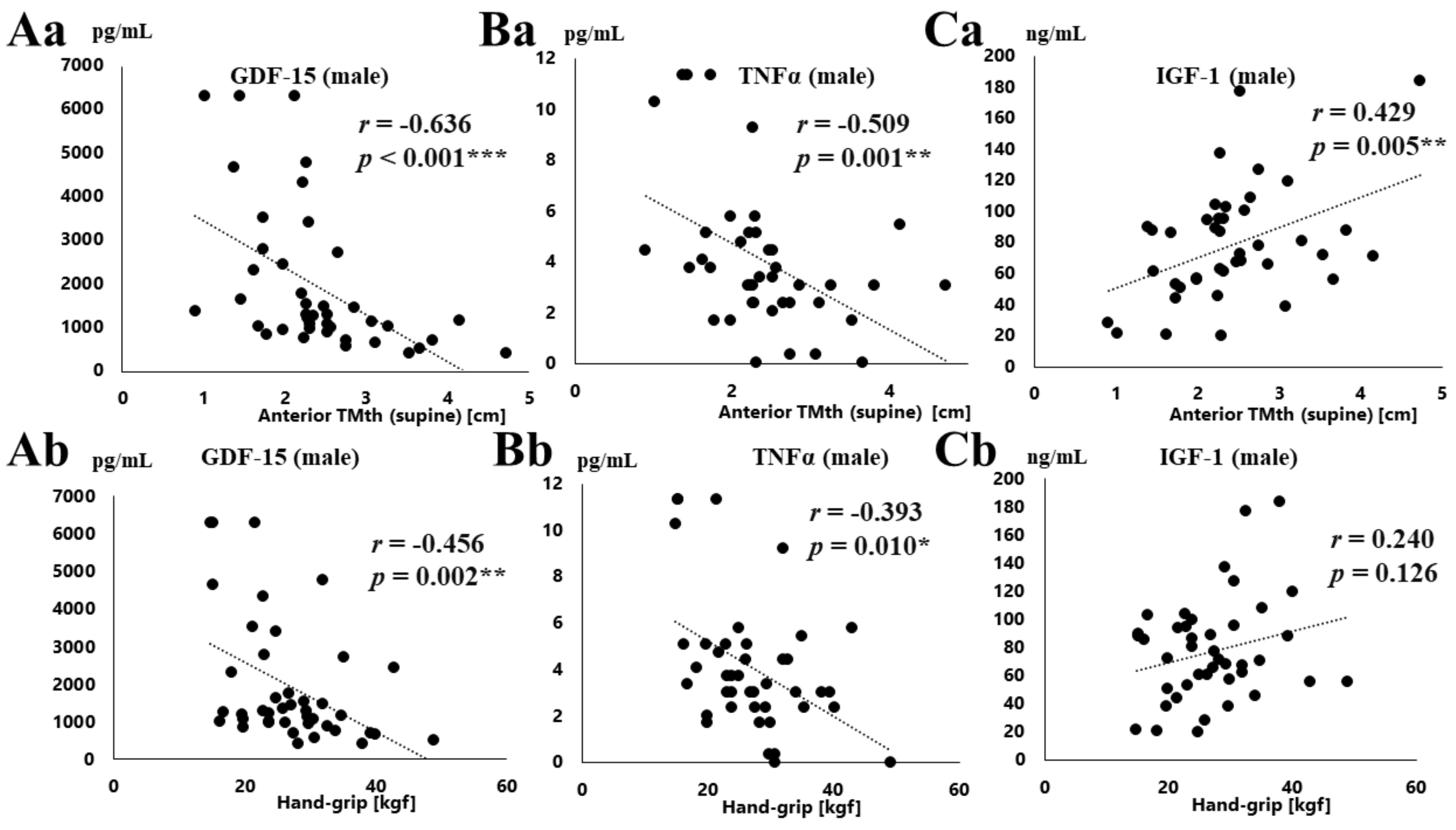
3.2. Correlation between Various Parameters and Serum GDF-15, TNFα, and IGF-1 Concentration
3.3. Relationships among Serum GDF-15, TNFα, and IGF-1 Concentration
3.4. Multiple Regression Analysis of Serum GDF-15 Levels and the Clinical Parameters
3.5. Relationships between Sarcopenia and Serum Concentration of GDF-15, TNFα and IGF-1
4. Discussion
4.1. Association of Serum GDF-15 Levels with eGFR
4.2. Association of Serum GDF-15 Levels with Muscle Loss
4.3. Relationships between GDF-15 and TNFα, IGF-1 Concentration
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CVD | cardiovascular disease |
| CABG | coronary artery bypass graft |
| AVR | aortic valve replacement |
| MVR | mitral valve replacement |
| MVP | mitral valve plasty |
| TVP | tricuspid valve plasty |
| TAVI | transcatheter valve implantation procedure |
| TVR | tricuspid valve replacement |
| TAR | total arch replacement |
| EVAR | endovascular aneurysm repair |
| BMI | body mass index |
| NYHA | New York Heart Association |
| TNFα | tumor necrosis factor α |
| GDF-15 | growth differentiation factor-15 |
| IGF-1 | insulin growth factor 1 |
| TGF-β; | transforming growth factor β |
| SMI | skeletal muscle mass index |
| BNP | brain natriuretic peptide |
| eGFR | estimated glomerular filtration rate |
| hsCRP | high-sensitivity C-reactive protein |
| ROC | receiver operating characteristic |
| AUC | area under the curve |
| TMth | thigh muscle thickness |
| COPD | chronic obstructive pulmonary disease |
| HbA1c | hemoglobin A1c |
| MVIC | maximum voluntary isometric contraction |
| ELISA | enzyme-linked immunosorbent assay |
| ICU | intensive care unit |
| ACS | acute coronary syndrome |
| AKI | acute kidney disease |
| BIA | bioelectric impedance analyzer |
| HF | heart failure |
| HT | hypertension |
| HD | hemodialysis |
| CKD | chronic kidney disease |
References
- Partridge, J.S.; Harari, D.; Dhesi, J.K. Frailty in the older surgical patient: A review. Age Ageing 2012, 41, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Makary, M.A.; Segev, D.L.; Pronovost, P.J.; Syin, D.; Bandeen-Roche, K.; Patel, P.; Takenaga, R.; Devgan, L.; Holzmueller, C.G.; Tian, J.; et al. Frailty as a predictor of surgical outcomes in older patients. J. Am. Coll. Surg. 2010, 210, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Shimura, T.; Yamamoto, M.; Kano, S.; Kagase, A.; Kodama, A.; Koyama, Y.; Tsuchikane, E.; Suzuki, T.; Otsuka, T.; Kohsaka, S.; et al. OCEAN-TAVI Investigators. Impact of the Clinical Frailty Scale on Outcomes After Transcatheter Aortic Valve Replacement. Circulation 2017, 135, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Afilalo, J.; Alexander, K.P.; Mack, M.J.; Maurer, M.S.; Green, P.; Allen, L.A. Frailty assessment in the cardiovascular care of older adults. J. Am. Coll. Cardiol. 2014, 63, 747–762. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127 (Suppl. 5), 990S–991S. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Mimeault, M.; Batra, S.K. Divergent molecular mechanisms underlying the pleiotropic functions of macrophage inhibitory cytokine-1 in cancer. J. Cell. Physiol. 2010, 224, 626–635. [Google Scholar] [CrossRef]
- Shi, Y.; Massagué, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Bootcov, M.R.; Bauskin, A.R.; Valenzuela, S.M.; Moore, A.G.; Bansal, M.; He, X.Y.; Zhang, H.P.; Donnellan, M.; Mahler, S.; Pryor, K.; et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc. Natl. Acad. Sci. USA 1997, 94, 11514–11519. [Google Scholar] [CrossRef]
- Dandrea, T.; Hellmold, H.; Jonsson, C.; Zhivotovsky, B.; Hofer, T.; Wärngård, L.; Cotgreave, I. The transcriptosomal response of human A549 lung cells to a hydrogen peroxide-generating system: Relationship to DNA damage, cell cycle arrest, and caspase activation. Free Radic. Biol. Med. 2004, 36, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Kempf, T.; Eden, M.; Strelau, J.; Naguib, M.; Willenbockel, C.; Tongers, J.; Heineke, J.; Kotlarz, D.; Xu, J.; Molkentin, J.D.; et al. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ. Res. 2006, 98, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Wallentin, L.; Kempf, T.; Tapken, H.; Quint, A.; Lindahl, B.; Olofsson, S.; Venge, P.; Larsson, A.; Hulthe, J.; et al. Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: Results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) Study. Eur. Heart J. 2009, 30, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Eggers, K.M.; Kempf, T.; Wallentin, L.; Wollert, K.C.; Lind, L. Change in growth differentiation factor 15 concentrations over time independently predicts mortality in community-dwelling elderly individuals. Clin. Chem. 2013, 59, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Kempf, T.; Wallentin, L. Growth Differentiation Factor 15 as a Biomarker in Cardiovascular Disease. Clin. Chem. 2017, 63, 140–151. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Jena, A.; Srivatsan, V.; Muthukumar, R.; Dhandapani, V.E. GDF 15-A Novel Biomarker in the Offing for Heart Failure. Curr. Cardiol. Rev. 2016, 12, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Preeshagul, I.; Gharbaran, R.; Jeong, K.H.; Abdel-Razek, A.; Lee, L.Y.; Elman, E.; Suh, K.S. Potential biomarkers for predicting outcomes in CABG cardiothoracic surgeries. J. Cardiothorac. Surg. 2013, 8, 176. [Google Scholar] [CrossRef]
- Wollert, K.C.; Kempf, T.; Lagerqvist, B.; Lindahl, B.; Olofsson, S.; Allhoff, T.; Peter, T.; Siegbahn, A.; Venge, P.; Drexler, H.; et al. Growth differentiation factor 15 for risk stratification and selection of an invasive treatment strategy in non ST-elevation acute coronary syndrome. Circulation 2007, 116, 1540–1548. [Google Scholar] [CrossRef]
- Khan, S.Q.; Ng, K.; Dhillon, O.; Kelly, D.; Quinn, P.; Squire, I.B.; Davies, J.E.; Ng, L.L. Growth differentiation factor-15 as a prognostic marker in patients with acute myocardial infarction. Eur. Heart J. 2009, 30, 1057–1065. [Google Scholar] [CrossRef]
- Kempf, T.; von Haehling, S.; Peter, T.; Allhoff, T.; Cicoira, M.; Doehner, W.; Ponikowski, P.; Filippatos, G.S.; Rozentryt, P.; Drexler, H.; et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007, 50, 1054–1060. [Google Scholar] [CrossRef]
- Breit, S.N.; Carrero, J.J.; Tsai, V.W.; Yagoutifam, N.; Luo, W.; Kuffner, T.; Bauskin, A.R.; Wu, L.; Jiang, L.; Barany, P.; et al. Macrophage inhibitory cytokine-1 (MIC-1/GDF15) and mortality in end-stage renal disease. Nephrol. Dial. Transplant. 2012, 27, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Hwang, S.J.; Wollert, K.C.; Larson, M.G.; Cheng, S.; Kempf, T.; Vasan, R.S.; Januzzi, J.L.; Wang, T.J.; Fox, C.S. Biomarkers of cardiovascular stress and incident chronic kidney disease. Clin. Chem. 2013, 59, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Robinson-Cohen, C.; Smith, M.R.; Bellovich, K.A.; Bhat, Z.Y.; Bobadilla, M.; Brosius, F.; de Boer, I.H.; Essioux, L.; Formentini, I.; et al. Growth Differentiation Factor-15 and Risk of CKD Progression. J. Am. Soc. Nephrol. 2017, 28, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Heringlake, M.; Charitos, E.I.; Gatz, N.; Käbler, J.H.; Beilharz, A.; Holz, D.; Schön, J.; Paarmann, H.; Petersen, M.; Hanke, T. Growth differentiation factor 15: A novel risk marker adjunct to the EuroSCORE for risk stratification in cardiac surgery patients. J. Am. Coll. Cardiol. 2013, 66, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Kahli, A.; Guenancia, C.; Zeller, M.; Grosjean, S.; Stamboul, K.; Rochette, L.; Girard, C.; Vergely, C. Growth differentiation factor-15 (GDF-15) levels are associated with cardiac and renal injury in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. PLoS ONE 2014, 9, e105759. [Google Scholar] [CrossRef] [PubMed]
- Krau, N.C.; Lünstedt, N.S.; Freitag-Wolf, S.; Brehm, D.; Petzina, R.; Lutter, G.; Bramlage, P.; Dempfle, A.; Frey, N.; Frank, D. Elevated growth differentiation factor 15 levels predict outcome in patients undergoing transcatheter aortic valve implantation. Eur. J. Heart Fail. 2015, 17, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Guenancia, C.; Kahli, A.; Laurent, G.; Hachet, O.; Malapert, G.; Grosjean, S.; Girard, C.; Vergely, C.; Bouchot, O. Pre-operative growth differentiation factor 15 as a novel biomarker of acute kidney injury after cardiac bypass surgery. Int. J. Cardiol. 2015, 197, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, H.; Qi, Q.; Gong, W.; Qian, C.; Dong, R.; Zang, Y.; Li, J.; Zhou, M.; Cai, J.; et al. Plasma levels of growth differentiation factor-15 are associated with myocardial injury in patients undergoing off-pump coronary artery bypass grafting. Sci. Rep. 2016, 6, 28221. [Google Scholar] [CrossRef]
- Fujita, Y.; Taniguchi, Y.; Shinkai, S.; Tanaka, M.; Ito, M. Secreted growth differentiation factor 15 as a potential biomarker for mitochondrial dysfunctions in aging and age-related disorders. Geriatr. Gerontol. Int. 2016, 16 (Suppl. 1), 17–29. [Google Scholar] [CrossRef]
- Patel, M.S.; Lee, J.; Baz, M.; Wells, C.E.; Bloch, S.; Lewis, A.; Donaldson, A.V.; Garfield, B.E.; Hopkinson, N.S.; Natanek, A.; et al. Growth differentiation factor-15 is associated with muscle mass in chronic obstructive pulmonary disease and promotes muscle wasting in vivo. J. Cachexia Sarcopenia Muscle 2016, 7, 436–448. [Google Scholar] [CrossRef]
- Bloch, S.A.; Lee, J.Y.; Wort, S.J.; Polkey, M.I.; Kemp, P.R.; Griffiths, M.J. Sustained elevation of circulating growth and differentiation factor-15 and a dynamic imbalance in mediators of muscle homeostasis are associated with the development of acute muscle wasting following cardiac surgery. Crit. Care Med. 2013, 41, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Bloch, S.A.; Lee, J.Y.; Syburra, T.; Rosendahl, U.; Griffiths, M.J.; Kemp, P.R.; Polkey, M.I. Increased expression of GDF-15 may mediate ICU-acquired weakness by down-regulating muscle microRNAs. Thorax 2015, 70, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Lerner, L.; Hayes, T.G.; Tao, N.; Krieger, B.; Feng, B.; Wu, Z.; Nicoletti, R.; Chiu, M.I.; Gyuris, J.; Garcia, J.M. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J. Cachexia Sarcopenia Muscle 2015, 6, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Nakajima, T.; Sawaguchi, T.; Nozawa, N.; Arakawa, T.; Takahashi, R.; Mizushima, Y.; Katayanagi, S.; Matsumoto, K.; Toyoda, S.; et al. Short Physical Performance Battery for cardiovascular disease inpatients: Implications for critical factors and sarcopenia. Sci. Rep. 2017, 7, 17425. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Gona, P.; Larson, M.G.; Tofler, G.H.; Levy, D.; Newton-Cheh, C.; Jacques, P.F.; Rifai, N.; Selhub, J.; Robins, S.J.; et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N. Engl. J. Med. 2006, 355, 2631–2639. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003, 108, 2154–2169. [Google Scholar] [PubMed]
- Wali, R.K.; Henrich, W.L. Chronic kidney disease: A risk factor for cardiovascular disease. Cardiol. Clin. 2005, 23, 343–362. [Google Scholar] [CrossRef]
- Ibáñez, J.; Riera, M.; Saez de Ibarra, J.I.; Carrillo, A.; Fernández, R.; Herrero, J.; Fiol, M.; Bonnin, O. Effect of preoperative mild renal dysfunction on mortality and morbidity following valve cardiac surgery. Interact. Cardiovasc. Thorac. Surg. 2007, 6, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Thourani, V.; Keeling, W.B.; Sarin, E.L.; Guyton, R.A.; Kilgo, P.D.; Dara, A.B.; Puskas, J.D.; Chen, E.P.; Cooper, W.A.; Vega, J.D.; et al. Impact of preoperative renal dysfunction on long-term survival for patients undergoing aortic valve replacement. Ann. Thorac. Surg. 2011, 91, 1798–1806. [Google Scholar] [CrossRef]
- Gibson, P.H.; Croal, B.L.; Cuthbertson, B.H.; Chiwara, M.; Scott, A.E.; Buchan, K.G.; El-Shafei, H.; Gibson, G.; Jeffrey, R.R.; Hillis, G.S. The relationship between renal function and outcome from heart valve surgery. Am. Heart J. 2008, 156, 893–899. [Google Scholar] [CrossRef]
- Bauskin, A.R.; Brown, D.A.; Kuffner, T.; Johnen, H.; Luo, X.W.; Hunter, M.; Breit, S.N. Role of macrophage inhibitory cytokine-1 in tumorigenesis and diagnosis of cancer. Cancer Res. 2006, 66, 4983–4986. [Google Scholar] [CrossRef]
- Krintus, M.; Braga, F.; Kozinski, M.; Borille, S.; Kubica, J.; Sypniewska, G.; Panteghini, M. A study of biological and lifestyle factors, including within-subject variation, affecting concentrations of growth differentiation factor 15 in serum. Clin. Chem. Lab. Med. 2019, 57, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Rothenbacher, D.; Dallmeier, D.; Christow, H.; Koenig, W.; Denkinger, M.; Klenk, J.; ActiFE study group. Association of growth differentiation factor 15 with other key biomarkers, functional parameters and mortality in community-dwelling older adults. Age Ageing 2019, 48, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Gonzalez-Freire, M.; Tanaka, T.; Biancotto, A.; Zhang, P.; Shardell, M.; Moaddel, R.; Ferrucci, L.; CHI Consortium. Elevated Plasma Growth and Differentiation Factor-15 is Associated with Slower Gait Speed and Lower Physical Performance in Healthy Community-Dwelling Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2019. [Google Scholar] [CrossRef]
- Welsh, J.B.; Sapinoso, L.M.; Kern, S.G.; Brown, D.A.; Liu, T.; Bauskin, A.R.; Ward, R.L.; Hawkins, N.J.; Quinn, D.I.; Russell, P.J.; et al. Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc. Natl. Acad. Sci. USA 2003, 100, 3410–3415. [Google Scholar] [CrossRef]
- Johnen, H.; Lin, S.; Kuffner, T.; Brown, D.A.; Tsai, V.W.; Bauskin, A.R.; Wu, L.; Pankhurst, G.; Jiang, L.; Junankar, S.; et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat. Med. 2007, 13, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Tsai, V.W.; Macia, L.; Johnen, H.; Kuffner, T.; Manadhar, R.; Jørgensen, S.B.; Lee-Ng, K.K.; Zhang, H.P.; Wu, L.; Marquis, C.P.; et al. TGF-b superfamily cytokine MIC-1/GDF15 is a physiological appetite and body weight regulator. PLoS ONE 2013, 8, e55174. [Google Scholar] [CrossRef]
- Hubbard, R.E.; O’Mahony, M.S.; Savva, G.M.; Calver, B.L.; Woodhouse, K.W. Inflammation and frailty measures in older people. J. Cell. Mol. Med. 2009, 13, 3103–3109. [Google Scholar] [CrossRef] [PubMed]
- Utech, A.E.; Tadros, E.M.; Hayes, T.G.; Garcia, J.M. Predicting survival in cancer patients: The role of cachexia and hormonal, nutritional and inflammatory markers. J. Cachexia Sarcopenia Muscle 2012, 3, 245–251. [Google Scholar] [CrossRef]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Harris, T.B.; Kritchevsky, S.B.; Newman, A.B.; Colbert, L.H.; Pahor, M.; Rubin, S.M.; Tylavsky, F.A.; et al. Health ABC Study. Higher inflammatory marker levels in older persons: Associations with 5-year change in muscle mass and muscle strength. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- von Haehling, S.; Steinbeck, L.; Doehner, W.; Springer, J.; Anker, S.D. Muscle wasting in heart failure: An overview. Int. J. Biochem. Cell Biol. 2013, 45, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.X.; Cappola, A.R.; Andersen, R.E.; Blackman, M.R.; Koenig, K.; Blair, M.; Walston, J.D. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin. Exp. Res. 2004, 16, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, J.; McDonald, C.; Lupino, K.; Zhai, X.; Wilkins, B.J.; Hakonarson, H.; Pei, L. GDF15 is a heart-derived hormone that regulates body growth. EMBO. Mol. Med. 2017, 9, 1150–1164. [Google Scholar] [CrossRef] [PubMed]

| Number | 72 |
| Male, Female | 42, 30 |
| Age, y | 69.9 ± 13.1 |
| BMI, kg/m2 | 24.3 ± 3.9 |
| Risk factors (percentage) | |
| Hypertension | 75 |
| Diabetes | 35 |
| Dyslipidemia | 46 |
| Smoking | 11 |
| Hemodialysis | 8 |
| NYHA classification | 2.2 ± 1.0 |
| Coronary artery disease (percentage) | |
| 0-vessel disease | 53 |
| 1-vessel disease | 11 |
| 2-vessel disease | 6 |
| 3-vessel disease | 30 |
| Cardiovascular surgery (percentage) | |
| CABG | 26 |
| AVR | 21 |
| Other valve replacement/repair (MVR, MVP, TAP, TAR, LAAAC) | 17 |
| CABG combined with valve replacement/repair (AVR, MVP, TAP) | 8 |
| AVR combined with other valve (MVP, TAP, LAAC) or aortic diseases (TAR) | 11 |
| Aortic disease (TAR, TEVAR, et al.) | 7 |
| Others | 10 |
| Drugs (percentage) | |
| β-blockers | 49 |
| Ca-blockers | 38 |
| ACE-I/ARB | 58 |
| Diuretics | 49 |
| Statins | 53 |
| Oral antidiabetic drugs | 31 |
| Insulin | 8 |
| Total (n = 72) | Male (n = 42) | Female (n = 30) | |
|---|---|---|---|
| Age, years | 69.9 (13.1) | 66.9 (14.4) | 73.7 (10.0) * |
| BMI, kg/m2 | 24.3 (3.9) | 24.9 (4.5) | 24.7 (5.5) |
| NYHA classification | 2.2 (1.0) | 2.3 (1.1) | 2.1 (0.9) |
| Gait speed, m/s | 0.93 (0.32) | 0.99 (0.34) | 0.86 (0.28) |
| Hand-grip strength, kgf | 22.8 (8.5) | 27.1 (7.9) | 16.5 (4.6) *** |
| Knee extension strength, kgf | 20.8 (9.5) | 24.4 (9.3) | 15.6 (7.1) *** |
| Body fat percentage, % | 32.3 (9.3) | 28.4 (7.8) | 37.6 (8.7) *** |
| Skeletal muscle mass index (SMI), kg/m2 | 6.5 (1.4) | 7.2 (1.3) | 5.4 (0.9) *** |
| Anterior thigh muscle thickness (TMth) (supine), cm | 2.28 (0.75) | 2.41 (0.80) | 2.1 (0.6) |
| Anterior thigh muscle thickness (TMth) (standing), cm | 3.47 (0.95) | 3.68 (0.97) | 3.2 (0.9) * |
| HbA1c, % | 6.2 (0.9) | 6.3 (1.0) | 6.0 (0.8) |
| BNP, pg/mL | 355 (570) | 399 (673) | 268 (345) |
| eGFR, ml/min/1.73 m2 | 58.2 (24.0) | 55.7 (26.6) | 63.4 (19.3) |
| Hb, g/dL | 12.2 (1.8) | 12.3 (1.9) | 11.9 (1.7) |
| HOMA-IR | 2.75 (4.20) | 3.46 (5.29) | 1.64 (1.29) |
| hsCRP, mg/L | 5.9 (12) | 7.4 (13.8) | 3.3 (7.9) |
| GDF-15, pg/mL | 1676 (1465) | 1928 (1655) | 1325 (1078) |
| TNFα, pg/mL | 3.5 (2.8) | 4.1 (2.9) | 2.6 (1.9) * |
| IGF-1, ng/mL | 74.4 (33.4) | 77.3 (36.5) | 70.4 (28.5) |
| GDF-15 Males/Females | TNFα Males/Females | IGF-1 Males/Females | |
|---|---|---|---|
| Age | 0.436 (0.004) **/0.637 (<0.001) *** | 0.244 (0.120)/0.261 (0.164) | −0.319 (0.036) */−0.339 (0.066) |
| BMI | −0.143 (0.367)/−0.062 (0.745) | −0.263 (0.092)/−0.055 (0.772) | 0.047 (0.769)/0.194 (0.303) |
| HbA1C | −0.155 (0.340)/−0.229 (0.223) | −0.154 (0.342)/−0.127 (0.503) | 0.261 (0.104)/0.344 (0.063) |
| BNP | 0.427 (0.005) **/0.480 (0.007) ** | 0.465 (0.002) **/0.214 (0.257) | −0.059 (0.710)/−0.353 (0.055) |
| eGFR | −0.792 (<0.001) ***/−0.726 (<0.001) *** | −0.642 (<0.001) ***/−0.394 (0.031) * | 0.144 (0.363)/0.301 (0.106) |
| Hb | −0.560 (<0.001) ***/−0.370 (0.044) * | −0.566 (<0.001) ***/−0.065 (0.732) | 0.079 (0.617)/−0.006 (0.977) |
| Body fat percentage | −0.140 (0.403)/0.300 (0.128) | −0.146 (0.382)/0.135 (0.502) | −0.119 (0.479)/0.197 (0.326) |
| SMI | −0.392 (0.014) */−0.529 (0.005) ** | −0.368 (0.021) */−0.189 (0.346) | 0.313 (0.053)/0.153 (0.446) |
| Hand-grip | −0.456 (0.002) **/−0.656 (<0.001) *** | −0.393 (0.010) */−0.298 (0.117) | 0.240 (0.126)/0.244 (0.202) |
| Knee extension | −0.222 (0.169)/−0.541 (0.003) ** | −0.431 (0.005) **/−0.192 (0.329) | 0.140 (0.390)/0.061 (0.758) |
| Gait speed | −0.218 (0.165)/−0.558 (0.002) ** | −0.190 (0.229)/−0.336 (0.074) | 0.256 (0.102)/0.253 (0.186) |
| Anterior TMth (supine) | −0.636 (<0.001) ***/−0.391 (0.044) * | −0.509 (0.001) **/−0.200 (0.316) | 0.429 (0.005) **/0.057 (0.779) |
| Anterior TMth (standing) | −0.600 (<0.001) ***/−0.557 (0.003) ** | −0.434 (0.005) **/−0.267 (0.178) | 0.366 (0.020) */0.301 (0.127) |
| GDF-15 | -/- | 0.657 (<0.001) ***/0.434 (0.017) * | −0.203 (0.198)/−0.553 (0.002) ** |
| TNFα | 0.657 (<0.001) ***/0.434 (0.017) * | -/- | −0.233 (0.137)/−0.479 (0.007) ** |
| A: Multiple linear regression analysis of GDF15 and the clinical data | ||||
| Dependent variable: log (GDF−15) | ||||
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Independent variable. | β-value (p) | β-value (p) | β-value (p) | β-value (p) |
| eGFR | −0.650 (<0.001) *** | −0.655 (<0.001) *** | −0.613 (<0.001) *** | −0.597 (<0.001) *** |
| BNP (log) | −0.009 (0.929) | −0.011 (0.912) | −0.042 (0.649) | −0.040 (0.663) |
| Hb | −0.106 (0.257) | −0.110 (0.253) | −0.079 (0.372) | −0.084 (0.343) |
| SMI | 0.036 (0.722) | 0.031 (0.771) | −0.171 (0.139) | −0.196 (0.098) |
| Hand−grip strength | 0.106 (0.312) | 0.323 (0.104) | −0.105 (0.367) | −0.059 (0.632) |
| Anterior TMth (supine) | −0.358 (0.001) ** | −0.362 (0.001) ** | −0.233 (0.033) * | −0.272 (0.019) * |
| B: Multiple linear regression analysis of anterior thigh muscle thickness (supine) and serum markers | ||||
| Dependent variable: anterior thigh muscle thickness (supine) | ||||
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Independent variable | β-value (p) | β-value (p) | β-value (p) | β-value (p) |
| GDF−15 (log) | −0.401 (0.005) ** | −0.311 (0.024) * | −0.384 (0.007) ** | −0.390 (0.004) ** |
| TNFα (log) | −0.007 (0.955) | −0.054 (0.671) | −0.068 (0.584) | −0.054 (0.644) |
| IGF−1 | 0.256 (0.031) * | 0.094 (0.456) | 0.071 (0.656) | 0.078 (0.506) |
| C: Multiple linear regression analysis of eGFR and the clinical data | ||||
| Dependent variable: eGFR | ||||
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Independent variable | β-value (p) | β-value (p) | β-value (p) | β-value (p) |
| BNP (log) | −0.164 (0.070) | −0.149 (0.106) | −0.149 (0.113) | −0.170 (0.070) |
| hsCRP (log) | −0.070 (0.377) | −0.094 (0.262) | −0.097 (0.257) | −0.085 (0.999) |
| Hb | 0.035 (0.685) | 0.005 (0.958) | 0.002 (0.984) | 0.011 (0.657) |
| GDF−15 (log) | −0.583 (<0.001) *** | −0.571 (<0.001) *** | −0.577 (<0.001) *** | −0.565 (<0.001) *** |
| TNFα (log) | −0.177 (0.069) | −0.196 (0.050) | −0.198 (0.050) | −0.197 (0.050) |
| Male | Female | |||
|---|---|---|---|---|
| Sarcopenia (−) | Sarcopenia (+) | Sarcopenia (−) | Sarcopenia (+) | |
| Number | 28 | 11 | 14 | 13 |
| Age (years) | 63.4 (14.2) | 75.6 (10.8) *** | 68.9 (10.5) | 76.2 (7.3) * |
| BMI (kg/m2) | 26.0 (4.8) | 22.6 (3.0) * | 24.9 (3.1) | 25.9 (7.3) |
| Physical capacity | ||||
| Gait speed (m/s) | 1.08 (0.34) | 0.77 (0.28) * | 1.01 (0.17) | 0.73 (0.32) * |
| Grip strength (kgf) | 30.0 (7.4) | 20.2 (4.4) *** | 20.1 (3.1) | 13.9 (3.5) *** |
| Knee extension (kgf) | 26.9 (8.4) | 17.5 (7.3) ** | 19.2 (7.5) | 13.6 (4.8)* |
| BIA findings | ||||
| Body fat percentage (%) | 28.8 (7.4) | 27.9 (8.9) | 35.7 (6.6) | 39.8 (10.3) |
| Skeletal muscle mass index (SMI) (kg/m2) | 7.68 (1.15) | 5.90 (0.53) *** | 6.05 (0.53) | 4.74 (0.63) *** |
| Muscle thickness | ||||
| Anterior TMth (supine) (cm) | 2.64 (0.68) | 1.65 (0.53) *** | 2.45 (0.68) | 1.89 (0.48) * |
| Anterior TMth (standing) (cm) | 3.98 (0.88) | 2.78 (0.46) *** | 3.70 (0.74) | 2.77 (0.79) ** |
| HbA1c, % | 6.3 (0.9) | 6.4 (1.3) | 6.12 (0.98) | 5.97 (0.60) |
| BNP, pg/mL | 209 (285) | 646 (722) ** | 162 (250) | 366 (427) * |
| eGFR, ml/min/1.73 m2 | 59.7 (26.4) | 47.9 (26.1) | 70.6 (14.4) | 56.1 (22.1) |
| Hb, g/dL | 13.0 (1.6) | 11.0 (1.7) | 12.5 (1.7) | 11.5 (1.6) |
| hsCRP, mg/L | 7.1 (12.7) | 9.9 (18.5) | 4.5 (11.3) | 2.0 (2.3) |
| GDF-15, pg/mL | 1483 (1125) | 3053 (2346) * | 891 (700) | 1625 (1302) * |
| TNFα, pg/mL | 3.06 (1.95) | 6.07 (3.27) ** | 2.23 (2.10) | 2.92 (1.66) |
| IGF-1, ng/mL | 84.1 (40.0) | 62.7 (25.6) | 74.7 (23.6) | 70.6 (34.6) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakajima, T.; Shibasaki, I.; Sawaguchi, T.; Haruyama, A.; Kaneda, H.; Nakajima, T.; Hasegawa, T.; Arikawa, T.; Obi, S.; Sakuma, M.; et al. Growth Differentiation Factor-15 (GDF-15) Is a Biomarker of Muscle Wasting and Renal Dysfunction in Preoperative Cardiovascular Surgery Patients. J. Clin. Med. 2019, 8, 1576. https://doi.org/10.3390/jcm8101576
Nakajima T, Shibasaki I, Sawaguchi T, Haruyama A, Kaneda H, Nakajima T, Hasegawa T, Arikawa T, Obi S, Sakuma M, et al. Growth Differentiation Factor-15 (GDF-15) Is a Biomarker of Muscle Wasting and Renal Dysfunction in Preoperative Cardiovascular Surgery Patients. Journal of Clinical Medicine. 2019; 8(10):1576. https://doi.org/10.3390/jcm8101576
Chicago/Turabian StyleNakajima, Toshiaki, Ikuko Shibasaki, Tatsuya Sawaguchi, Akiko Haruyama, Hiroyuki Kaneda, Takafumi Nakajima, Takaaki Hasegawa, Takuo Arikawa, Syotaro Obi, Masashi Sakuma, and et al. 2019. "Growth Differentiation Factor-15 (GDF-15) Is a Biomarker of Muscle Wasting and Renal Dysfunction in Preoperative Cardiovascular Surgery Patients" Journal of Clinical Medicine 8, no. 10: 1576. https://doi.org/10.3390/jcm8101576
APA StyleNakajima, T., Shibasaki, I., Sawaguchi, T., Haruyama, A., Kaneda, H., Nakajima, T., Hasegawa, T., Arikawa, T., Obi, S., Sakuma, M., Ogawa, H., Toyoda, S., Nakamura, F., Abe, S., Fukuda, H., & Inoue, T. (2019). Growth Differentiation Factor-15 (GDF-15) Is a Biomarker of Muscle Wasting and Renal Dysfunction in Preoperative Cardiovascular Surgery Patients. Journal of Clinical Medicine, 8(10), 1576. https://doi.org/10.3390/jcm8101576





