Exploring the Ability of Electronic Nose Technology to Recognize Interstitial Lung Diseases (ILD) by Non-Invasive Breath Screening of Exhaled Volatile Compounds (VOC): A Pilot Study from the European IPF Registry (eurIPFreg) and Biobank
Abstract
:1. Introduction
2. Objectives of This Study
3. Materials and Methods
3.1. Study Design and Data Collection
3.2. Subject Selection
3.3. Sample Collection and Data Analysis
3.4. Statistical Analysis and Data Presentation
3.5. Aeonose® Data Presentation
4. Results
Demographics
5. Discussion
Study Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bonniaud, P.; Fabre, A.; Frossard, N.; Guignabert, C.; Inman, M.; Kuebler, W.M.; Maes, T.; Shi, W.; Stampfli, M.; Uhlig, S.; et al. Optimising experimental research in respiratory diseases: An ERS statement. Eur. Respir. J. 2018, 51, 1702133. [Google Scholar] [CrossRef] [PubMed]
- Barratt, S.L.; Creamer, A.; Hayton, C.; Chaudhuri, N. Idiopathic Pulmonary Fibrosis (IPF): An Overview. J. Clin. Med. 2018, 7, 201. [Google Scholar] [CrossRef] [PubMed]
- Kolb, M.; Vašáková, M. The natural history of progressive fibrosing interstitial lung diseases. Respir Res. 2019, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Krauss, E.; Gehrken, G.; Drakopanagiotakis, F.; Tello, S.; Dartsch, R.C.; Maurer, O.; Windhorst, A.; von der Beck, D.; Griese, M.; Seeger, W.; et al. Clinical characteristics of patients with familial idiopathic pulmonary fibrosis (f-IPF). BMC Pulm. Med. 2019, 19, 130. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.E.; Glaspole, I.; Moodley, Y.; Chapman, S.; Ellis, S.; Goh, N.; Hopkins, P.; Keir, G.; Mahar, A.; Cooper, W.; et al. Disease progression in idiopathic pulmonary fibrosis with mild physiological impairment: Analysis from the Australian IPF registry. BMC Pulm. Med. 2018, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef]
- Guenther, A.; Krauss, E.; Tello, S.; Wagner, J.; Paul, B.; Kuhn, S.; Maurer, O.; Heinemann, S.; Costabel, U.; Barbero, M.A.N.; et al. The European IPF registry (eurIPFreg): Baseline characteristics and survival of patients with idiopathic pulmonary fibrosis. Respir. Res. 2018, 19, 141. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Krauss, E.; Froehler, M.; Degen, M.; Mahavadi, P.; Dartsch, R.C.; Korfei, M.; Ruppert, C.; Seeger, W.; Guenther, A. Exhalative Breath Markers Do Not Offer for Diagnosis of Interstitial Lung Diseases: Data from the European IPF Registry (eurIPFreg) and Biobank. J. Clin. Med. 2019, 8, 643. [Google Scholar] [CrossRef]
- Loeh, B.; Brylski, L.T.; von der Beck, D.; Seeger, W.; Krauss, E.; Bonniaud, P.; Crestani, B.; Vancheri, C.; Wells, A.U.; Markart, P.; et al. Lung CT densitometry in idiopathic pulmonary fibrosis (IPF) for the prediction of natural course, severity and mortality. Chest 2019, 155, 972–981. [Google Scholar] [CrossRef]
- Drakopanagiotakis, F.; Wujak, L.; Wygrecka, M.; Markart, P. Biomarkers in idiopathic pulmonary fibrosis. Matrix Biol. 2018, 68–69, 404–421. [Google Scholar] [CrossRef] [PubMed]
- Kort, S.; Tiggeloven, M.M.; Brusse-Keizer, M.; Gerritsen, J.W.; Schouwink, J.H.; Citgez, E.; de Jongh, F.H.C.; Samii, S.; van der Maten, J.; van den Bogart, M.; et al. Multi-centre prospective study on diagnosing subtypes of lung cancer by exhaled-breath analysis. Lung Cancer 2018, 125, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Bos, L.D.; van der Schee, M.P.; van Schooten, F.-J.; Sterk, P.J. Exhaled Molecular Fingerprinting in Diagnosis and Monitoring: Validating Volatile Promises. Trends Mol. Med. 2015, 21, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Portable Electronic Nose Based on Electrochemical Sensors for Food Quality Assessment. Sensors 2017, 17, 2715. [Google Scholar] [CrossRef]
- Papadimitropoulos, M.-E.P.; Vasilopoulou, C.G.; Maga-Nteve, C.; Klapa, M.I. Untargeted GC-MS Metabolomics. Methods Mol. Biol. 2018, 1738, 133–147. [Google Scholar] [CrossRef]
- Amato, F.; López, A.; Peña-Méndez, E.M.; Vaňhara, P.; Hampl, A.; Havel, J. Artificial neural networks in medical diagnosis. J. Appl. Biomed. 2013, 11, 47–58. [Google Scholar] [CrossRef]
- Bruins, M.; Gerritsen, J.W.; van de Sande, W.W.J.; van Belkum, A.; Bos, A. Enabling a transferable calibration model for metal-oxide type electronic noses. Sens. Actuators B Chem. 2013, 188, 1187–1195. [Google Scholar] [CrossRef]
- Dragonieri, S.; Pennazza, G.; Carratu, P.; Resta, O. Electronic Nose Technology in Respiratory Diseases. Lung 2017, 195, 157–165. [Google Scholar] [CrossRef]
- Kort, S.; Brusse-Keizer, M.; Gerritsen, J.-W.; van der Palen, J. Data analysis of electronic nose technology in lung cancer: Generating prediction models by means of Aethena. J. Breath Res. 2017, 11, 026006. [Google Scholar] [CrossRef]
- Boughorbel, S.; Jarray, F.; El-Anbari, M. Optimal classifier for imbalanced data using Matthews Correlation Coefficient metric. PLoS ONE 2017, 12, e0177678. [Google Scholar] [CrossRef]
- Leopold, J.H.; Bos, L.D.J.; Sterk, P.J.; Schultz, M.J.; Fens, N.; Horvath, I.; Bikov, A.; Montuschi, P.; Di Natale, C.; Yates, D.H.; et al. Comparison of classification methods in breath analysis by electronic nose. J. Breath Res. 2015, 9, 46002. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Joshi, R.; Anil Vishnu, G.K.; Bhalerao, S.; Pandya, H.J. Electronic nose: A non-invasive technology for breath analysis of diabetes and lung cancer patients. J. Breath Res. 2019, 13, 024001. [Google Scholar] [CrossRef] [PubMed]
- Biehl, W.; Hattesohl, A.; Jörres, R.A.; Duell, T.; Althöhn, U.; Koczulla, A.R.; Schmetzer, H. VOC pattern recognition of lung cancer: A comparative evaluation of different dog- and eNose-based strategies using different sampling materials. Acta Oncol. 2019, 58, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.H.; Philipp, A.; Bein, T.; Redel, A.; Gruber, M.; Schultz, M.J.; Abu-Hanna, A.; Brinkman, P.; Janssen, H.-G.; Bos, L.D.J. Volatile organic compound profiles in outlet air from extracorporeal life-support devices differ from breath profiles in critically ill patients. ERJ Open Res. 2019, 5, 00134-2018. [Google Scholar] [CrossRef] [PubMed]
- Beale, D.J.; Jones, O.A.H.; Karpe, A.V.; Dayalan, S.; Oh, D.Y.; Kouremenos, K.A.; Ahmed, W.; Palombo, E.A. A Review of Analytical Techniques and Their Application in Disease Diagnosis in Breathomics and Salivaomics Research. Int. J. Mol. Sci. 2016, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Cavaleiro Rufo, J.; Paciência, I.; Mendes, F.C.; Farraia, M.; Rodolfo, A.; Silva, D.; de Oliveira Fernandes, E.; Delgado, L.; Moreira, A. Exhaled breath condensate volatilome allows sensitive diagnosis of persistent asthma. Allergy 2019, 74, 527–534. [Google Scholar] [CrossRef]
- Finamore, P.; Pedone, C.; Scarlata, S.; Di Paolo, A.; Grasso, S.; Santonico, M.; Pennazza, G.; Incalzi, R.A. Validation of exhaled volatile organic compounds analysis using electronic nose as index of COPD severity. Int. J. Chron. Obstr. Pulm. Dis. 2018, 13, 1441–1448. [Google Scholar] [CrossRef]
- Besa, V.; Teschler, H.; Kurth, I.; Khan, A.M.; Zarogoulidis, P.; Baumbach, J.I.; Sommerwerck, U.; Freitag, L.; Darwiche, K. Exhaled volatile organic compounds discriminate patients with chronic obstructive pulmonary disease from healthy subjects. Int. J. Chron. Obstr. Pulm. Dis. 2015, 10, 399–406. [Google Scholar] [CrossRef] [Green Version]
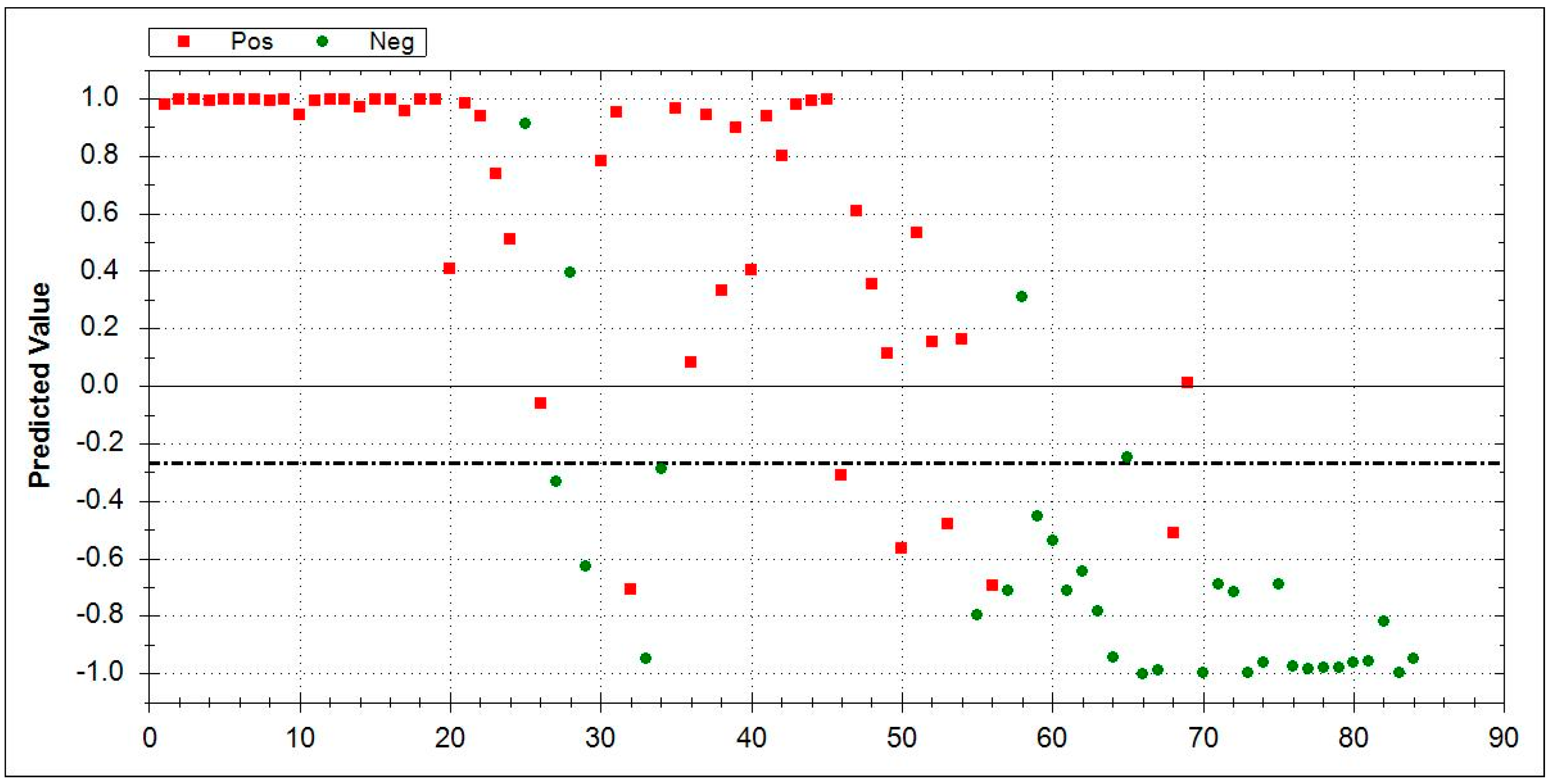

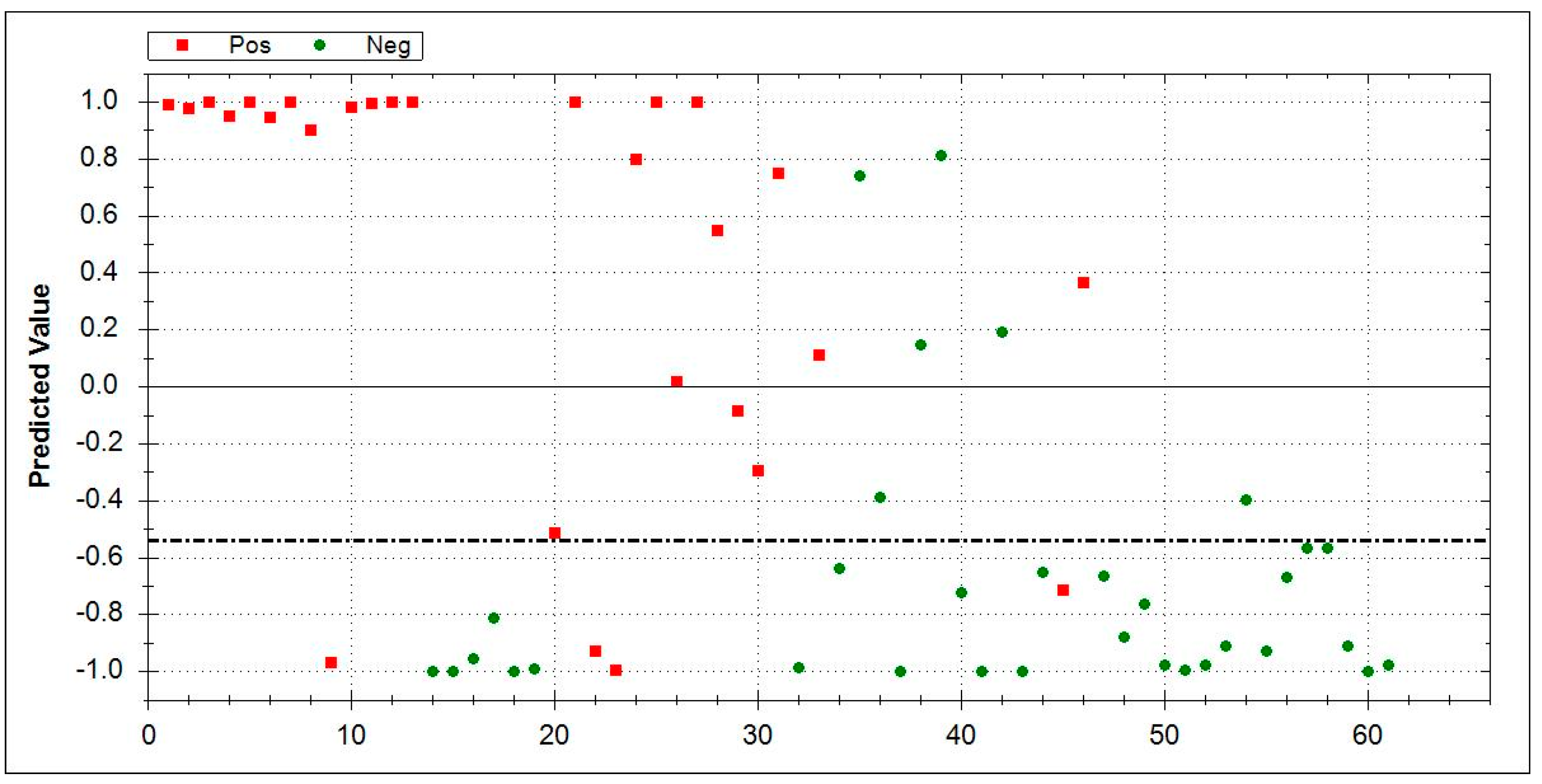



| Group | Number | Mean Age at Baseline ± SD | Male | Smoking History | |||
|---|---|---|---|---|---|---|---|
| (n) | (years) | (n) | Current Smoker (n) | Ex-Smoker (n) | Never-Smoked (n) | Smoking History Unknown (n) | |
| ILD | 174 | ||||||
| 25 | 66.4 ± 11.2 | 6 | 1 | 13 | 10 | 1 |
| 28 | 67.2 ± 7.7 | 13 | - | 20 | 8 | - |
| 20 | 63.2 ± 12.7 | 12 | - | 9 | 8 | 3 |
| 51 | 68.6 ± 8.3 | 37 | 2 | 33 | 15 | 1 |
| 19 | 56.7 ± 14.3 | 9 | 2 | 6 | 11 | - |
| 20 | 65.5 ± 11.7 | 14 | 5 | 5 | 10 | - |
| 5 | 72 ± 3.9 | 5 | - | 3 | 2 | - |
| 6 | 66.8 ± 11.9 | 3 | 1 | 2 | 3 | - |
| Healthy controls | 33 | 34.4 ± 14.9 | 1 | 8 | 2 | 10 | 13 |
| COPD | 23 | 64.4 ± 9.4 | 18 | 2 | 17 | 2 | 2 |
| Significance (2-tailed) | Mean Difference | 95% Confidence Interval (Lower) | 95% Confidence Interval (Upper) | |
|---|---|---|---|---|
| Mean Age at baseline | 0.000 | 62.5200 | Lower | Upper |
| Male | 0.006 | 11.800 | 4.41 | 19.19 |
| Ex-smoker (n) | 0.007 | 11.000 | 3.86 | 18.14 |
| Never-smoked (n) | 0.000 | 7.900 | 4.82 | 10.98 |
| Current smoker (n) | 0.022 | 3.,000 | .61 | 5..39 |
| CTD-ILD (n = 25) | COP (n = 28) | IPF (n = 51) | COPD (n = 23) | |
|---|---|---|---|---|
| VC (% predicted), mean value ± SD | 57.33 ± 6.51 | 87.38 ± 21.70 | 65.58 ± 17.46 | 87.00 ± 17.35 |
| FVC (% predicted), mean value ± SD | 50.67 ± 11.37 | 74.88 ± 24.89 | 57.33 ± 17.58 | 66.00 ± 23.52 |
| FEV 1 (% predicted), mean value ± SD | 52.67 ± 22.03 | 80.63 ± 30.31 | 62.13 ± 20.04 | 55.67 ± 18.01 |
| DLCO (% predicted), mean value ± SD | 49.67 ± 9.50 | 72.88 ± 14.87 | 56.71 ± 19.91 | 72.67 ± 25.82 |
| pO2 (mm Hg) at rest, mean value ± SD | 66.50 ± 13.94 | 74.42 ± 4.69 | 68.90 ± 9.07 | 65.03 ± 9.19 |
| 6MWD (meters), mean value ± SD | 180 ± 158.74 | 386.25 ± 98.12 | 395.42 ± 106.65 | 320 ± 183.30 |
| Groups | Number (n) | Sensitivity (%) | Specificity (%) | AUC | MCC |
|---|---|---|---|---|---|
| IPF vs. HC | 51 vs. 33 | 0.88 | 0.85 | 0.95 | 0.73 |
| CTD-ILD vs. HC | 25 vs. 33 | 0.84 | 0.85 | 0.9 | 0.69 |
| COP vs. HC | 28 vs.33 | 0.86 | 0.82 | 0.89 | 0.67 |
| COPD vs. HC | 23 vs. 33 | 0.86 | 0.88 | 0.91 | 0.73 |
| COP vs. COPD | 28 vs. 23 | 0.75 | 0.71 | 0.77 | 0.46 |
| CTD-ILD vs. COPD | 25 vs. 23 | 0.88 | 0.71 | 0.85 | 0.61 |
| IPF vs. COP | 51 vs. 28 | 0.84 | 0.64 | 0.82 | 0.49 |
| IPF vs. CTD-ILD | 51 vs.25 | 0.86 | 0.64 | 0.84 | 0.55 |
| COP vs. CTD-ILD | 28 vs. 25 | 0.82 | 0.56 | 0.75 | 0.40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krauss, E.; Haberer, J.; Maurer, O.; Barreto, G.; Drakopanagiotakis, F.; Degen, M.; Seeger, W.; Guenther, A. Exploring the Ability of Electronic Nose Technology to Recognize Interstitial Lung Diseases (ILD) by Non-Invasive Breath Screening of Exhaled Volatile Compounds (VOC): A Pilot Study from the European IPF Registry (eurIPFreg) and Biobank. J. Clin. Med. 2019, 8, 1698. https://doi.org/10.3390/jcm8101698
Krauss E, Haberer J, Maurer O, Barreto G, Drakopanagiotakis F, Degen M, Seeger W, Guenther A. Exploring the Ability of Electronic Nose Technology to Recognize Interstitial Lung Diseases (ILD) by Non-Invasive Breath Screening of Exhaled Volatile Compounds (VOC): A Pilot Study from the European IPF Registry (eurIPFreg) and Biobank. Journal of Clinical Medicine. 2019; 8(10):1698. https://doi.org/10.3390/jcm8101698
Chicago/Turabian StyleKrauss, Ekaterina, Jana Haberer, Olga Maurer, Guillermo Barreto, Fotios Drakopanagiotakis, Maria Degen, Werner Seeger, and Andreas Guenther. 2019. "Exploring the Ability of Electronic Nose Technology to Recognize Interstitial Lung Diseases (ILD) by Non-Invasive Breath Screening of Exhaled Volatile Compounds (VOC): A Pilot Study from the European IPF Registry (eurIPFreg) and Biobank" Journal of Clinical Medicine 8, no. 10: 1698. https://doi.org/10.3390/jcm8101698
APA StyleKrauss, E., Haberer, J., Maurer, O., Barreto, G., Drakopanagiotakis, F., Degen, M., Seeger, W., & Guenther, A. (2019). Exploring the Ability of Electronic Nose Technology to Recognize Interstitial Lung Diseases (ILD) by Non-Invasive Breath Screening of Exhaled Volatile Compounds (VOC): A Pilot Study from the European IPF Registry (eurIPFreg) and Biobank. Journal of Clinical Medicine, 8(10), 1698. https://doi.org/10.3390/jcm8101698






