Innate Immunity and Alcohol
Abstract
:1. Introduction
2. Experimental Section
3. Innate Immunity Factors—Preserved through Time
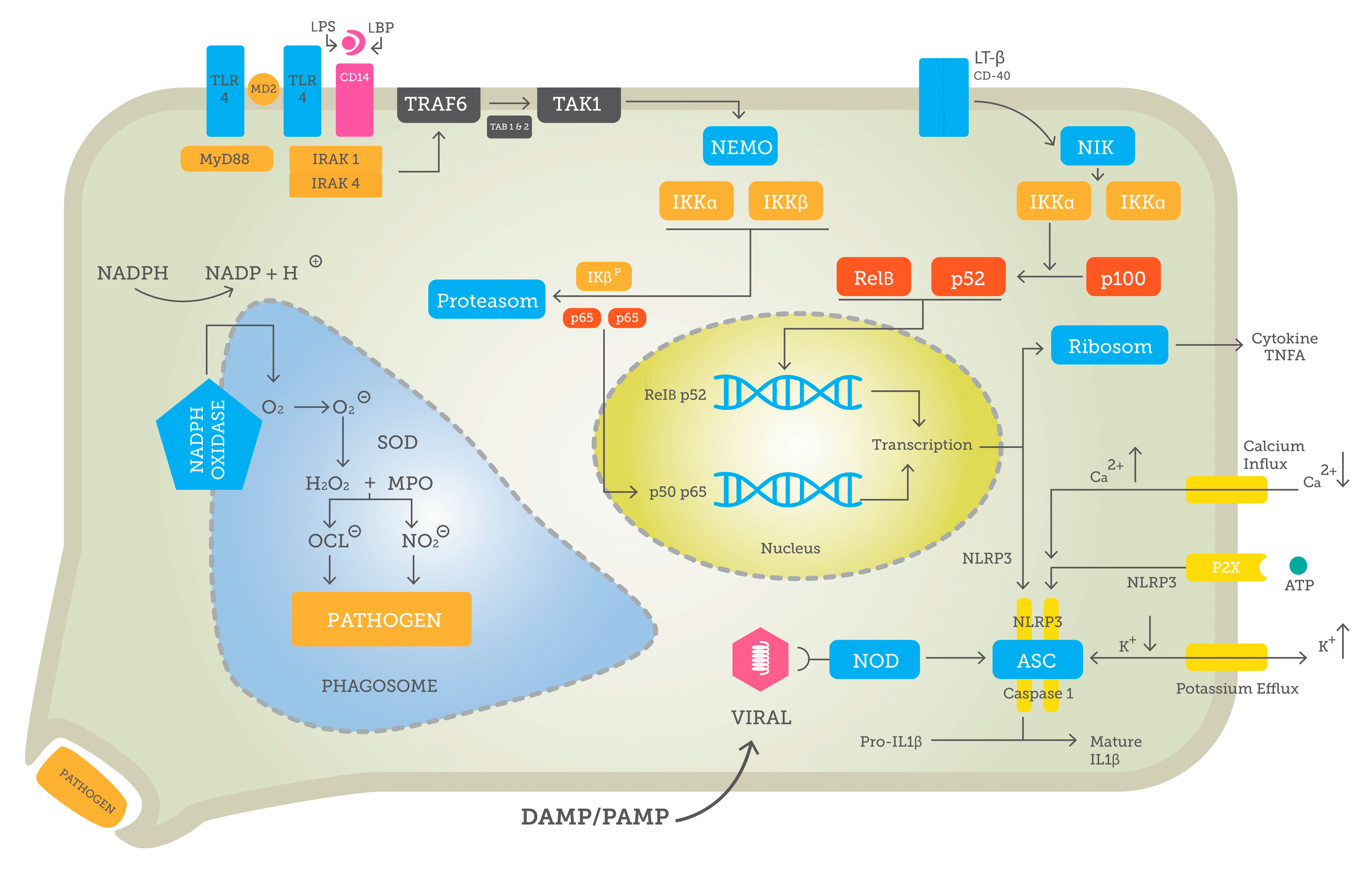
3.1. Pattern Recognition and Downstream Signaling
3.2. NF-κB—Key to Inflammation
4. Pathogen-Associated and Sterile Inflammation
5. Cytokines
6. Cellular Responses
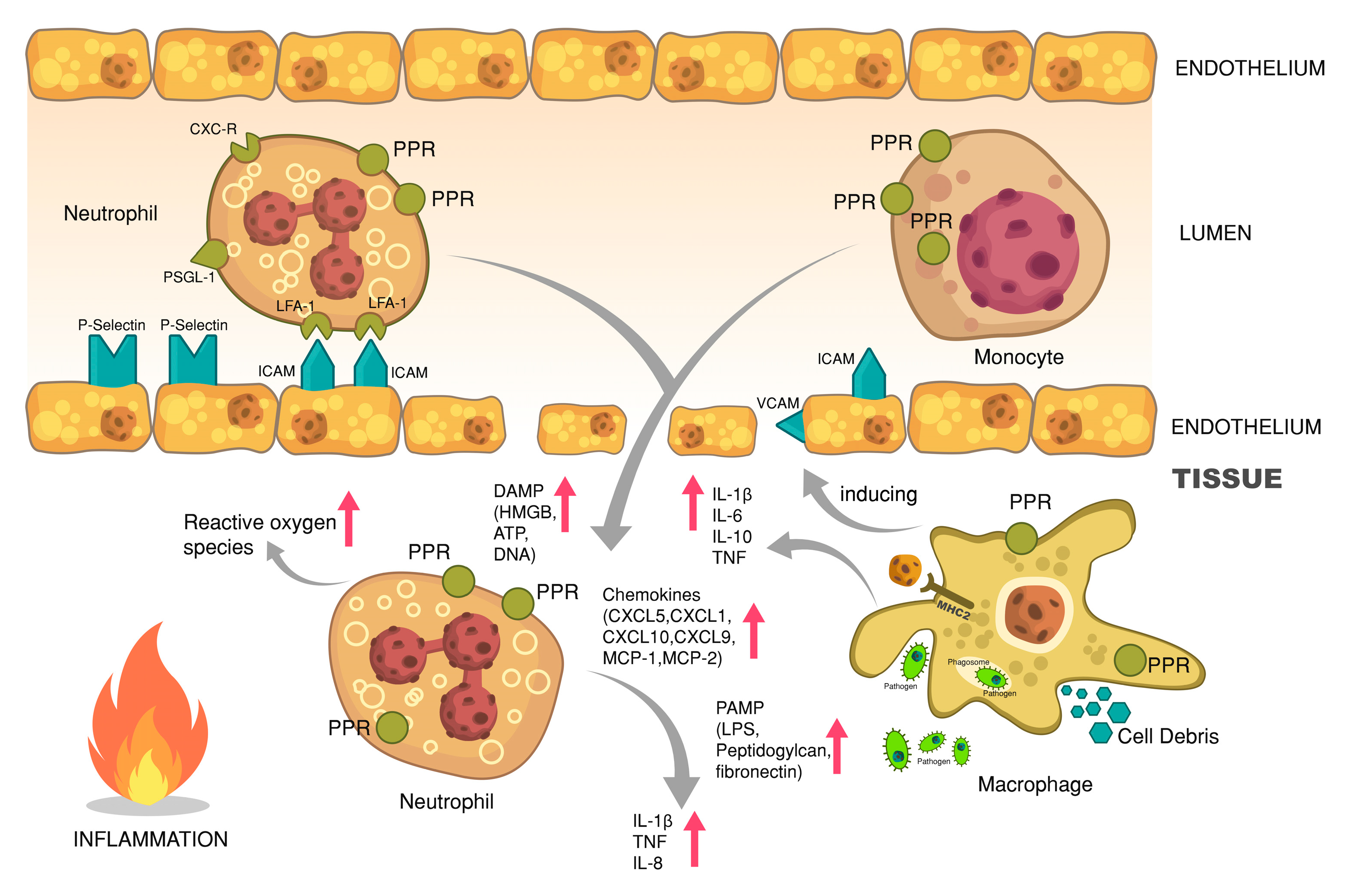
6.1. Cellular Responses—Phagocytosis and Oxidative Burst
6.2. Cellular Responses—Leukocyte Recruitment and Extravasation
7. Structural Responses
8. Limitations
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rehm, J.; Room, R.; Graham, K.; Monteiro, M.; Gmel, G.; Sempos, C.T. The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease: An overview. Addiction 2003, 98, 1209–1228. [Google Scholar] [CrossRef]
- Hadfield, R.J.; Mercer, M.; Parr, M.J. Alcohol and drug abuse in trauma. Resuscitation 2001, 48, 25–36. [Google Scholar] [CrossRef]
- Bagnardi, V.; Blangiardo, M.; La Vecchia, C.; Corrao, G. Alcohol consumption and the risk of cancer: A meta-analysis. Alcohol Res. Health 2001, 25, 263–271. [Google Scholar]
- Edwards, G. Alcohol policy and the public good. Addiction 1997, 92, S73–S79. [Google Scholar] [CrossRef]
- Doll, R.; Peto, R.; Hall, E.; Wheatley, K.; Gray, R. Mortality in relation to consumption of alcohol: 13 years’ observations on male British doctors. BMJ Br. Med. J. 1994, 309, 911–918. [Google Scholar] [CrossRef]
- Rehm, J.T.; Bondy, S.J.; Sempos, C.T.; Vuong, C.V. Alcohol consumption and coronary heart disease morbidity and mortality. Am. J. Epidemiol. 1997, 146, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. Ethanol and hormesis. Crit. Rev. Toxicol. 2003, 33, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Khabsa, M.; Giles, C.L. The number of scholarly documents on the public web. PLoS ONE 2014. [Google Scholar] [CrossRef]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997, 388, 394–397. [Google Scholar] [CrossRef]
- Janeway, C.A. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989, 54, 1–13. [Google Scholar] [CrossRef]
- Häcker, G.; Redecke, V.; Häcker, H. Activation of the immune system by bacterial CpG-DNA. Immunology 2002, 105, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Akashi, S.; Nagafuku, M.; Ogata, M.; Iwakura, Y.; Akira, S.; Kitamura, T.; Kosugi, A.; Kimoto, M.; Miyake, K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 2002, 3, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. TLR signaling. Cell Death Differ. 2006, 13, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Warner, N.; Viani, K.; Nuñez, G. Function of Nod-like receptors in microbial recognition and host defense. Immunol. Rev. 2009, 227, 106–128. [Google Scholar] [CrossRef] [PubMed]
- Creagh, E.M.; O’Neill, L.A.J. TLRs, NLRs and RLRs: A trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006, 27, 352–357. [Google Scholar] [CrossRef]
- Delgado, M.A.; Elmaoued, R.A.; Davis, A.S.; Kyei, G.; Deretic, V. Toll-like receptors control autophagy. EMBO J. 2008, 27, 1110–1121. [Google Scholar] [CrossRef]
- Gay, N.J.; Packman, L.C.; Weldon, M.A.; Barna, J.C. A leucine-rich repeat peptide derived from the Drosophila Toll receptor forms extended filaments with a beta-sheet structure. FEBS Lett. 1991, 291, 87–91. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef]
- Nishiyama, D.; Ikejima, K.; Honda, H.; Hirose, M.; Takei, Y.; Sato, N. Acute ethanol administration down-regulates toll-like receptor-4 in the murine liver. Hepatol. Res. 2002, 23, 130–137. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, J.; Li, J.; Wang, H.; Tang, C. Acute ethanol administration inhibits Toll-like receptor 4 signaling pathway in rat intestinal epithelia. Alcohol 2013, 47, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Bhatty, M.; Jan, B.L.; Tan, W.; Pruett, S.B.; Nanduri, B. Role of acute ethanol exposure and TLR4 in early events of sepsis in a mouse model. Alcohol 2011, 45, 795–803. [Google Scholar] [CrossRef]
- Fernandez-Lizarbe, S.; Montesinos, J.; Guerri, C. Ethanol induces TLR4/TLR2 association, triggering an inflammatory response in microglial cells. J. Neurochem. 2013, 126, 261–273. [Google Scholar] [CrossRef]
- Goral, J.; Kovacs, E.J. In vivo ethanol exposure down-regulates TLR2-, TLR4-, and TLR9-mediated macrophage inflammatory response by limiting p38 and ERK1/2 activation. J. Immunol. 2005, 174, 456–463. [Google Scholar] [CrossRef]
- Szabo, G.; Mandrekar, P.; Girouard, L.; Catalano, D. Regulation of human monocyte functions by acute ethanol treatment: Decreased tumor necrosis factor-alpha, interleukin-1 beta and elevated interleukin-10, and transforming growth factor-beta production. Alcohol Clin. Exp. Res. 1996, 20, 900–907. [Google Scholar] [CrossRef]
- Oak, S.; Mandrekar, P.; Catalano, D.; Kodys, K.; Szabo, G. TLR2-and TLR4-mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J. Immunol. 2006, 176, 7628–7635. [Google Scholar] [CrossRef]
- Pang, M.; Bala, S.; Kodys, K.; Catalano, D.; Szabo, G. Inhibition of TLR8- and TLR4-induced Type I IFN induction by alcohol is different from its effects on inflammatory cytokine production in monocytes. BMC Immunol. 2011, 12, 55. [Google Scholar] [CrossRef]
- Gustot, T.; Lemmers, A.; Moreno, C.; Nagy, N.; Quertinmont, E.; Nicaise, C.; Franchimont, D.; Louis, H.; Devière, J.; Le Moine, O. Differential liver sensitization to Toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology 2006, 43, 989–1000. [Google Scholar] [CrossRef]
- Byun, J.-S.; Suh, Y.-G.; Yi, H.-S.; Lee, Y.-S.; Jeong, W.-I. Activation of toll-like receptor 3 attenuates alcoholic liver injury by stimulating Kupffer cells and stellate cells to produce interleukin-10 in mice. J. Hepatol. 2013, 58, 342–349. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Hoshino, K.; Kaisho, T.; Sanjo, H.; Takeuchi, O.; Sugiyama, M.; Okabe, M.; Takeda, K.; et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 2003, 301, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Xiong, J.; Takeuchi, M.; Kurama, T.; Goeddel, D.V. TRAF6 is a signal transducer for interleukin-1. Nature 1996, 383, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Deng, L.; Hong, M.; Akkaraju, G.R.; Inoue, J.; Chen, Z.J. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 2001, 412, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, D.; Yeh, W.-C.; Wakeham, A.; Rudolph, B.; Nallainathan, D.; Potter, J.; Elia, A.J.; Mak, T.W. Severe liver degeneration and lack of NF-κB activation in NEMO/IKKγ-deficient mice. Genes Dev. 2000, 14, 854–862. [Google Scholar]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Huang, T.T.; Kudo, N.; Yoshida, M.; Miyamoto, S. A nuclear export signal in the N-terminal regulatory domain of IkappaBalpha controls cytoplasmic localization of inactive NF-kappaB/IkappaBalpha complexes. Proc. Natl. Acad. Sci. USA 2000, 97, 1014–1019. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.; Harhaj, E.W.; Sun, S.C. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol. Cell 2001, 7, 401–409. [Google Scholar] [CrossRef]
- Zhao, Y.; Lei, M.; Wang, Z.; Qiao, G.; Yang, T.; Zhang, J. TCR-induced, PKC-θ-mediated NF-κB activation is regulated by a caspase-8-caspase-9-caspase-3 cascade. Biochem. Biophys. Res. Commun. 2014, 450, 526–531. [Google Scholar] [CrossRef] [Green Version]
- Claudio, E.; Brown, K.; Park, S.; Wang, H.; Siebenlist, U. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat. Immunol. 2002, 3, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Novack, D.V.; Yin, L.; Hagen-Stapleton, A.; Schreiber, R.D.; Goeddel, D.V.; Ross, F.P.; Teitelbaum, S.L. The IkappaB function of NF-kappaB2 p100 controls stimulated osteoclastogenesis. J. Exp. Med. 2003, 198, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Remouchamps, C.; Dejardin, E. Methods to assess the activation of the alternative (noncanonical) NF-κB pathway by non-death TNF receptors. Methods Mol. Biol. 2015, 1280, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Yin, L.; Wu, L.; Wesche, H.; Arthur, C.D.; White, J.M.; Goeddel, D.V.; Schreiber, R.D. Defective lymphotoxin-beta receptor-induced NF-kappaB transcriptional activity in NIK-deficient mice. Science 2001, 291, 2162–2165. [Google Scholar] [CrossRef]
- Senftleben, U.; Cao, Y.; Xiao, G.; Greten, F.R.; Krähn, G.; Bonizzi, G.; Chen, Y.; Hu, Y.; Fong, A.; Sun, S.C.; et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 2001, 293, 1495–1499. [Google Scholar] [CrossRef]
- Matsushima, A.; Kaisho, T.; Rennert, P.D.; Nakano, H.; Kurosawa, K.; Uchida, D.; Takeda, K.; Akira, S.; Matsumoto, M. Essential Role of Nuclear Factor (NF)-κB–Inducing Kinase and Inhibitor of κb (Iκb) Kinase α in Nf-κb Activation through Lymphotoxin β Receptor, but Not through Tumor Necrosis Factor Receptor I. J. Exp. Med. 2001, 193, 631–636. [Google Scholar] [CrossRef]
- Crews, F.T.; Vetreno, R.P. Addiction, adolescence, and innate immune gene induction. Front. Psychiatry 2011, 2, 19. [Google Scholar] [CrossRef] [Green Version]
- Crews, F.T.; Zou, J.; Qin, L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav. Immun. 2011, 25 (Suppl. 1), S4–S12. [Google Scholar] [CrossRef] [Green Version]
- Joosten, M.M.; van Erk, M.J.; Pellis, L.; Witkamp, R.F.; Hendriks, H.F. Moderate alcohol consumption alters both leucocyte gene expression profiles and circulating proteins related to immune response and lipid metabolism in men. Br. J. Nutr. 2012, 108, 620–627. [Google Scholar] [CrossRef] [Green Version]
- Barr, T.; Girke, T.; Sureshchandra, S.; Nguyen, C.; Grant, K.; Messaoudi, I. Alcohol Consumption Modulates Host Defense in Rhesus Macaques by Altering Gene Expression in Circulating Leukocytes. J. Immunol. 2016, 196, 182–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sureshchandra, S.; Rais, M.; Stull, C.; Grant, K.; Messaoudi, I. Transcriptome Profiling Reveals Disruption of Innate Immunity in Chronic Heavy Ethanol Consuming Female Rhesus Macaques. PLoS ONE 2016, 11, e0159295. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.S.; Cantrell, C.H.; Leingang, K.A. Inhibition of the Kupffer Cell Inflammatory Response by Acute Ethanol: NF-κB Activation and Subsequent Cytokine Production. Biochem. Biophys. Res. Commun. 1996, 225, 134–140. [Google Scholar] [CrossRef]
- Zakhari, S.; Szabo, G. NF-kB, a Prototypical Cytokine-Regulated Transcription Factor: Implications for Alcohol-Mediated Responses. Alcohol. Clin. Exp. Res. 1996, 20, 236a–242a. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, P.; Catalano, D.; Szabo, G. Alcohol-induced regulation of nuclear regulatory factor-kappa beta in human monocytes. Alcohol Clin. Exp. Res. 1997, 21, 988–994. [Google Scholar]
- Rappa, G.; Anzanello, F.; Lorico, A. Ethanol induces upregulation of the nerve growth factor receptor CD271 in human melanoma cells via nuclear factor-κB activation. Oncol. Lett. 2015, 10, 815–821. [Google Scholar] [CrossRef] [Green Version]
- Szabo, G.; Mandrekar, P. Ethanol-mediated regulation of transcription factors in immunocompetent cells. Front. Biosci. 2002, 7, a80–a89. [Google Scholar] [CrossRef]
- Mandrekar, P.; Catalano, D.; White, B.; Szabo, G. Moderate alcohol intake in humans attenuates monocyte inflammatory responses: Inhibition of nuclear regulatory factor kappa B and induction of interleukin 10. Alcohol Clin. Exp. Res. 2006, 30, 135–139. [Google Scholar] [CrossRef]
- Pruett, S.B.; Fan, R. Ethanol inhibits LPS-induced signaling and modulates cytokine production in peritoneal macrophages in vivo in a model for binge drinking. BMC Immunol. 2009, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.E.; Odden, A.R.; Jeyaseelan, S.; Zhang, P.; Bagby, G.J.; Nelson, S.; Happel, K.I. Ethanol exposure impairs LPS-induced pulmonary LIX expression: Alveolar epithelial cell dysfunction as a consequence of acute intoxication. Alcohol. Clin. Exp. Res. 2009, 33, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Maraslioglu, M.; Oppermann, E.; Blattner, C.; Weber, R.; Henrich, D.; Jobin, C.; Schleucher, E.; Marzi, I.; Lehnert, M. Chronic ethanol feeding modulates inflammatory mediators, activation of nuclear factor-kappaB, and responsiveness to endotoxin in murine Kupffer cells and circulating leukocytes. Mediat. Inflamm. 2014, 2014, 808695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Relja, B.; Weber, R.; Maraslioglu, M.; Wagner, N.; Borsello, T.; Jobin, C.; Marzi, I.; Lehnert, M. Differential Relevance of NF-κB and JNK in the Pathophysiology of Hemorrhage/Resususcitation-Induced Liver Injury after Chronic Ethanol Feeding. PLoS ONE 2015, 10, e0137875. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, P.; Bala, S.; Catalano, D.; Kodys, K.; Szabo, G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J. Immunol. 2009, 183, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Yamashina, S.; Takei, Y.; Ikejima, K.; Enomoto, N.; Kitamura, T.; Sato, N. Ethanol-induced sensitization to endotoxin in Kupffer cells is dependent upon oxidative stress. Alcohol. Clin. Exp. Res. 2005, 29, 246S–250S. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, P. Tolerance, Danger, and the Extended Family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef]
- Zong, M.; Bruton, J.D.; Grundtman, C.; Yang, H.; Li, J.H.; Alexanderson, H.; Palmblad, K.; Andersson, U.; Harris, H.E.; Lundberg, I.E.; et al. TLR4 as receptor for HMGB1 induced muscle dysfunction in myositis. Ann. Rheum. Dis. 2013, 72, 1390–1399. [Google Scholar] [CrossRef] [Green Version]
- Seong, S.-Y.; Matzinger, P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004, 4, 469–478. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Bianchi, M.E.; Agresti, A. HMG proteins: Dynamic players in gene regulation and differentiation. Curr. Opin. Genet. Dev. 2005, 15, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Shi, Y.; Kang, R.; Li, T.; Xiao, W.; Wang, H.; Xiao, X. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J. Leukoc. Biol. 2007, 81, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Wang, H.; Yuan, R.; Li, H.; Ochani, M.; Ochani, K.; Rosas-Ballina, M.; Czura, C.J.; Huston, J.M.; Miller, E.; et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J. Exp. Med. 2006, 203, 1637–1642. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wang, H.; Chavan, S.S.; Andersson, U. High Mobility Group Box Protein 1 (HMGB1): The Prototypical Endogenous Danger Molecule. Mol. Med. 2015, 21, S6–S12. [Google Scholar] [CrossRef]
- Lu, B.; Antoine, D.J.; Kwan, K.; Lundbäck, P.; Wähämaa, H.; Schierbeck, H.; Robinson, M.; Van Zoelen, M.A.D.; Yang, H.; Li, J.; et al. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc. Natl. Acad. Sci. USA 2014, 111, 3068–3073. [Google Scholar] [CrossRef] [Green Version]
- Bours, M.J.L.; Swennen, E.L.R.; Di Virgilio, F.; Cronstein, B.N.; Dagnelie, P.C. Adenosine 5’-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 2006, 112, 358–404. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic nerves. Pharm. Rev. 1972, 24, 509–581. [Google Scholar]
- Sattin, A.; Rall, T.W. The effect of adenosine and adenine nucleotides on the cyclic adenosine 3’, 5’-phosphate content of guinea pig cerebral cortex slices. Mol. Pharm. 1970, 6, 13–23. [Google Scholar]
- Li, M.; Silberberg, S.D.; Swartz, K.J. Subtype-specific control of P2X receptor channel signaling by ATP and Mg2+. Proc. Natl. Acad. Sci. USA 2013, 110, E3455–E3463. [Google Scholar] [CrossRef] [Green Version]
- Riteau, N.; Gasse, P.; Fauconnier, L.; Gombault, A.; Couegnat, M.; Fick, L.; Kanellopoulos, J.; Quesniaux, V.F.J.; Marchand-Adam, S.; Crestani, B.; et al. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 2010, 182, 774–783. [Google Scholar] [CrossRef]
- Boe, D.M.; Nelson, S.; Zhang, P.; Bagby, G.J. Acute ethanol intoxication suppresses lung chemokine production following infection with Streptococcus pneumoniae. J. Infect. Dis. 2001, 184, 1134–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.; Behara, R.; Swanson, G.; Forsyth, C.; Voigt, R.; Keshavarzian, A. Alcohol and the Intestine. Biomolecules 2015, 5, 2573–2588. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Marcos, M.; Gattu, A.; Catalano, D.; Szabo, G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS ONE 2014, 9, e96864. [Google Scholar] [CrossRef] [PubMed]
- Jong, G.M.; Hsiue, T.R.; Chen, C.R.; Chang, H.Y.; Chen, C.W. Rapidly fatal outcome of bacteremic Klebsiella pneumoniae pneumonia in alcoholics. Chest 1995, 107, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Griffin, R.; Poe, A.M.; Cross, J.M.; Rue, L.W.; McGwin, G. The Association Between Blood Alcohol Level and Infectious Complications Among Burn Patients. J. Burn Care Res. 2009, 30, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Bull-Otterson, L.; Feng, W.; Kirpich, I.; Wang, Y.; Qin, X.; Liu, Y.; Gobejishvili, L.; Joshi-Barve, S.; Ayvaz, T.; Petrosino, J.; et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS ONE 2013, 8, e53028. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Farhadi, A.; Forsyth, C.B.; Rangan, J.; Jakate, S.; Shaikh, M.; Banan, A.; Fields, J.Z. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J. Hepatol. 2009, 50, 538–547. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.J.; Zakhari, S.; Jung, M.K. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J. Gastroenterol. 2010, 16, 1304–1313. [Google Scholar] [CrossRef]
- Faunce, D.E.; Llanas, J.N.; Patel, P.J.; Gregory, M.S.; Duffner, L.A.; Kovacs, E.J. Neutrophil chemokine production in the skin following scald injury. Burns 1999, 25, 403–410. [Google Scholar] [CrossRef]
- Kavanaugh, M.J.; Clark, C.; Goto, M.; Kovacs, E.J.; Gamelli, R.L.; Sayeed, M.M.; Choudhry, M.A. Effect of acute alcohol ingestion prior to burn injury on intestinal bacterial growth and barrier function. Burns 2005, 31, 290–296. [Google Scholar] [CrossRef]
- Wang, X.; Chu, G.; Yang, Z.; Sun, Y.; Zhou, H.; Li, M.; Shi, J.; Tian, B.; Zhang, C.; Meng, X. Ethanol directly induced HMGB1 release through NOX2/NLRP1 inflammasome in neuronal cells. Toxicology 2015, 334, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Asatryan, L.; Khoja, S.; Rodgers, K.E.; Alkana, R.L.; Tsukamoto, H.; Davies, D.L. Chronic ethanol exposure combined with high fat diet up-regulates P2X7 receptors that parallels neuroinflammation and neuronal loss in C57BL/6J mice. J. Neuroimmunol. 2015, 285, 169–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, D.L.; Kochegarov, A.A.; Kuo, S.T.; Kulkarni, A.A.; Woodward, J.J.; King, B.F.; Alkana, R.L. Ethanol differentially affects ATP-gated P2X(3) and P2X(4) receptor subtypes expressed in Xenopus oocytes. Neuropharmacology 2005, 49, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Syed, N.-i.-H.; Kennedy, C. Pharmacology of P2X receptors. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 16–30. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Schaper, F.; Rose-John, S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 2015, 26, 475–487. [Google Scholar] [CrossRef]
- Hurst, S.M.; Wilkinson, T.S.; McLoughlin, R.M.; Jones, S.; Horiuchi, S.; Yamamoto, N.; Rose-John, S.; Fuller, G.M.; Topley, N.; Jones, S.A. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 2001, 14, 705–714. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Fisher, D.T.; Clancy, K.A.; Gauguet, J.-M.M.; Wang, W.-C.; Unger, E.; Rose-John, S.; von Andrian, U.H.; Baumann, H.; Evans, S.S. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat. Immunol. 2006, 7, 1299–1308. [Google Scholar] [CrossRef]
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.-Y.; Vallabhapurapu, S.; Scheller, J.; Rose-John, S.; Cheroutre, H.; Eckmann, L.; et al. IL-6 and Stat3 Are Required for Survival of Intestinal Epithelial Cells and Development of Colitis-Associated Cancer. Cancer Cell 2009, 15, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Neuhöfer, P.; Song, L.; Rabe, B.; Lesina, M.; Kurkowski, M.U.; Treiber, M.; Wartmann, T.; Regnér, S.; Thorlacius, H.; et al. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J. Clin. Investig. 2013, 123, 1019–1031. [Google Scholar] [CrossRef] [Green Version]
- Luig, M.; Kluger, M.A.; Goerke, B.; Meyer, M.; Nosko, A.; Yan, I.; Scheller, J.; Mittrucker, H.W.; Rose-John, S.; Stahl, R.A.K.; et al. Inflammation-Induced IL-6 Functions as a Natural Brake on Macrophages and Limits GN. J. Am. Soc. Nephrol. 2015, 26, 1597–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinarello, C.A.; Goldin, N.P.; Wolff, S.M. Demonstration and Characterization of Two Distinct Human Leukocytic Pyrogens. J. Exp. Med. 1974, 139, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Inukai, Y.; Takii, T.; Furutani, Y.; Shibata, Y.; Hayashi, H.; Sakurada, S.; Okamoto, T.; Inoue, J.; Oomoto, Y.; et al. Molecular analysis of constitutive IL-1alpha gene expression in human melanoma cells: Autocrine stimulation through NF-kappaB activation by endogenous IL-1alpha. Cytokine 1998, 10, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Agostini, L.; Martinon, F.; Burns, K.; McDermott, M.F.; Hawkins, P.N.; Tschopp, J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 2004, 20, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef] [PubMed]
- Horai, R.; Asano, M.; Sudo, K.; Kanuka, H.; Suzuki, M.; Nishihara, M.; Takahashi, M.; Iwakura, Y. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J. Exp. Med. 1998, 187, 1463–1475. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A.; Donath, M.Y.; Mandrup-Poulsen, T. Role of IL-1beta in type 2 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Carswell, E.A.; Old, L.J.; Kassel, R.L.; Green, S.; Fiore, N.; Williamson, B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl. Acad. Sci. USA 1975, 72, 3666–3670. [Google Scholar] [CrossRef] [Green Version]
- Zelová, H.; Hošek, J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Zhou, T.; Mountz, J.D.; Kimberly, R.P. Immunobiology of tumor necrosis factor receptor superfamily. Immunol. Res. 2002, 26, 323–336. [Google Scholar] [CrossRef]
- Tracey, D.; Klareskog, L.; Sasso, E.H.; Salfeld, J.G.; Tak, P.P. Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharm. Ther. 2008, 117, 244–279. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, K.; Matsuyama, T.; Kündig, T.M.; Wakeham, A.; Kishihara, K.; Shahinian, A.; Wiegmann, K.; Ohashi, P.S.; Krönke, M.; Mak, T.W. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 1993, 73, 457–467. [Google Scholar] [CrossRef]
- Mootoo, A.; Stylianou, E.; Arias, M.A.; Reljic, R. TNF-alpha in tuberculosis: A cytokine with a split personality. Inflamm. Allergy Drug Targets 2009, 8, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, D.F.; Bond, M.W.; Mosmann, T.R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989, 170, 2081–2095. [Google Scholar] [CrossRef] [PubMed]
- Siewe, L.; Bollati-Fogolin, M.; Wickenhauser, C.; Krieg, T.; Müller, W.; Roers, A. Interleukin-10 derived from macrophages and/or neutrophils regulates the inflammatory response to LPS but not the response to CpG DNA. Eur. J. Immunol. 2006, 36, 3248–3255. [Google Scholar] [CrossRef] [PubMed]
- Geginat, J.; Larghi, P.; Paroni, M.; Nizzoli, G.; Penatti, A.; Pagani, M.; Gagliani, N.; Meroni, P.; Abrignani, S.; Flavell, R.A. The light and the dark sides of Interleukin-10 in immune-mediated diseases and cancer. Cytokine Growth Factor Rev. 2016, 30, 87–93. [Google Scholar] [CrossRef]
- Chaudhry, A.; Samstein, R.M.; Treuting, P.; Liang, Y.; Pils, M.C.; Heinrich, J.M.; Jack, R.S.; Wunderlich, F.T.; Bruning, J.C.; Muller, W.; et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 2011, 34, 566–578. [Google Scholar] [CrossRef] [Green Version]
- Sabat, R.; Grütz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginat, J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010, 21, 331–344. [Google Scholar] [CrossRef] [Green Version]
- Martire-Greco, D.; Rodriguez-Rodrigues, N.; Landoni, V.I.; Rearte, B.; Isturiz, M.A.; Fernández, G.C. Interleukin-10 controls human peripheral PMN activation triggered by lipopolysaccharide. Cytokine 2013, 62, 426–432. [Google Scholar] [CrossRef]
- Huet, O.; Laemmel, E.; Fu, Y.; Dupic, L.; Aprico, A.; Andrews, K.L.; Moore, X.L.; Harrois, A.; Meikle, P.L.; Vicaut, E.; et al. IL-10 anti oxidant effect decreases leukocytes/ endothelial interaction induced by TNF-α. Shock 2013, 1. [Google Scholar] [CrossRef] [PubMed]
- Baggiolini, M.; Clark-Lewis, I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992, 307, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, E.; Dittrich-Breiholz, O.; Holtmann, H.; Kracht, M. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 2002, 72, 847–855. [Google Scholar] [PubMed]
- Lehner, M.; Morhart, P.; Stilper, A.; Petermann, D.; Weller, P.; Stachel, D.; Holter, W. Efficient chemokine-dependent migration and primary and secondary IL-12 secretion by human dendritic cells stimulated through Toll-like receptors. J. Immunother. 2007, 30, 312–322. [Google Scholar] [CrossRef]
- Hol, J.; Küchler, A.M.; Johansen, F.-E.; Dalhus, B.; Haraldsen, G.; Oynebråten, I. Molecular requirements for sorting of the chemokine interleukin-8/CXCL8 to endothelial Weibel–Palade bodies. J. Biol. Chem. 2009, 284, 23532–23539. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, Y. The role of chemokines in neutrophil biology. Front. Biosci. 2008, 13, 2400–2407. [Google Scholar] [CrossRef] [Green Version]
- Relja, B.; Omid, N.; Schaible, A.; Perl, M.; Meier, S.; Oppermann, E.; Lehnert, M.; Marzi, I. Pre-or post-treatment with ethanol and ethyl pyruvate results in distinct anti-inflammatory responses of human lung epithelial cells triggered by interleukin-6. Mol. Med. Rep. 2015, 12, 2991–2998. [Google Scholar] [CrossRef] [Green Version]
- Relja, B.; Omid, N.; Wagner, N.; Mors, K.; Werner, I.; Juengel, E.; Perl, M.; Marzi, I. Ethanol, ethyl and sodium pyruvate decrease the inflammatory responses of human lung epithelial cells via Akt and NF-kappaB in vitro but have a low impact on hepatocellular cells. Int. J. Mol. Med. 2016, 37, 517–525. [Google Scholar] [CrossRef]
- Pruett, S.B.; Zheng, Q.; Fan, R.; Matthews, K.; Schwab, C. Ethanol suppresses cytokine responses induced through Toll-like receptors as well as innate resistance to Escherichia coli in a mouse model for binge drinking. Alcohol 2004, 33, 147–155. [Google Scholar] [CrossRef]
- Massey, V.L.; Poole, L.G.; Siow, D.L.; Torres, E.; Warner, N.L.; Schmidt, R.H.; Ritzenthaler, J.D.; Roman, J.; Arteel, G.E. Chronic Alcohol Exposure Enhances Lipopolysaccharide-Induced Lung Injury in Mice: Potential Role of Systemic Tumor Necrosis Factor-Alpha. Alcohol. Clin. Exp. Res. 2015, 39, 1978–1988. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, C.; Andersen, O.; Krag, A.; Bendtsen, F.; Møller, S. High-sensitivity C-reactive protein levels predict survival and are related to haemodynamics in alcoholic cirrhosis. Eur. J. Gastroenterol. Hepatol. 2012, 24, 619–626. [Google Scholar] [CrossRef] [PubMed]
- González-Reimers, E.; Sánchez-Pérez, M.J.; Santolaria-Fernández, F.; Abreu-González, P.; De la Vega-Prieto, M.J.; Viña-Rodríguez, J.; Alemán-Valls, M.R.; Rodríguez-Gaspar, M. Changes in cytokine levels during admission and mortality in acute alcoholic hepatitis. Alcohol 2012, 46, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Khoruts, A.; Stahnke, L.; McClain, C.J.; Logan, G.; Allen, J.I. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology 1991, 13, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Afshar, M.; Richards, S.; Mann, D.; Cross, A.; Smith, G.B.; Netzer, G.; Kovacs, E.; Hasday, J. Acute immunomodulatory effects of binge alcohol ingestion. Alcohol 2015, 49, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neupane, S.P.; Skulberg, A.; Skulberg, K.R.; Aass, H.C.D.; Bramness, J.G. Cytokine Changes following Acute Ethanol Intoxication in Healthy Men: A Crossover Study. Mediat. Inflamm. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Bell, S.; Mehta, G.; Moore, K.; Britton, A. Ten-year alcohol consumption typologies and trajectories of C-reactive protein, interleukin-6 and interleukin-1 receptor antagonist over the following 12 years: A prospective cohort study. J. Intern. Med. 2017, 281, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Relja, B.; Menke, J.; Wagner, N.; Auner, B.; Voth, M.; Nau, C.; Marzi, I. Effects of positive blood alcohol concentration on outcome and systemic interleukin-6 in major trauma patients. Injury 2016, 47, 640–645. [Google Scholar] [CrossRef]
- Wagner, N.; Akbarpour, A.; Mors, K.; Voth, M.; Stormann, P.; Auner, B.; Lehnert, M.; Marzi, I.; Relja, B. Alcohol Intoxication Reduces Systemic Interleukin-6 Levels and Leukocyte Counts After Severe TBI Compared With Not Intoxicated TBI Patients. Shock 2016, 46, 261–269. [Google Scholar] [CrossRef]
- Bird, M.D.; Zahs, A.; Deburghgraeve, C.; Ramirez, L.; Choudhry, M.A.; Kovacs, E.J. Decreased pulmonary inflammation following ethanol and burn injury in mice deficient in TLR4 but not TLR2 signaling. Alcohol. Clin. Exp. Res. 2010, 34, 1733–1741. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Hamilton, J.L.; Bird, M.D.; Chen, M.M.; Ramirez, L.; Zahs, A.; Kovacs, E.J.; Makowski, L. Adipose Inflammation and Macrophage Infiltration After Binge Ethanol and Burn Injury. Alcohol. Clin. Exp. Res. 2014, 38, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Schwacha, M.G.; Chaudry, I.H.; Choudhry, M.A. Acute Alcohol Intoxication Potentiates Neutrophil-Mediated Intestinal Tissue Damage after Burn Injury. Shock 2007, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahs, A.; Bird, M.D.; Ramirez, L.; Turner, J.R.; Choudhry, M.A.; Kovacs, E.J. Inhibition of long myosin light-chain kinase activation alleviates intestinal damage after binge ethanol exposure and burn injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G705–G712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahs, A.; Bird, M.D.; Ramirez, L.; Choudhry, M.A.; Kovacs, E.J. Anti-IL-6 antibody treatment but not IL-6 knockout improves intestinal barrier function and reduces inflammation after binge ethanol exposure and burn injury. Shock 2013, 39, 373–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoyt, L.R.; Ather, J.L.; Randall, M.J.; DePuccio, D.P.; Landry, C.C.; Wewers, M.D.; Gavrilin, M.A.; Poynter, M.E. Ethanol and Other Short-Chain Alcohols Inhibit NLRP3 Inflammasome Activation through Protein Tyrosine Phosphatase Stimulation. J. Immunol. 2016, 197, 1322–1334. [Google Scholar] [CrossRef] [Green Version]
- Nurmi, K.; Virkanen, J.; Rajamäki, K.; Niemi, K.; Kovanen, P.T.; Eklund, K.K. Ethanol inhibits activation of NLRP3 and AIM2 inflammasomes in human macrophages—A novel anti-inflammatory action of alcohol. PLoS ONE 2013, 8, e78537. [Google Scholar] [CrossRef] [Green Version]
- Barros, F.R.; Castro-Faria-Neto, H.C.; Castro, C.L.; Aguiar Nemer, A.S.; Rocha, E.M.S.; Silva Fonseca, V.A. Effects of Chronic Ethanol Consumption in Experimental Sepsis. Alcohol Alcohol. 2012, 47, 677–682. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.-Y.; Jiang, Z.-A.; Zhao, C.-Y.; Zhen, Z.; Wang, W.; Nanji, A.A. Long-Term Binge and Escalating Ethanol Exposure Causes Necroinflammation and Fibrosis in Rat Liver. Alcohol. Clin. Exp. Res. 2013, 37, 213–222. [Google Scholar] [CrossRef]
- Glavind, E.; Vilstrup, H.; Grønbaek, H.; Hamilton-Dutoit, S.; Magnusson, N.E.; Thomsen, K.L. Long-Term Ethanol Exposure Decreases the Endotoxin-Induced Hepatic Acute Phase Response in Rats. Alcohol. Clin. Exp. Res. 2017, 41, 562–570. [Google Scholar] [CrossRef]
- O’Halloran, E.B.; Curtis, B.J.; Afshar, M.; Chen, M.M.; Kovacs, E.J.; Burnham, E.L. Alveolar macrophage inflammatory mediator expression is elevated in the setting of alcohol use disorders. Alcohol 2016, 50, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Asquith, M.; Pasala, S.; Engelmann, F.; Haberthur, K.; Meyer, C.; Park, B.; Grant, K.A.; Messaoudi, I. Chronic Ethanol Consumption Modulates Growth Factor Release, Mucosal Cytokine Production, and MicroRNA Expression in Nonhuman Primates. Alcohol. Clin. Exp. Res. 2014, 38, 980–993. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S. Phagocytosis: An Immunobiologic Process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Yona, S.; Kim, K.-W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef]
- Jakubzick, C.; Gautier, E.L.; Gibbings, S.L.; Sojka, D.K.; Schlitzer, A.; Johnson, T.E.; Ivanov, S.; Duan, Q.; Bala, S.; Condon, T.; et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity 2013, 39, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, D.; Chow, A.; Noizat, C.; Teo, P.; Beasley, M.B.; Leboeuf, M.; Becker, C.D.; See, P.; Price, J.; Lucas, D.; et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013, 38, 792–804. [Google Scholar] [CrossRef] [Green Version]
- Davies, L.C.; Taylor, P.R. Tissue-resident macrophages: Then and now. Immunology 2015, 144, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Flannagan, R.S.; Jaumouille, V.; Grinstein, S. The cell biology of phagocytosis. Annu. Rev. Pathol. 2012, 7, 61–98. [Google Scholar] [CrossRef]
- Parlato, M.; Cavaillon, J.-M. Host response biomarkers in the diagnosis of sepsis: A general overview. Methods Mol. Biol. 2015, 1237, 149–211. [Google Scholar] [CrossRef]
- Mildner, A.; Jung, S. Development and Function of Dendritic Cell Subsets. Immunity 2014, 40, 642–656. [Google Scholar] [CrossRef] [Green Version]
- Goodridge, H.S.; Underhill, D.M.; Touret, N. Mechanisms of Fc receptor and dectin-1 activation for phagocytosis. Traffic 2012, 13, 1062–1071. [Google Scholar] [CrossRef]
- Brown, G.D. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006, 6, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, A.M.; Brown, G.D. C-type lectins and phagocytosis. Immunobiology 2009, 214, 562–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Laan, L.J.; Döpp, E.A.; Haworth, R.; Pikkarainen, T.; Kangas, M.; Elomaa, O.; Dijkstra, C.D.; Gordon, S.; Tryggvason, K.; Kraal, G. Regulation and functional involvement of macrophage scavenger receptor MARCO in clearance of bacteria in vivo. J. Immunol. 1999, 162, 939–947. [Google Scholar] [PubMed]
- Tohyama, Y.; Yamamura, H. Complement-mediated phagocytosis--the role of Syk. Iubmb Life 2006, 58, 304–308. [Google Scholar] [CrossRef]
- Beningo, K.A.; Wang, Y.L. Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J. Cell Sci. 2002, 115, 849–856. [Google Scholar]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Hitt, N.D.; Kleinberg, M.E. Stoichiometry of p22-phox and gp91-phox in phagocyte cytochrome b558. Biochemistry 1995, 34, 16753–16757. [Google Scholar] [CrossRef]
- Wientjes, F.B.; Segal, A.W. NADPH oxidase and the respiratory burst. Semin. Cell Biol. 1995, 6, 357–365. [Google Scholar] [CrossRef]
- Borgstahl, G.E.; Parge, H.E.; Hickey, M.J.; Johnson, M.J.; Boissinot, M.; Hallewell, R.A.; Lepock, J.R.; Cabelli, D.E.; Tainer, J.A. Human mitochondrial manganese superoxide dismutase polymorphic variant Ile58Thr reduces activity by destabilizing the tetrameric interface. Biochemistry 1996, 35, 4287–4297. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Xie, G.-H.; Chen, Q.-X.; Cheng, B.-L.; Fang, X.-M. Defensins and sepsis. Biomed. Res. Int. 2014, 2014, 180109. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, D.J.; Radek, K.A.; Chaar, M.; Faunce, D.E.; DiPietro, L.A.; Kovacs, E.J. Effects of acute ethanol exposure on the early inflammatory response after excisional injury. Alcohol. Clin. Exp. Res. 2007, 31, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Pruett, S.B.; Fan, R.; Cheng, B.; Glover, M.; Tan, W.; Deng, X. Innate immunity and inflammation in sepsis: Mechanisms of suppressed host resistance in mice treated with ethanol in a binge-drinking model. Toxicol. Sci. 2010, 117, 314–324. [Google Scholar] [CrossRef]
- Jareo, P.W.; Preheim, L.C.; Gentry, M.J. Ethanol Ingestion Impairs Neutrophil Bactericidal Mechanisms against Streptococcus pneumoniae. Alcohol. Clin. Exp. Res. 1996, 20, 1646–1652. [Google Scholar] [CrossRef]
- Molina, P.E.; Zambell, K.L.; Norenberg, K.; Eason, J.; Phelan, H.; Zhang, P.; Stouwe, C.V.; Carnal, J.W.; Porreta, C. Consequences of alcohol-induced early dysregulation of responses to trauma/hemorrhage. Alcohol 2004, 33, 217–227. [Google Scholar] [CrossRef]
- Chiu, C.-H.; Wang, Y.-C.; Yeh, K.-M.; Lin, J.-C.; Siu, L.K.; Chang, F.-Y. Influence of ethanol concentration in the phagocytic function of neutrophils against Klebsiella pneumoniae isolates in an experimental model. J. Microbiol. Immunol. Infect. 2016. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Sun, A.; Simonyi, A.; Kalogeris, T.; Miller, D.; Sun, G.; Korthuis, R. Ethanol preconditioning protects against ischemia/reperfusion-induced brain damage: Role of NADPH oxidase-derived ROS. Free Radic. Biol. Med. 2007, 43, 1048–1060. [Google Scholar] [CrossRef] [Green Version]
- Thakur, V. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: Role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF- production. J. Leukoc. Biol. 2006, 79, 1348–1356. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Crews, F.T. Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. J. Neuroinflamm. 2012, 9. [Google Scholar] [CrossRef] [Green Version]
- Alfonso-Loeches, S.; Ureña-Peralta, J.; Morillo-Bargues, M.J.; Gómez-Pinedo, U.; Guerri, C. Ethanol-Induced TLR4/NLRP3 Neuroinflammatory Response in Microglial Cells Promotes Leukocyte Infiltration Across the BBB. Neurochem. Res. 2016, 41, 193–209. [Google Scholar] [CrossRef]
- He, J.; Crews, F.T. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp. Neurol. 2008, 210, 349–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Yeligar, S.M.; Brown, L.A.S. Chronic-alcohol-abuse-induced oxidative stress in the development of acute respiratory distress syndrome. Sci. World J. 2012, 2012, 740308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeligar, S.M.; Harris, F.L.; Hart, C.M.; Brown, L.A.S. Glutathione attenuates ethanol-induced alveolar macrophage oxidative stress and dysfunction by downregulating NADPH oxidases. AJP Lung Cell. Mol. Physiol. 2014, 306, L429–L441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeligar, S.M.; Mehta, A.J.; Harris, F.L.; Brown, L.A.S.; Hart, C.M. Peroxisome Proliferator–Activated Receptor γ Regulates Chronic Alcohol-Induced Alveolar Macrophage Dysfunction. Am. J. Respir. Cell Mol. Biol. 2016, 55, 35–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saikia, P.; Bellos, D.; McMullen, M.R.; Pollard, K.A.; de la Motte, C.; Nagy, L.E. miR181b-3p and its target importin α5 regulate TLR4 signaling in Kupffer cells and liver injury in mice in response to ethanol. Hepatology 2017. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, N.; Ikejima, K.; Bradford, B.; Rivera, C.; Kono, H.; Brenner, D.A.; Thurman, R.G. Alcohol causes both tolerance and sensitization of rat Kupffer cells via mechanisms dependent on endotoxin. Gastroenterology 1998, 115, 443–451. [Google Scholar] [CrossRef]
- Enomoto, N.; Ikejima, K.; Kitamura, T.; Oide, H.; Takei, Y.; Sato, N.; Thurman, R.G. Alcohol enhances lipopolysaccharide-induced increases in nitric oxide production by Kupffer cells via mechanisms dependent on endotoxin. Alcohol. Clin. Exp. Res. 2000, 24, 55S–58S. [Google Scholar] [CrossRef]
- Husain, K.; Ferder, L.; Ansari, R.A.; Lalla, J. Chronic ethanol ingestion induces aortic inflammation/oxidative endothelial injury and hypertension in rats. Hum. Exp. Toxicol. 2011, 30, 930–939. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Huang, H.; Zhang, Z.; Wang, F.S. The role of neutrophils in the development of liver diseases. Cell. Mol. Immunol. 2014, 11, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, L.; Sperandio, M.; Yago, T.; McDaniel, J.M.; Cummings, R.D.; Pearson-White, S.; Ley, K.; McEver, R.P. P-selectin glycoprotein ligand-1–deficient mice have impaired leukocyte tethering to E-selectin under flow. J. Clin. Investig. 2002, 109, 939–950. [Google Scholar] [CrossRef]
- Phillipson, M.; Heit, B.; Colarusso, P.; Liu, L.; Ballantyne, C.M.; Kubes, P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J. Exp. Med. 2006, 203, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.H.; Carman, C.V.; Springer, T.A. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007, 25, 619–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkos, C.A.I. Neutrophil adhesive interactions with intestinal epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 1997, 273, G763–G768. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.I.; Hu, Y.; Vestweber, D.; Smith, C.W. Neutrophil tethering on E-selectin activates β2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J. Immunol. 2000, 164, 4348–4358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taooka, Y.; Chen, J.; Yednock, T.; Sheppard, D. The integrin α9β1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J. Cell Biol. 1999, 145, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Woodfin, A.; Voisin, M.B.; Imhof, B.A.; Dejana, E.; Engelhardt, B.; Nourshargh, S. Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A, and PECAM-1. Blood 2009, 113, 6246–6257. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Place, A.T.; Chen, Z.; Brovkovych, V.M.; Vogel, S.M.; Muller, W.A.; . Skidgel, R.A.; Malik, A.B.; Minshall, R.D. ICAM-1–activated Src and eNOS signaling increase endothelial cell surface PECAM-1 adhesivity and neutrophil transmigration. Blood 2012, 120, 1942–1952. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, C.D.; Lim, P.; Sun, J.; Albelda, S.M. PECAM-1-dependent neutrophil transmigration is independent of monolayer PECAM-1 signaling or localization. Blood 2003, 101, 2816–2825. [Google Scholar] [CrossRef] [Green Version]
- Keck, T.; Balcom IV, J.H.; Fernández–Del Castillo, C.; Antoniu, B.A.; Warshaw, A.L. Matrix metalloproteinase-9 promotes neutrophil migration and alveolar capillary leakage in pancreatitis-associated lung injury in the rat. Gastroenterology 2002, 122, 188–201. [Google Scholar] [CrossRef]
- Gacouin, A.; Roussel, M.; Le Priol, J.; Azzaoui, I.; Uhel, F.; Fest, T.; Yves, L.T.; Tadie, J.M. Acute alcohol exposure has an independent impact on C-reactive protein levels, neutrophil CD64 expression, and subsets of circulating white blood cells differentiated by flow cytometry in nontrauma patients. Shock 2014, 42, 192–198. [Google Scholar] [CrossRef]
- Saeed, R.W.; Varma, S.; Peng, T.; Tracey, K.J.; Sherry, B.; Metz, C.N. Ethanol blocks leukocyte recruitment and endothelial cell activation in vivo and in vitro. J. Immunol. 2004, 173, 6376–6383. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Bagby, G.J.; Xie, M.; Stoltz, D.A.; Summer, W.R.; Nelson, S. Acute ethanol intoxication inhibits neutrophil β2-integrin expression in rats during endotoxemia. Alcohol. Clin. Exp. Res. 1998, 22, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Bertola, A.; Park, O.; Gao, B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Hepatology 2013, 58, 1814–1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lister, P.D.; Gentry, M.J.; Preheim, L.C. Ethanol impairs neutrophil chemotaxis in vitro but not adherence or recruitment to lungs of rats with experimental pneumococcal pneumonia. J. Infect. Dis. 1993, 167, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Diamond, S.L. Ethanol enhances neutrophil membrane tether growth and slows rolling on P-selectin but reduces capture from flow and firm arrest on IL-1-treated endothelium. J. Immunol. 2008, 181, 2472–2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bautista, A.P. Chronic alcohol intoxication enhances the expression of CD18 adhesion molecules on rat neutrophils and release of a chemotactic factor by Kupffer cells. Alcohol. Clin. Exp. Res. 1995, 19, 285–290. [Google Scholar] [CrossRef]
- Raasch, C.E.; Zhang, P.; Siggins, R.W.; LaMotte, L.R.; Nelson, S.; Bagby, G.J. Acute alcohol intoxication impairs the hematopoietic precursor cell response to pneumococcal pneumonia. Alcohol. Clin. Exp. Res. 2010, 34, 2035–2043. [Google Scholar] [CrossRef] [Green Version]
- Siggins, R.W.; Melvan, J.N.; Welsh, D.A.; Bagby, G.J.; Nelson, S.; Zhang, P. Alcohol suppresses the granulopoietic response to pulmonary Streptococcus pneumoniae infection with enhancement of STAT3 signaling. J. Immunol. 2011, 186, 4306–4313. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, K.J.; Rao, R.K. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G1280–G1288. [Google Scholar] [CrossRef]
- Tang, Y.; Banan, A.; Forsyth, C.B.; Fields, J.Z.; Lau, C.K.; Zhang, L.J.; Keshavarzian, A. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin. Exp. Res. 2008, 32, 355–364. [Google Scholar] [CrossRef]
- Casafont Morencos, F.; de las Heras Castano, G.; Martin Ramos, L.; Lopez Arias, M.J.; Ledesma, F.; Pons Romero, F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig. Dis. Sci. 1996, 41, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.; Keshavarzian, A.; Engen, P.; Forsyth, C.B.; Sikaroodi, M.; Gillevet, P. Intestinal dysbiosis: A possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin. Exp. Res. 2009, 33, 1836–1846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjorkhaug, S.T.; Skar, V.; Medhus, A.W.; Tollisen, A.; Bramness, J.G.; Valeur, J. Chronic alcohol overconsumption may alter gut microbial metabolism: A retrospective study of 719 (13)C-D-xylose breath test results. Microb. Ecol. Health Dis. 2017, 28, 1301725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meroni, M.; Longo, M.; Dongiovanni, P. Alcohol or Gut Microbiota: Who Is the Guilty? Int. J. Mol. Sci. 2019, 20, 4568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadlbauer, V.; Horvath, A.; Komarova, I.; Schmerboeck, B.; Feldbacher, N.; Wurm, S.; Klymiuk, I.; Durdevic, M.; Rainer, F.; Blesl, A.; et al. A single alcohol binge impacts on neutrophil function without changes in gut barrier function and gut microbiome composition in healthy volunteers. PLoS ONE 2019, 14, e0211703. [Google Scholar] [CrossRef] [Green Version]
- Molina, P.E.; Happel, K.I.; Zhang, P.; Kolls, J.K.; Nelson, S. Focus on: Alcohol and the immune system. Alcohol Res. Health 2010, 33, 97–108. [Google Scholar]
- Elliott, M.K.; Sisson, J.H.; Wyatt, T.A. Effects of cigarette smoke and alcohol on ciliated tracheal epithelium and inflammatory cell recruitment. Am. J. Respir. Cell Mol. Biol. 2007, 36, 452–459. [Google Scholar] [CrossRef]
- Szabo, G.; Saha, B. Alcohol’s Effect on Host Defense. Alcohol Res. 2015, 37, 159–170. [Google Scholar]
- Boule, L.A.; Kovacs, E.J. Alcohol, aging, and innate immunity. J. Leukoc. Biol. 2017, 102, 41–55. [Google Scholar] [CrossRef] [Green Version]
- Pasala, S.; Barr, T.; Messaoudi, I. Impact of Alcohol Abuse on the Adaptive Immune System. Alcohol Res. 2015, 37, 185–197. [Google Scholar]
- Mors, K.; Horauf, J.A.; Kany, S.; Wagner, N.; Sturm, R.; Woschek, M.; Perl, M.; Marzi, I.; Relja, B. Ethanol Decreases Inflammatory Response in Human Lung Epithelial Cells by Inhibiting the Canonical NF-kB-Pathway. Cell Physiol. Biochem. 2017, 43, 17–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Alcohol Intake | Pattern Recognition | ||||
|---|---|---|---|---|---|
| Species | Study | Cell Type/Organ | Major Finding | Reference | |
| acute | human | in vitro | monocytes | ↓ TLR8- and TLR4-mediated TNFα synthesis (mRNA, protein) | [26,27] |
| in vitro | monocytes | ↓ TLR4-dependent NFκB activation | [27] | ||
| mouse | in vivo | liver | ↓ TLR4 gene expression | [21] | |
| in vivo | intestine | ↓ TLR4-mediated cytokine production (sepsis model) | [23] | ||
| in vitro | microglial cells | ↑ TLR4 and TLR2 mRNA at 1 h ↓ TLR4 and TLR2 mRNA at 3 h | [24] | ||
| in vivo | macrophages | ↓ TLR2, TLR4 and TLR9 induced inflammatory response mediated by p38 and ERK1/2 | [25] | ||
| rat | in vivo | intestine | ↓ TLR4 sensitivity to ligands (LPS) | [22] | |
| chronic | mouse | in vivo | liver macrophages | ↑ TLR4 protein expression | [29] |
| in vivo | liver | ↑ all TLR mRNAs except TLR3 and TLR5 (TLR10 and TLR11 not tested) ↓ antibiotics effectiveness toward TLR mRNA expression ↓ TNFα protein level by NADPH oxidase inhibition | [29] | ||
| NF-κB signaling | |||||
| acute | human | in vitro | monocytes | ↑ p50 translocation to the nucleus ↓ cytokine levels = p65/RelA | [54,55] |
| in vitro | FEMX-I melanoma cells | ↑ NF-κB activation | [56] | ||
| in vitro | monocytes | ↓ NF-κB activity independently on IκB α degradation | [57] | ||
| in vitro | monocytes | ↑ IRAK-M ↓ IκB α kinase activity, NF-κB DNA binding, and NF-κB driven cellular responses | [63] | ||
| mouse | in vitro | alveolar type 2 epithelial cells | ↓ CXCL5 expression ↓ p65 phosphorylation (following LPS and TNFα exposure) | [60] | |
| in vivo | kupffer cells | ↓ IRAK-1 and LPS-mediated activation of NF-κB at 1 h in ↑ IRAK-1 and LPS-induced NF-κB activity at 24 h | [64] | ||
| rat | in vitro | kupffer cells | ↓ LPS-induced NF-κB activation ↓ TNFα mRNA and TNFα secretion | [53] | |
| in vivo | peritoneal macrophages | ↓ NF-κB activation = LPS-induced p65 translocation | [59] | ||
| chronic | human | in vitro | monocytes | ↓IRAK-M activity ↑IRAK-1 and IKK kinase levels, NF-κB DNA binding and responses ↑ ERK activation | [63] |
| mouse | in vivo | kupffer cells, leukocytes, liver | ↑NF-κB phosphorylation ↑ NF-κB activation | [61,62] | |
| Bacterial clearance | |||||
| acute | human | healthy volunteers | blood | ↑ serum endotoxin ↑ 16 S rDNA levels | [83] |
| mouse | in vivo | peritoneal cavity + spleen; intestinal barrier, blood | ↓ bacterial clearance ↑ overgrowth of bacteria, and ↑ translocation of pathogens into the bloodstream | [23,90] | |
| rat | in vivo | bacteria numbers in the lungs, blood | delayed lung neutrophil recruitment ↑ elevated bacterial burden, systemic dissemination to spleen, blood, and liver | [81] | |
| chronic | human | ↑ bacterial infections | [85] | ||
| rats | in vivo | systemic, gut mucosa | ↑ endotoxemia | [87] | |
| Cytokines | |||||
| acute | human | in vitro | lung epithelial cells | ↓ IL-8 and IL-6 release | [127,128] |
| in vitro | monocytes | ↓ TNFα release and gene expression ↑ IL-10 | [27,63] | ||
| healthy volunteers | blood | ↑ TNFα after 20 minutes, followed by ↓ IL-1β after 2-5 hours | [134] | ||
| trauma patients | blood | ↓ IL-6 ↑ TNFα after 20 minutes, followed by ↓ IL-1β after 2-5 hours | [137,138] | ||
| mouse | in vivo | blood | ↓ CXCL9, IL-6 and IL-12 ↑ IL-10 | [129] | |
| in vivo | peritoneal lavage fluid | ↓ IL-15, TNFα, IL-9, IL-1β & IL-1α, IL-13, IL-17, and IL-6 ↑ IL-10 and MIP-2 | [23] | ||
| in vivo | blood, lung | ↑ IL-6 and TNFα ↑ IL-6 | [139,140] | ||
| in vivo | ileal tissue | ↑ IL-6, IL-1β and IL-18 protein | [141,142] | ||
| chronic | human | in vitro | monocytes | ↑ TNFα = IL-10 protein level | [28,63] |
| alcoholic liver disease | blood | ↑ TNFα, IL-6 and IL-1 | [131] | ||
| acute alcoholic hepatitis | blood | ↑ IL-8, IL-4, and IFNγ | [132] | ||
| mouse | in vivo | liver | ↑ mRNA expression of TNFα, IL-6 and IL-10 ↑ TNFα level | [29,130] | |
| rat | in vivo | blood | ↑ IL-1, IL-10 and TNFα | [147] | |
| in vivo | blood | = TNFα, IL-10, IL-6 or CXCL1 without inflammatory trigger, but ↑of IL-6 and TNF upon stimulation | [148] | ||
| in vivo | blood | ↑ IL-6, MCP-1 and TNFα | [61] | ||
| Cellular responses | |||||
| acute | human | precarious ill patients | blood | ↓ CRP, circulating neutrophils and neutrophilic CD64 | [200] |
| mouse | in vivo | wounds | ↓ MPO and neutrophilic infiltration | [172] | |
| in vivo | leukocytes | ↓ cellular recruitment ↓ adhesion molecules ICAM-1, VCAM1, and E-selectin ↓ chemokines like CXCL8, MCP-1 and RANTES | [201] | ||
| in vivo | lung neutrophils | ↓ recruitment | [207] | ||
| rat | in vivo | isolated PMN | ↑ chemotaxis and superoxide release ↓ ingestion and intracellular killing | [175] | |
| in vivo | isolated PMN | ↓ reduced phagocytosis of virulent K. pneumoniae | [176] | ||
| chronic | mouse | in vivo | brain tissue, microglia | ↑ microglia activation | [180] |
| in vitro | microglia | ↑ mitochondrial ROS production | [180] | ||
| in vivo | alveolar macrophages | ↓ cellular functions e.g. phagocytosis | [183,184] | ||
| in vivo | liver | ↑ E-selectin expression = expression of P-selectin, ICAM-1, and VCAM1 | [184] | ||
| rat | in vivo | isolated PMN | ↓ ROS | [174] | |
| in vivo | systemic, gut mucosa | ↑ ROS | [87] | ||
| in vivo | liver | modulation of different steps of neutrophil infiltration | [186] | ||
| in vivo | isolated PMN | ↑ CD18 expression | [187] | ||
| Others | |||||
| acute | human | in vitro | neuroblastoma cells | ↑ HMGB1 expression and release | [91] |
| in vitro | macrophages | ↑ IL-1β and inflammasome activation | [145] | ||
| mice | in vivo | brain | ↑ P2X7R in alcohol sensitive brain regions | [92] | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kany, S.; Janicova, A.; Relja, B. Innate Immunity and Alcohol. J. Clin. Med. 2019, 8, 1981. https://doi.org/10.3390/jcm8111981
Kany S, Janicova A, Relja B. Innate Immunity and Alcohol. Journal of Clinical Medicine. 2019; 8(11):1981. https://doi.org/10.3390/jcm8111981
Chicago/Turabian StyleKany, Shinwan, Andrea Janicova, and Borna Relja. 2019. "Innate Immunity and Alcohol" Journal of Clinical Medicine 8, no. 11: 1981. https://doi.org/10.3390/jcm8111981
APA StyleKany, S., Janicova, A., & Relja, B. (2019). Innate Immunity and Alcohol. Journal of Clinical Medicine, 8(11), 1981. https://doi.org/10.3390/jcm8111981






