AAV Mediated Delivery of Myxoma Virus M013 Gene Protects the Retina against Autoimmune Uveitis
Abstract
1. Introduction
2. Experimental Section
2.1. Animals
2.2. Adeno-Associated Viral (AAV) Vector Production
2.3. Intravitreal Injection
2.4. Electroretinogram
2.5. Spectral Domain Optical Coherence Tomography
2.6. Quantification of Infiltrative Cells within the Vitreous Humor Using SD-OCT Images
2.7. Fundoscopy
2.8. Clinical Scoring of Fundus Images
2.9. Histological Evaluation
2.10. Retinal RNA Extraction and cDNA Synthesis
2.11. Reverse Transcribed Quantitative PCR (RT-qPCR)
2.12. Retinal Protein Extraction and Protein Determination
2.13. Protein Determination
2.14. Multiplex ELISA (MAGPIX) Assay
2.15. Western Blot
2.16. IL-21 Enzyme Linked Immunosorbent Assay (ELISA)
2.17. Statistical Analysis
3. Results
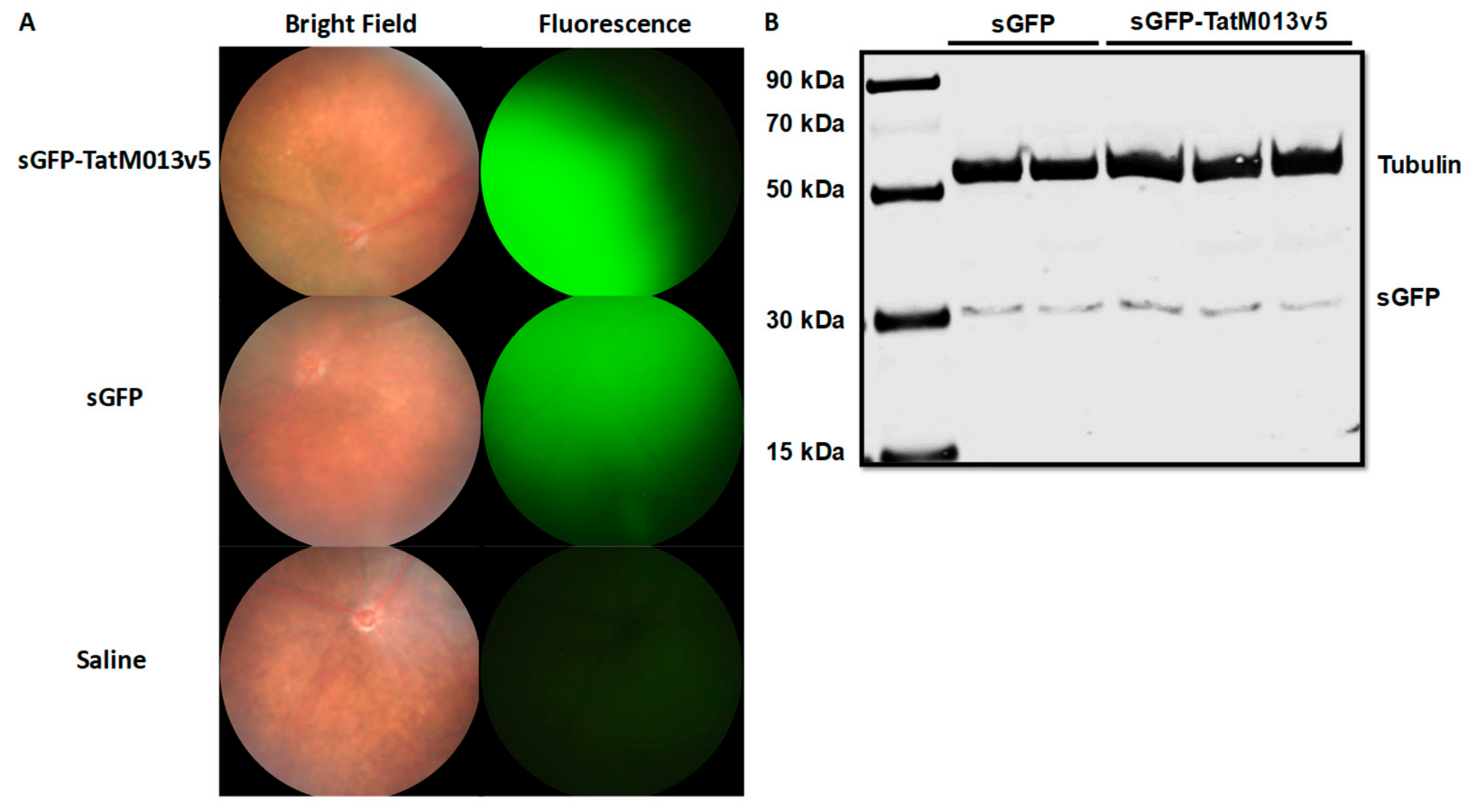
3.1. Retinal Gene Delivery of a Secretable TatM013 Does Not Alter the Mouse Retina
3.1.1. Effect of TatM013 Expression on Retina Function
3.1.2. TatM013 Does Not Alter the Retina Structure
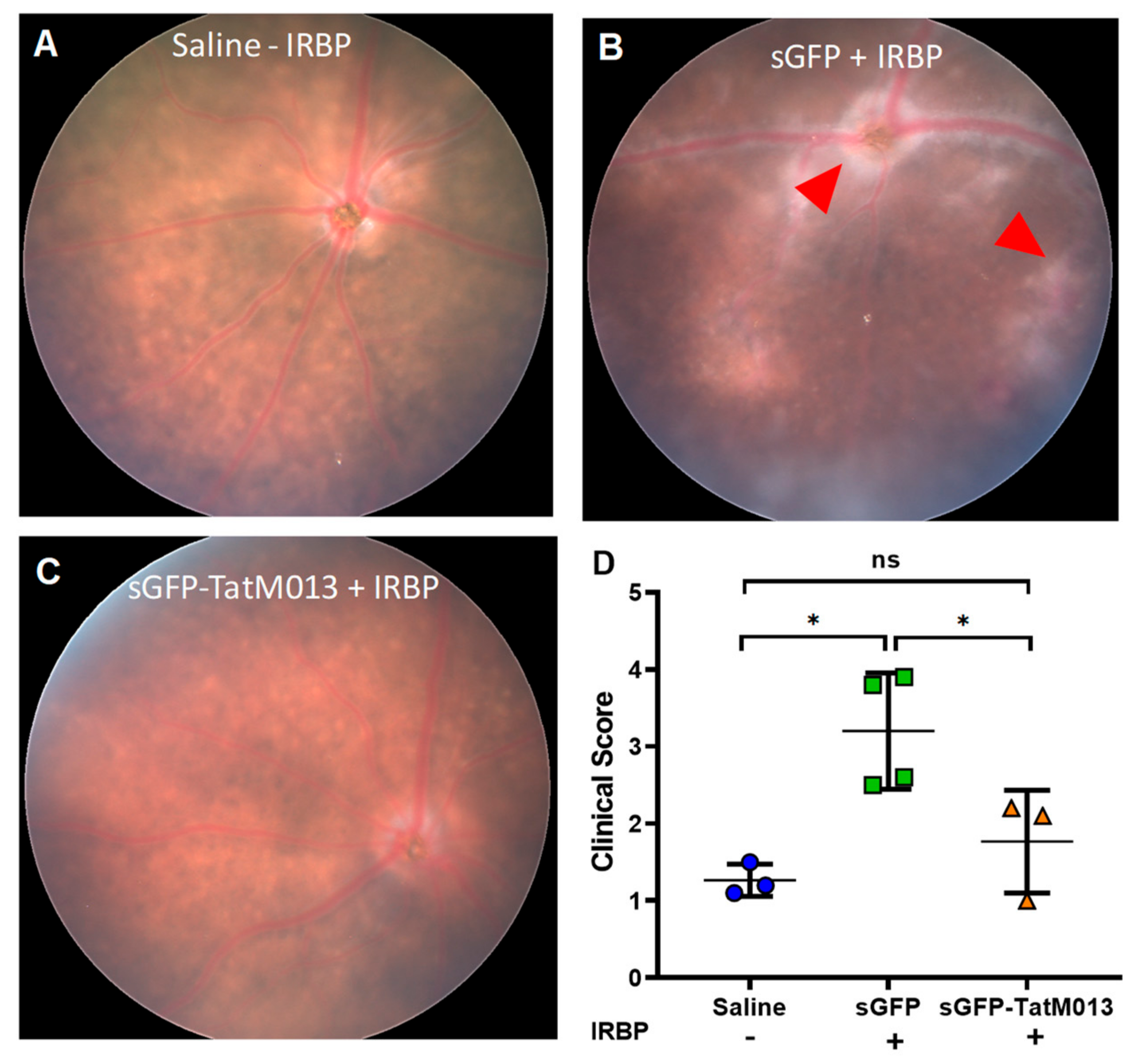
3.2. Retinal Gene Expression of TatM013 Protects the Retina in an Experimental Autoimmune Uveitis (EAU) Mouse Model
3.2.1. Retinal Expression of TatM013 Reduces the Clinical Signs of the EAU Mouse Model
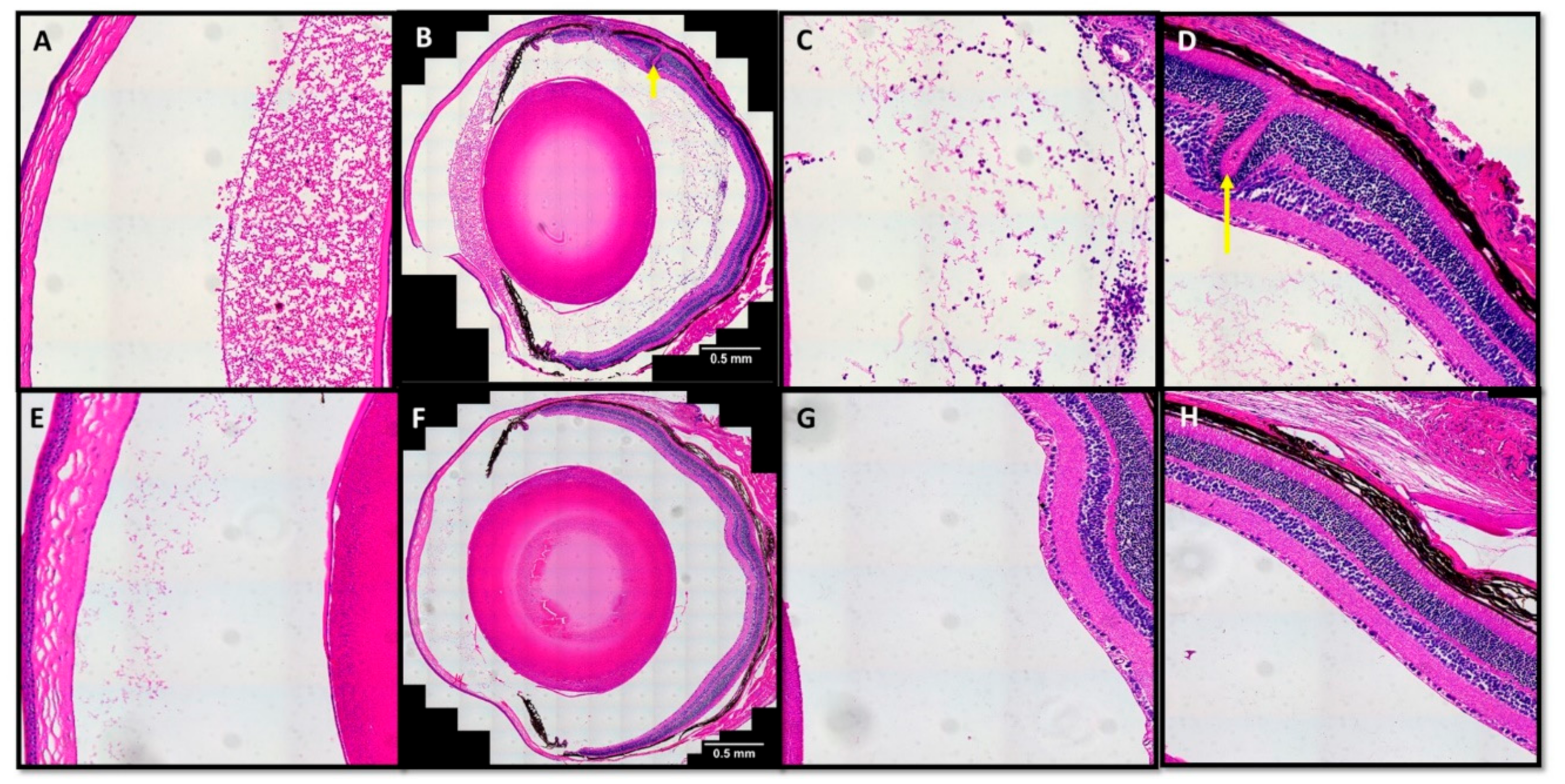
3.2.2. TatM013 Decreases the Recruitment of Infiltrative Cells in the EAU Mouse Model
3.3. M013 Modulates Inflammatory and Anti-Inflammatory Genes in the Retina of the EAU Mouse Model
3.3.1. M013 Changes Gene Expression Pattern in the Retina of EAU Mice
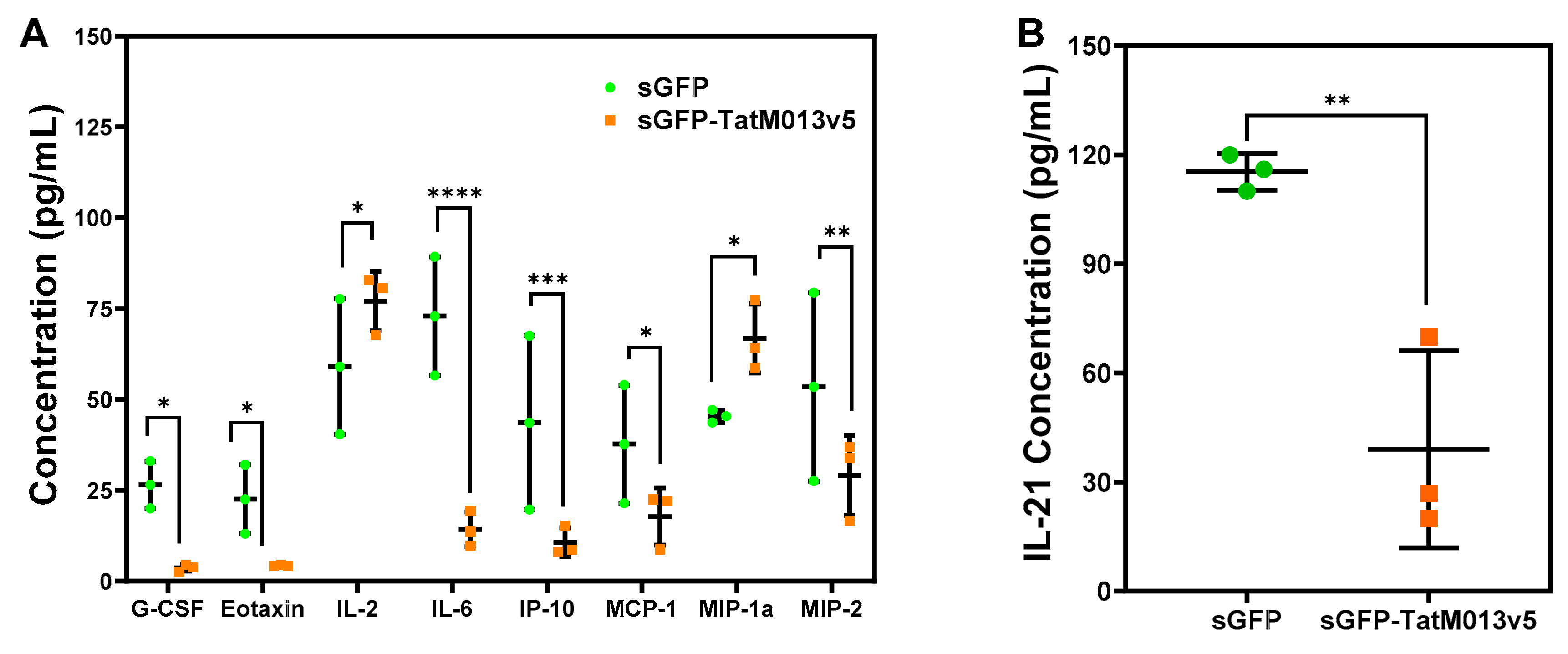
3.3.2. M013 Modulates Multiple Cytokines and Chemokines in the Retina of the EAU Mouse Model
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kerr, P.J. Myxomatosis in Australia and Europe: A model for emerging infectious diseases. Antivir. Res. 2012, 93, 387–415. [Google Scholar] [CrossRef] [PubMed]
- Ogbomo, H.; Zemp, F.J.; Lun, X.; Zhang, J.; Stack, D.; Rahman, M.M.; McFadden, G.; Mody, C.H.; Forsyth, P.A. Myxoma virus infection promotes NK lysis of malignant gliomas in vitro and in vivo. PLoS ONE 2013, 8, e66825. [Google Scholar] [CrossRef] [PubMed]
- Stanford, M.M.; Shaban, M.; Barrett, J.W.; Werden, S.J.; Gilbert, P.A.; Bondy-Denomy, J.; Mackenzie, L.; Graham, K.C.; Chambers, A.F.; McFadden, G. Myxoma Virus Oncolysis of Primary and Metastatic B16F10 Mouse Tumors. In Vivo. Mol. Ther. 2008, 16, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Stanford, M.M.; McFadden, G. Myxoma virus and oncolytic virotherapy: A new biologic weapon in the war against cancer. Expert Opin. Biol. Ther. 2007, 7, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.W.; Cao, J.X.; Hota-Mitchell, S.; McFadden, G. Immunomodulatory proteins of myxoma virus. Semin. Immunol. 2001, 13, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Mohamed, M.R.; Kim, M.; Smallwood, S.; McFadden, G. Co-regulation of NF-kappaB and inflammasome-mediated inflammatory responses by myxoma virus pyrin domain-containing protein M013. PLoS Pathog. 2009, 5, e1000635. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; McFadden, G. Myxoma virus lacking the pyrin-like protein M013 is sensed in human myeloid cells by both NLRP3 and multiple Toll-like receptors, which independently activate the inflammasome and NF-kappaB innate response pathways. J. Virol. 2011, 85, 12505–12517. [Google Scholar] [CrossRef]
- Garg, R.R.; Jackson, C.B.; Rahman, M.M.; Khan, A.R.; Lewin, A.S.; McFadden, G. Myxoma virus M013 protein antagonizes NF-κB and inflammasome pathways via distinct structural motifs. J. Biol. Chem. 2019, 294, 8480–8489. [Google Scholar] [CrossRef]
- Ildefonso, C.J.; Jaime, H.; Rahman, M.M.; Li, Q.; Boye, S.E.; Hauswirth, W.W.; Lucas, A.R.; McFadden, G.; Lewin, A.S. Gene Delivery of a Viral Anti-Inflammatory Protein to Combat Ocular Inflammation. Hum. Gene Ther. 2014, 26, 59–68. [Google Scholar] [CrossRef]
- Taylor, A.W. Ocular Immune Privilege; Nature Publishing Group: Berlin/Heidelberg, Germany, 2009; pp. 1885–1889. [Google Scholar]
- Medawar, P.B. Immunity to Homologous Grafted Skin. III. The Fate of Skin Homographs Transplanted to the Brain, to Subcutaneous Tissue, and to the Anterior Chamber of the Eye. Br. J. Exp. Pathol. 1948, 29, 58–69. [Google Scholar]
- Heckenlively, J.R.; Ferreyra, H.A. Autoimmune retinopathy: A review and summary. Semin. Immunopathol. 2008, 30, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.G.; Frederiksen, J. The association between multiple sclerosis and uveitis. Surv. Ophthalmol. 2017, 62, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sen, E.S.; Ramanan, A.V. Juvenile idiopathic arthritis-associated uveitis. Best Pract. Res. Clin. Rheumatol. 2017, 31, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Ksiaa, I.; Abroug, N.; Kechida, M.; Zina, S.; Jelliti, B.; Khochtali, S.; Attia, S.; Khairallah, M. Eye and Behçet’s disease. J. Fr. Ophtalmol. 2019, 42, e133–e146. [Google Scholar] [CrossRef] [PubMed]
- Horai, R.; Silver, P.B.; Chen, J.; Agarwal, R.K.; Chong, W.P.; Jittayasothorn, Y.; Mattapallil, M.J.; Nguyen, S.; Natarajan, K.; Villasmil, R.; et al. Breakdown of immune privilege and spontaneous autoimmunity in mice expressing a transgenic T cell receptor specific for a retinal autoantigen. J. Autoimmun. 2013, 44, 21–33. [Google Scholar] [CrossRef]
- Chen, J.; Qian, H.; Horai, R.; Chan, C.C.; Caspi, R.R. Mouse Models of Experimental Autoimmune Uveitis: Comparative Analysis of Adjuvant-Induced vs Spontaneous Models of Uveitis. Curr. Mol. Med. 2015, 15, 550–557. [Google Scholar] [CrossRef]
- Touzot, M.; Cacoub, P.; Bodaghi, B.; Soumelis, V.; Saadoun, D. IFN-α induces IL-10 production and tilt the balance between Th1 and Th17 in Behçet disease. Autoimmun. Rev. 2015, 14, 370–375. [Google Scholar] [CrossRef]
- Okunuki, Y.; Mukai, R.; Nakao, T.; Tabor, S.J.; Butovsky, O.; Dana, R.; Ksander, B.R.; Connor, K.M. Retinal microglia initiate neuroinflammation in ocular autoimmunity. Proc. Natl. Acad. Sci. USA 2019, 116, 9989–9998. [Google Scholar] [CrossRef]
- Uchi, S.H.; Yanai, R.; Kobayashi, M.; Hatano, M.; Kobayashi, Y.; Yamashiro, C.; Nagai, T.; Tokuda, K.; Connor, K.M.; Sonoda, K.H.; et al. Dendritic cells mediate the anti-inflammatory action of omega-3 long-chain polyunsaturated fatty acids in experimental autoimmune uveitis. PLoS ONE 2019, 14, e0219405. [Google Scholar] [CrossRef]
- Hasegawa, E.; Takeda, A.; Yawata, N.; Sonoda, K.H. The effectiveness of adalimumab treatment for non-infectious uveitis. Immunol. Med. 2019, 42, 79–83. [Google Scholar] [CrossRef]
- Wan, C.K.; He, C.; Sun, L.; Egwuagu, C.E.; Leonard, W.J. Cutting Edge: IL-1 Receptor Signaling is Critical for the Development of Autoimmune Uveitis. J. Immunol. 2016, 196, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhou, H.; Zhang, J.; Liu, X.; Su, S.B. Interleukin-1β promotes the induction of retinal autoimmune disease. Int. Immunopharmacol. 2014, 22, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Al-Janabi, A.; El Nokrashy, A.; Sharief, L.; Nagendran, V.; Lightman, S.; Tomkins-Netzer, O. Long-Term Outcomes of Treatment with Biological Agents in Eyes with Refractory, Active, Noninfectious Intermediate Uveitis, Posterior Uveitis, or Panuveitis. Ophthalmology 2019. [Google Scholar] [CrossRef] [PubMed]
- Simonelli, F.; Maguire, A.M.; Testa, F.; Pierce, E.A.; Mingozzi, F.; Bennicelli, J.L.; Rossi, S.; Marshall, K.; Banfi, S.; Surace, E.M.; et al. Gene Therapy for Leber’s Congenital Amaurosis is Safe and Effective Through 1.5 Years After Vector Administration. Mol. Ther. 2010, 18, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.N.; Ryals, R.C.; Aslanidi, G.V.; Min, S.H.; Ruan, Q.; Sun, J.; Dyka, F.M.; Kasuga, D.; Ayala, A.E.; Van Vliet, K.; et al. Targeting Photoreceptors via Intravitreal Delivery Using Novel, Capsid-Mutated AAV Vectors. PLoS ONE 2013, 8, e62097. [Google Scholar] [CrossRef]
- Maguire, A.M.; Russell, S.; Wellman, J.A.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; Marshall, K.A.; et al. Efficacy, Safety, and Durability of Voretigene Neparvovec-rzyl in RPE65 Mutation-Associated Inherited Retinal Dystrophy: Results of Phase 1 and 3 Trials. Ophthalmology 2019, 126, 1273–1285. [Google Scholar] [CrossRef]
- Caspi, R.R.; Roberge, F.G.; Chan, C.C.; Wiggert, B.; Chader, G.J.; Rozenszajn, L.A.; Lando, Z.; Nussenblatt, R.B. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J. Immunol. 1988, 140, 1490–1495. [Google Scholar]
- Chen, J.; Qian, H.; Horai, R.; Chan, C.C.; Caspi, R.R. Use of optical coherence tomography and electroretinography to evaluate retinal pathology in a mouse model of autoimmune uveitis. PLoS ONE 2013, 8, e63904. [Google Scholar] [CrossRef]
- Mattapallil, M.J.; Silver, P.B.; Cortes, L.M.; Leger, A.J.S.; Jittayasothorn, Y.; Kielczewski, J.L.; Moon, J.J.; Chan, C.C.; Caspi, R.R. Characterization of a New Epitope of IRBP That Induces Moderate to Severe Uveoretinitis in Mice with H-2b Haplotype. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5439–5449. [Google Scholar] [CrossRef]
- Zolotukhin, S.; Potter, M.; Zolotukhin, I.; Sakai, Y.; Loiler, S.; Fraites, T.J., Jr.; Chiodo, V.A.; Phillipsberg, T.; Muzyczka, N.; Hauswirth, W.W.; et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 2002, 28, 158–167. [Google Scholar] [CrossRef]
- Chu, C.J.; Herrmann, P.; Carvalho, L.S.; Liyanage, S.E.; Bainbridge, J.W.B.; Ali, R.R.; Dick, A.D.; Luhmann, U.F.O. Assessment and in vivo scoring of murine experimental autoimmune uveoretinitis using optical coherence tomography. PLoS ONE 2013, 8, e63002. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Koch, P.; Chen, M.; Lau, A.; Reid, D.M.; Forrester, J.V. A clinical grading system for retinal inflammation in the chronic model of experimental autoimmune uveoretinitis using digital fundus images. Exp. Eye Res. 2008, 87, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Ildefonso, C.J.; Jaime, H.; Biswal, M.R.; Boye, S.E.; Li, Q.; Hauswirth, W.W.; Lewin, A.S. Gene therapy with the caspase activation and recruitment domain reduces the ocular inflammatory response. Mol. Ther. 2015, 23, 875–884. [Google Scholar] [CrossRef]
- Rückerl, D.; Hessmann, M.; Yoshimoto, T.; Ehlers, S.; Hölscher, C. Alternatively activated macrophages express the IL-27 receptor alpha chain WSX-1. Immunobiology 2006, 211, 427–436. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, J.; Zhang, Z.; Li, F.; Zhang, C.; Lai, B.; Xiao, L.; Wang, N. Peroxisome proliferator–activated receptor γ (PPARγ) induces the gene expression of integrin αVβ5 to promote macrophage M2 polarization. J. Biol. Chem. 2018, 293, 16572–16582. [Google Scholar] [CrossRef]
- Natoli, R.; Fernando, N.; Jiao, H.; Racic, T.; Madigan, M.; Barnett, N.L.; Chu-Tan, J.A.; Valter, K.; Provis, J.; Rutar, M. Retinal Macrophages Synthesize C3 and Activate Complement in AMD and in Models of Focal Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2977–2990. [Google Scholar] [CrossRef]
- Hein, H.; Schlüter, C.; Kulke, R.; Christophers, E.; Schröder, J.M.; Bartels, J. Genomic organization, sequence, and transcriptional regulation of the human eotaxin gene. Biochem. Biophys. Res. Commun. 1997, 237, 537–542. [Google Scholar] [CrossRef]
- Nishizawa, M.; Nagata, S. Regulatory elements responsible for inducible expression of the granulocyte colony-stimulating factor gene in macrophages. Mol. Cell. Biol. 1990, 10, 2002–2011. [Google Scholar] [CrossRef]
- Son, Y.H.; Jeong, Y.T.; Lee, K.A.; Choi, K.H.; Kim, S.M.; Rhim, B.Y.; Kim, K. Roles of MAPK and NF-kappaB in interleukin-6 induction by lipopolysaccharide in vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 2008, 51, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Long, D.; Chen, Y.; Wu, H.; Zhao, M.; Lu, Q. Clinical significance and immunobiology of IL-21 in autoimmunity. J. Autoimmun. 2019, 99, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, C.R.; Kim, H.P.; Liao, W.; Telford, W.G.; Egwuagu, C.E.; Leonard, W.J. Key role for IL-21 in experimental autoimmune uveitis. Proc. Natl. Acad. Sci. USA 2011, 108, 9542–9547. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.R.; Parikh, A.A.; Pritts, T.A.; Fischer, J.E.; Cottongim, S.; Szabo, C.; Salzman, A.L.; Hasselgren, P.O. Complement component C3 production in IL-1beta-stimulated human intestinal epithelial cells is blocked by NF-kappaB inhibitors and by transfection with ser 32/36 mutant IkappaBalpha. J. Surg. Res. 1999, 82, 48–55. [Google Scholar] [CrossRef]
- Shen, F.; Hu, Z.; Goswami, J.; Gaffen, S.L. Identification of Common Transcriptional Regulatory Elements in Interleukin-17 Target Genes. J. Biol. Chem. 2006, 281, 24138–24148. [Google Scholar] [CrossRef]
- Letko, E.; Yeh, S.; Foster, C.S.; Pleyer, U.; Brigell, M.; Grosskreutz, C.L. Efficacy and Safety of Intravenous Secukinumab in Noninfectious Uveitis Requiring Steroid-Sparing Immunosuppressive Therapy. Ophthalmology 2015, 122, 939–948. [Google Scholar] [CrossRef]


| Gene | Forward Oligo (5′-3′) | Reverse Oligo (5′-3′) |
|---|---|---|
| Arg1 | AAGACAGGGCTCCTTTCAGG | CTGTGATGCCCCAGATGGTT |
| C3 | CAGAAACGCCCTGAAGCTG | CAGTTGGGACAACCATAAAC |
| Fcgr1 | CAAGTGCTTGGTCCCCAGTC | ACACGCCATCGCTTCTAACT |
| ICAM-1 | AAGGTGGTTCTTCTGAGCGG | TCCAGCCGAGGACCATACAG |
| IL-17A | GGAGAGCTTCATCTGTGTCTCTG | ACTTTTGCGCCAAGGGAGTT |
| IL27ra | TGCAGGAACGTGAGCAGTC | CCCATCAGAAGCAAACCCCA |
| Marco | ATCCAGGGATTGCAGGTGTG | CTGGCCAGAAGACCCTTTCA |
| Mrc1 | TTGCACTTTGAGGGAAGCGA | AGTCCAATCCAGAGTCCCGA |
| Pparg | CGCTGGGGTATTGGGTCG | TTTCAAATCTTGTCTGTCACACAGT |
| Retn1a | CCCCAGGATGCCAACTTTGA | CAGTAGCAGTCATCCCAGCA |
| Slamf1 | GAGCTTCTTCCTTGGGGGTAA | CCATCACACCTCCACCTGTTC |
| SOCS3 | CCCTTCCCGGCCCAG | CGGGGAGCTAGTCCCGAA |
| STAT1 | TGCAGTGAGTGAGTGAGAGC | GAACCACTGTGACATCCTTGAG |
| STAT6 | GCTACTGGTCAGATCGGCTG | CAGTGAGCGAATGGACAGGT |
| TGF-β | GCCTGAGTGGCTGTCTTTTG | TTTGGGGCTGATCCCGTTG |
| β-Actin | CTGTCGAGTCGCGTCCACC | ATTCCCACCATCACACCCTGG |
| Temperature (°C) | Time (min) | Cycles |
|---|---|---|
| 95.0 | 0:30 | 1 |
| 95.0 | 0:15 | 40 |
| 60.0 | 0:30 | |
| 65.0 | 0:05 | 1 |
| 0.5 increases | ||
| 95.0 |
| Gene | sGFP | sGFP-TatM013v5 | Multiple T-Test | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Average | ± StDev | n | Average | ± StDev | n | Difference | p-Value | Significance | |
| Arg1 | 1.00 | 0.11 | 3 | 1.75 | 0.77 | 3 | 0.75 | 0.17022 | ns |
| C3 | 1.00 | 0.10 | 3 | 0.20 | 0.10 | 3 | −0.8 | 0.000608 | *** |
| Fcgr1 | 1.00 | 0.83 | 3 | 0.40 | 0.34 | 3 | −0.6 | 0.311063 | ns |
| ICAM-1 | 1.00 | 0.30 | 3 | 1.50 | 0.30 | 3 | 0.5 | 0.110787 | ns |
| IL-17A | 1.00 | 0.30 | 3 | 0.20 | 0.10 | 3 | −0.8 | 0.011858 | * |
| IL-27Ra | 1.00 | 0.58 | 3 | 5.19 | 0.73 | 3 | 4.19 | 0.001469 | *** |
| Marco | 1.00 | 0.14 | 3 | 0.91 | 0.64 | 3 | −0.09 | 0.823617 | ns |
| Mrc1 | 1.00 | 0.15 | 3 | 0.65 | 0.52 | 3 | −0.35 | 0.325368 | ns |
| Ppar-γ | 1.00 | 0.97 | 3 | 15.29 | 0.78 | 3 | 14.29 | 0.000038 | **** |
| Retn1a | 1.00 | 0.09 | 3 | 1.21 | 0.53 | 3 | 0.21 | 0.535767 | ns |
| Slamf1 | 1.00 | 0.86 | 3 | 0.37 | 0.57 | 3 | −0.63 | 0.349871 | ns |
| Socs3 | 1.00 | 0.09 | 3 | 0.56 | 0.33 | 3 | −0.44 | 0.089808 | ns |
| Stat1 | 1.00 | 0.84 | 3 | 1.01 | 0.61 | 3 | 0.01 | 0.987487 | ns |
| Stat6 | 1.00 | 0.42 | 3 | 1.23 | 0.84 | 3 | 0.23 | 0.69325 | ns |
| TGF-β | 1.00 | 0.50 | 3 | 0.40 | 0.20 | 3 | -0.6 | 0.125844 | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ridley, R.B.; Young, B.M.; Lee, J.; Walsh, E.; Ahmed, C.M.; Lewin, A.S.; Ildefonso, C.J. AAV Mediated Delivery of Myxoma Virus M013 Gene Protects the Retina against Autoimmune Uveitis. J. Clin. Med. 2019, 8, 2082. https://doi.org/10.3390/jcm8122082
Ridley RB, Young BM, Lee J, Walsh E, Ahmed CM, Lewin AS, Ildefonso CJ. AAV Mediated Delivery of Myxoma Virus M013 Gene Protects the Retina against Autoimmune Uveitis. Journal of Clinical Medicine. 2019; 8(12):2082. https://doi.org/10.3390/jcm8122082
Chicago/Turabian StyleRidley, Raela B., Brianna M. Young, Jieun Lee, Erin Walsh, Chulbul M. Ahmed, Alfred S. Lewin, and Cristhian J. Ildefonso. 2019. "AAV Mediated Delivery of Myxoma Virus M013 Gene Protects the Retina against Autoimmune Uveitis" Journal of Clinical Medicine 8, no. 12: 2082. https://doi.org/10.3390/jcm8122082
APA StyleRidley, R. B., Young, B. M., Lee, J., Walsh, E., Ahmed, C. M., Lewin, A. S., & Ildefonso, C. J. (2019). AAV Mediated Delivery of Myxoma Virus M013 Gene Protects the Retina against Autoimmune Uveitis. Journal of Clinical Medicine, 8(12), 2082. https://doi.org/10.3390/jcm8122082





