Bioengineered Skin Substitutes: The Role of Extracellular Matrix and Vascularization in the Healing of Deep Wounds
Abstract
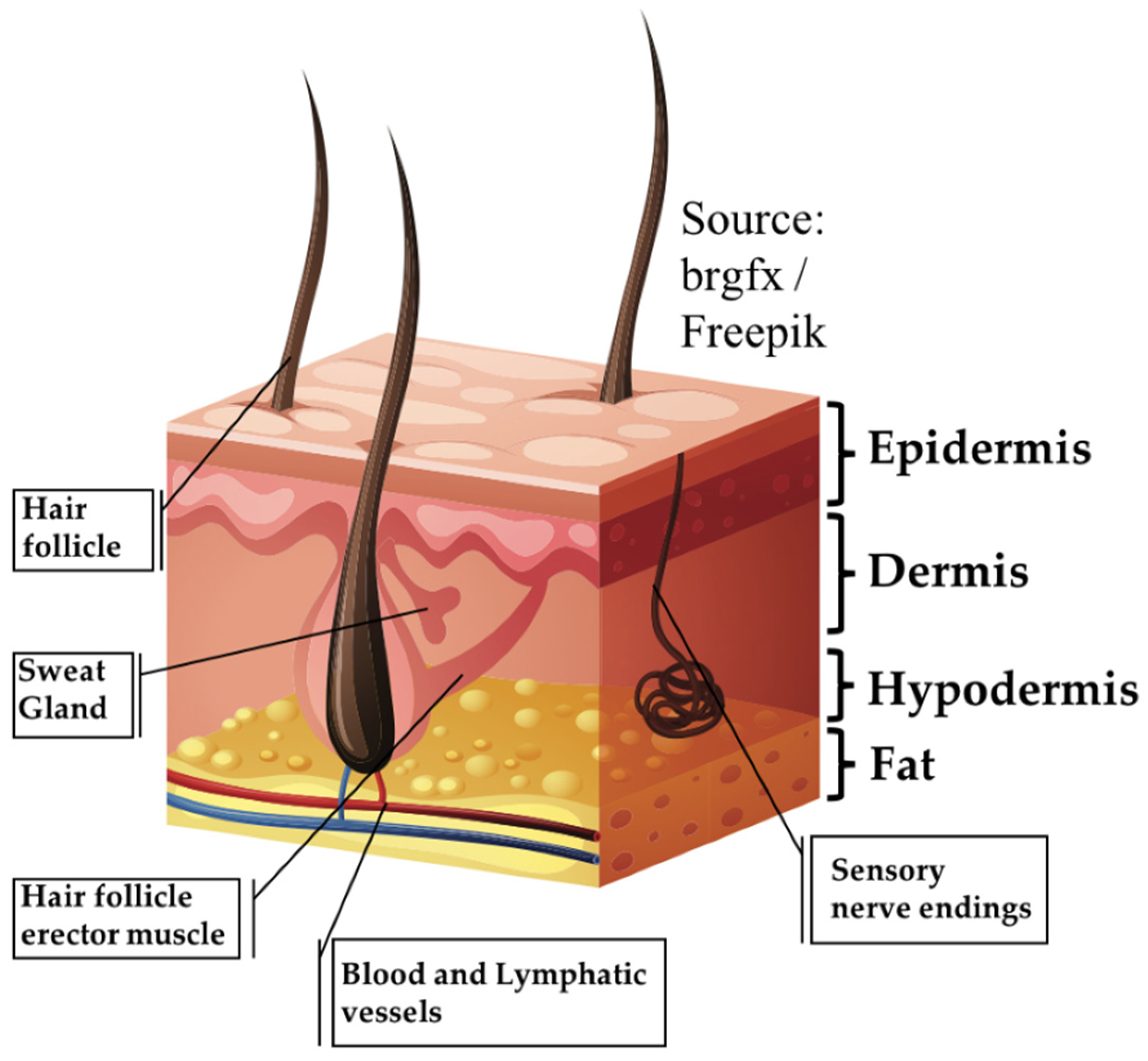
1. Introduction
2. Tissue Engineering Strategies for Skin Regeneration
- Cellularized epithelial tissues: used for superficial wounds, when the dermis is not (or is partially) damaged; autologous, allogenic or xenogeneic epithelial tissues are cultured in vitro and then implanted. In this case, the application of an STSG is not required. Engineered epithelial tissues are in general formed by cell sheets two or three cell layers thick [41,42].
- Cellularized composite skin (or full thickness): engineered tissues containing both epithelial and dermal tissues. They are composed of epithelial tissue grown on a dermis surrogate composed of fibroblasts entrapped in a biomaterial [4,41,42,43]. Due to the presence of an epidermis layer, the application of an STSG can be avoided.
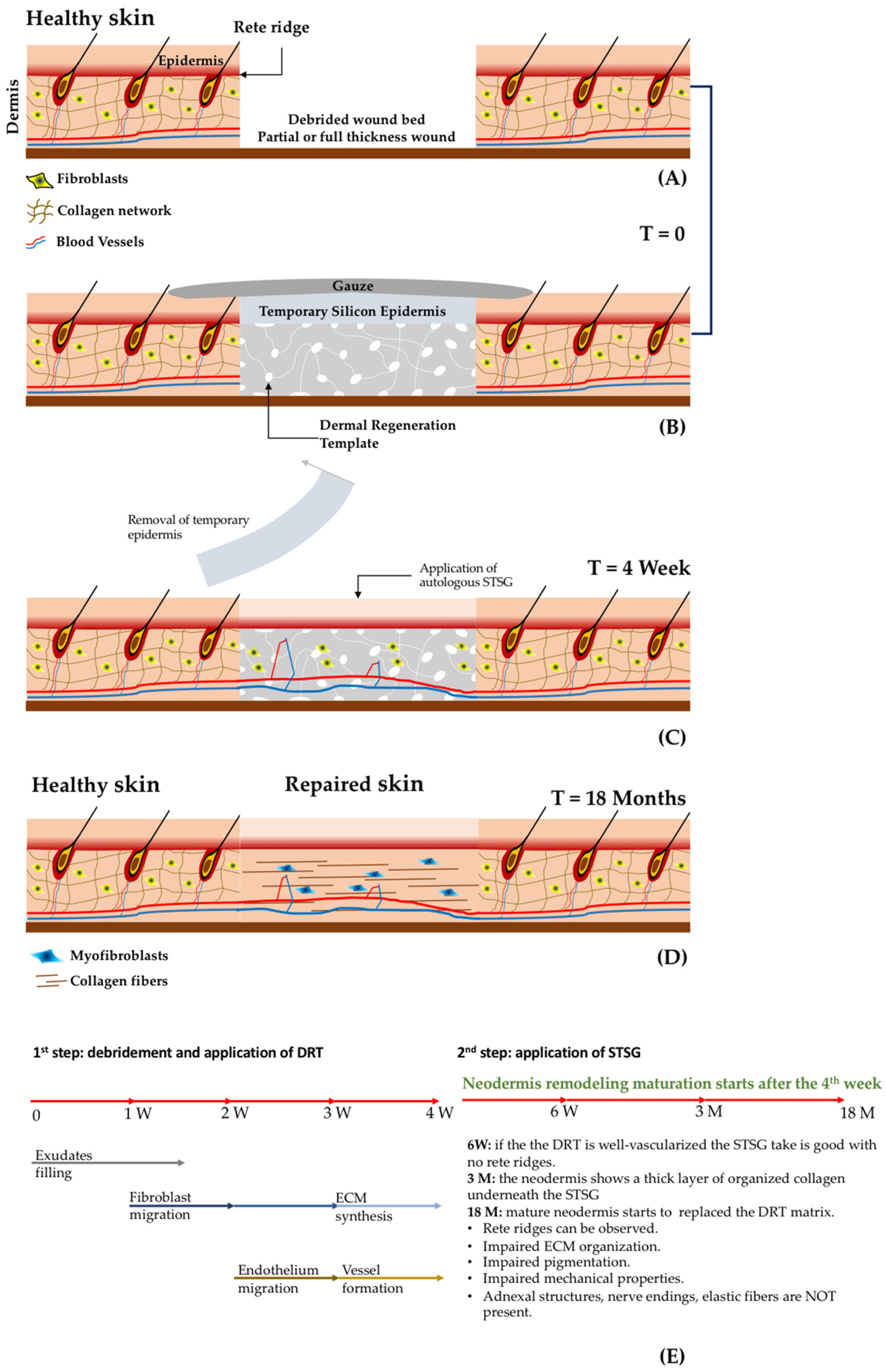
- Acellular dermal substitutes: or derma regeneration templates (DRT) that are porous 3D biomaterials (non-containing cells) applied in the missing dermis after wound debridement. This procedure implies a second surgical step for the application of an epithelial layer coming from an STSG, as shown in Figure 2 [4,41,42,43].
2.1. Dermal Regeneration Templates (DRT): Materials and Fabrication Techniques
2.2. Tissue Engineering Strategies
2.2.1. Traditional Tissue Engineering
2.2.2. Modular Tissue Engineering: Building a Tissue from the Bottom Up
Three-Dimensional Bio-Printing
3. Commercially Available Skin Substitutes
3.1. Acellular Dermal Substitutes
3.2. Cellularized Dermal Substitutes
3.3. Clinical Effectiveness of Skin Substitutes
3.3.1. Effectiveness of Acellular Dermal Regeneration Templates
- avoid the immune response, inflammation and any kind of rejection;
- protect the wounds from infection and loss of fluid;
- easy to handle and flexible, but stiff enough to withstand surgical procedures;
- enable the influx of cells (fibroblasts and endothelial cells) that will build the neodermis;
- stable enough to guarantee the correct neo-synthesis and assembly of immature extracellular matrix; on the other hand, its rate of degradation should be synchronous with the rate of formation of the neo-tissue;
- enable a correct vascularization in less than 14 days post implantation in order to increase the probability of the take of the STSG;
- be able to guide the regeneration avoiding the formation of scar tissue.
3.3.2. Effectiveness of Cellularized Skin Substitutes
- Safety concerns: any cultured cell material carries the risk of transmitting viral or bacterial infection, and some support materials (such as bovine collagen and murine feeder cells) may also have a disease risk.
- Clinical efficacy: since the biological risk is higher, the benefits in terms of quality of the healed tissue must be significantly superior, and not only equal, to conventional therapies.
- Convenience: in general, the cost of tissue engineered products is at least ten-fold higher compared to that of non-cellularized materials; in order to achieve clinical uptake, the benefits must include the reduction of hospitalization time, surgical operations after the implants, pain and the associated cost of the treatment.
4. Advanced Bioengineered Skin Equivalents: A Future Perspective
4.1. Pre–Vascularization of Dermis Substitutes
4.2. Engineered Skin Composed of Fibroblast-Assembled Extracellular Matrix
5. Discussion and Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kanitakis, J. Anatomy, histology and immunohistochemistry of normal human skin. Eur. J. Dermatol. 2002, 12, 390–401. [Google Scholar]
- Vig, K.; Atul, C.; Shweta, T.; Saurabh, D.; Rajnish, S.; Shreekumar, P.; Vida, A.D.; Shree, R.S. Advances in Skin Regeneration Using Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Michniak-kohn, B.B. Tissue Engineered Human Skin Equivalents. Pharmaceutics 2012, 4, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Matrices, D.; Skin, B. Dermal Matrices and Bioengineered Skin Substitutes: A Critical Review of Current Options. Plast. Reconstr. Surg. Glob. Open 2015, 3, e284. [Google Scholar]
- Heimbach, D.; Luterman, A.; Burke, J.; Cram, A.; Herndon, D.; Hunt, J.; Jordan, M.; McManus, W.; Solem, L.; Warden, G.L. Artificial Dermis for Major Burns. Ann. Surg. 1988, 208, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhi, S.; Arno, A.; Jeschke, M.G. The use of dermal substitutes in burn surgery: Acute phase. Wound Repair Regen. 2014, 22, 14–22. [Google Scholar] [CrossRef]
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–946. [Google Scholar] [CrossRef]
- Dhivya, S.; Vijaya, V.; Santhini, E. Review article Wound dressings—A review. BioMedicine 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Van der Veen, V.C.; van der Wal, M.B.; van Leeuwen, M.C.; Ulrich, M.M.; Middelkoop, E. Biological background of dermal substitutes. Burns 2010, 36, 305–321. [Google Scholar] [CrossRef]
- Bloemen, M.C.; van Leeuwen, M.C.; van Vucht, N.E.; van Zuijlen, P.P.; Middelkoop, E. Dermal Substitution in Acute Burns and Reconstructive Surgery: A 12-Year Follow-Up. Plast. Reconstr. Surg. 2010, 125, 1450–1459. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Schlader, Z.J.; Vargas, N.T. Regulation of Body Temperature by Autonomic and Behavioral Thermoeffectors. Exerc. Sport Sci. Rev. 2019, 47, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Enoch, S.; Leaper, D.J. Basic science of wound healing. Surgery 2008, 26, 31–37. [Google Scholar]
- Tarnuzzer, R.W.; Gregory, S. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen. 1996, 4, 321–325. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Bowers, S.L.; Banerjee, I.; Baudino, T.A. The extracellular matrix: At the center of it all. J. Mol. Cell Cardiol. 2010, 48, 474–482. [Google Scholar] [CrossRef]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126. [Google Scholar] [CrossRef]
- Watt, F.M.; Fujiwara, H. Cell-Extracellular Matrix Interactions in Normal and Diseased Skin. Cold Spring Harb. Perspect. Biol. 2011, 3, a005124. [Google Scholar] [CrossRef]
- Lombardi, B.; Casale, C.; Imparato, G.; Urciuolo, F.; Netti, P.A. Spatiotemporal Evolution of the Wound Repairing Process in a 3D Human Dermis Equivalent. Adv. Healthc. Mater. 2017, 6, 1–11. [Google Scholar] [CrossRef]
- Moiemen, N.S.; Vlachou, E.; Staiano, J.J.; Thawy, Y.; Frame, J.D. Reconstructive Surgery with Integra Dermal Regeneration Template: Histologic Study, Clinical Evaluation, and Current Practice. Plast. Reconstr. Surg. 2006, 117, 160S–174S. [Google Scholar] [CrossRef]
- Clark, J.A.; Leung, K.S. Mechanical properties of normal skin and hypetrophic scars. Burns 1996, 22, 443–446. [Google Scholar] [CrossRef]
- Frueh, F.S.; Sanchez-Macedo, N.; Calcagni, M.; Giovanoli, P.; Lindenblatt, N. The crucial role of vascularization and lymphangiogenesis in skin reconstruction. Eur. Surg. Res. 2018, 59, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Menger, M.D. Vascularization in tissue engineering: Angiogenesis versus inosculation. Eur. Surg. Res. 2012, 48, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future Prospects for Scaffolding Methods and Biomaterials in Skin Tissue Engineering: A Review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef]
- Hendrickx, B.; Verdonck, K.; Van den Berge, S.; Dickens, S.; Eriksson, E.; Vranckx, J.J.; Luttun, A. Integration of blood outgrowth endothelial cells in dermal fibroblast sheets promotes full thickness wound healing. Stem Cells 2010, 28, 1165–1177. [Google Scholar] [CrossRef]
- Lagus, H.; Sarlomo-Rikala, M.; Böhling, T.; Vuola, J. Prospective study on burns treated with Integra®, a cellulose sponge and split thickness skin graft: Comparative clinical and histological study—Randomized controlled trial. Burns 2013, 39, 1577–1587. [Google Scholar] [CrossRef]
- Cui, H.; Chai, Y.; Yu, Y. Review Article Progress in developing decellularized bioscaffolds for enhancing skin construction. J. Biomed. Mater. Res. Part A 2019, 107, 1849–1859. [Google Scholar]
- Moore, M.A.; Samsell, B.; Wallis, G.; Triplett, S.; Chen, S.; Jones, A.L.; Qin, X. Decellularization of human dermis using non-denaturing anionic detergent and endonuclease: A review. Cell Tissue Bank. 2015, 16, 249–259. [Google Scholar] [CrossRef]
- Macneil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef]
- Cheung, H.; Lau, K.; Lu, T.; Hui, D. A critical review on polymer-based bio-engineered materials for scaffold development. Compos. Part B 2007, 38, 291–300. [Google Scholar] [CrossRef]
- Zhong, S.P.; Zhang, Y.Z.; Lim, C.T. Tissue scaffolds for skin wound healing and dermal reconstruction. Nanomed. Nanobiotechnol. 2010, 2, 510–525. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, C.A. The history of tissue engineering. J. Cell. Mol. Med. 2006, 10, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Domaszewska-Szostek, A.; Krzyżanowska, M.; Siemionow, M. Cell-Based Therapies for Chronic Wounds Tested in Clinical Studies. Ann. Plast. Surg. 2019, 83, e96–e109. [Google Scholar] [CrossRef] [PubMed]
- Mazio, C.; Casale, C.; Imparato, G.; Urciuolo, F.; Attanasio, C.; De Gregorio, M.; Rescigno, F.; Netti, P.A. Pre-vascularized dermis model for fast and functional anastomosis with host vasculature. Biomaterials 2019, 192, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Martorina, F.; Casale, C.; Urciuolo, F.; Netti, P.A.; Imparato, G. In vitro activation of the neuro-transduction mechanism in sensitive organotypic human skin model. Biomaterials 2017, 113, 217–229. [Google Scholar] [CrossRef]
- Martin, I.; Wendt, D.; Heberer, M. The role of bioreactors in tissue engineering. TRENDS Biotechnol. 2004, 22, 80–86. [Google Scholar] [CrossRef]
- Ratcliffe, A.; Niklason, L.E.E. Bioreactors and Bioprocessing for Tiosue Engineering. Ann. N. Y. Acad. Sci. 2002, 961, 210–215. [Google Scholar] [CrossRef]
- Widjaja, W.; Tan, J.; Maitz, P.K.M.M. Efficacy of dermal substitute on deep dermal to full thickness burn injury: A systematic review. ANZ J. Surg. 2017, 87, 446–452. [Google Scholar] [CrossRef]
- Ucare. Bioengineering Skin Substitutes—Medical Policy 2016; Ucare Medical Policy-Policy Number: 2016M0011B; Ucare: Minneapolis, MN, USA, 2016. [Google Scholar]
- Mohebichamkhorami, F.; Alizadeh, A. Skin Substitutes: An Updated Review of Products from Year 1980 to 2017. J. Appl. Biotechnol. Reports 2017, 4, 615–623. [Google Scholar]
- Boyce, S.T.; Lalley, A.L. Tissue engineering of skin and regenerative medicine for wound care. Burns Trauma 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Gibot, L.; Galbraith, T.; Huot, J.; Auger, F.A. A preexisting microvascular network benefits in vivo revascularization of a microvascularized tissue-engineered skin substitute. Tissue Eng. Part A 2010, 16, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Grijpma, D.W.; Feijen, J. Porous polymeric structures for tissue engineering prepared by a coagulation, compression moulding and salt leaching technique. Biomaterials 2003, 24, 1937–1947. [Google Scholar] [CrossRef]
- Braghirolli, D.I.; Steffens, D.; Pranke, P. Electrospinning for regenerative medicine: A review of the main topics. Drug Discov. Today 2014, 19, 743–753. [Google Scholar] [CrossRef]
- Pörtner, R.; Nagel-Heyer, S.; Goepfert, C.; Adamietz, P.; Meenen, N.M. Bioreactor Design for Tissue Engineering. J. Biosci. Bioeng. 2005, 100, 235–245. [Google Scholar] [CrossRef]
- Helmedag, M.; Weinandy, S.; Marquardt, Y.; Baron, J.M.; Pallua, N. The Effects of Constant Flow Bioreactor Cultivation and Keratinocyte Seeding Densities on Organotypic Skin Grafts Based on a Fibrin Scaffold Corresponding Author. Tissue Eng. Part A 2013, 21, 1–31. [Google Scholar]
- Lei, X.H.; Ning, L.N.; Cao, Y.J.; Liu, S.; Zhang, S.B.; Qiu, Z.F.; Hu, H.M.; Zhang, H.S.; Liu, S.; Duan, E.K. NASA-Approved Rotary Bioreactor Enhances Proliferation of Human Epidermal Stem Cells and Supports Formation of 3D Epidermis-Like Structure. PLoS ONE 2011, 6, 1–8. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Slaughter, B.B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Yang, C.; Xu, L.; Zhou, Y.; Zhang, X.; Huang, X.; Wang, M.; Han, Y.; Zhai, M.; Wei, S.; Li, J. A green fabrication approach of gelatin/CM-chitosan hybrid hydrogel for wound healing. Carbohydr. Polym. 2010, 82, 1297–1305. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, X.; Shen, Y.I.; Sebastian, R.; Dickinson, L.E.; Fox-Talbot, K.; Reinblatt, M.; Steenbergen, C.; Harmon, J.W.; Gerecht, S. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. PNAS 2011, 108, 20976–20981. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.P.; Morgado, P.I.; Miguel, S.P.; Coutinho, P.; Correia, I.J. Dextran-based hydrogel containing chitosan microparticles loaded with growth factors to be used in wound healing. Mater. Sci. Eng. C 2013, 33, 2958–2966. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Review Collagen-Based Biomaterials for Wound Healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Heo, D.N.; Yang, D.H.; Lee, J.B.; Bae, M.S.; Kim, J.H.; Moon, S.H.; Chun, H.J.; Kim, C.H.; Lim, H.N.; Kwon, I.K. Burn-Wound Healing Effect of Gelatin/Polyurethane Nanofiber Scaffold Containing Silver-Sulfadiazine. J. Biomed. Nanotechnol. 2013, 9, 511–515. [Google Scholar] [CrossRef]
- Nunes, P.S.; Rabelo, A.S.; de Souza, J.C.; Santana, B.V.; da Silva, T.M.; Serafini, M.R.; dos Passos Menezes, P.; dos Santos Lima, B.; Cardoso, J.C.; Alves, J.C.; et al. Gelatin-based membrane containing usnic acid-loaded liposome improves dermal burn healing in a porcine model. Int. J. Pharm. 2016, 513, 473–482. [Google Scholar] [CrossRef]
- Ulubayram, K.; Cakar, A.N.; Korkusuz, P.; Ertan, C.; Hasirci, N. EGF containing gelatin-based wound dressings. Biomaterials 2001, 22, 1345–1356. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Khademhosseini, A.; Kaplan, D.L. Overview of Silk Fibroin Use in Wound Dressings. Trends Biotechnol. 2018, 36, 907–922. [Google Scholar] [CrossRef]
- Boucard, N.; Viton, C.; Agay, D.; Mari, E.; Roger, T.; Chancerelle, Y.; Domard, A. The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials 2007, 28, 3478–3488. [Google Scholar] [CrossRef]
- Opasanon, S.; Muangman, P.; Namviriyachote, N. Clinical effectiveness of alginate silver dressing in outpatient management of partial-thickness burns. Int. Wound J. 2010, 7, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.; Driver, V.R. Hyaluronic acid derivatives and their healing effect on burns, epithelial surgical wounds, and chronic wounds: A systematic review and meta-analysis of randomized controlled trials. Wound Repair Regen. 2012, 20, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Di Francesco, F.; Barisoni, D.; Leigh, I.M.; Navsaria, H.A. Use of hyaluronic acid and cultured autologous keratinocytes and fibroblasts in extensive burns. Lancet 1999, 353, 35–36. [Google Scholar] [CrossRef]
- Sood, R.; Roggy, D.; Zieger, M.; Balledux, J.; Chaudhari, S.; Koumanis, D.J.; Mir, H.S.; Cohen, A.; Knipe, C.; Gabehart, K.; et al. Cultured Epithelial Autografts for Coverage of Large Burn Wounds in Eighty-Eight Patients: The Indiana University Experience. J. Burn Care Res. 1990, 31, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Alexandra, P.M.; Rogeério, P.P.; Mariana, T.C.; Rui, L.R. Skin Tissue Models, 1st ed.; Mica Haley: Amsterdam, The Netherlands, 2018; pp. 1–451. [Google Scholar]
- Mostow, E.N.; Haraway, G.D.; Dalsing, M.; Hodde, J.P.; King, D.; OASIS Venus Ulcer Study Group. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: A randomized clinical trial. J. Vasc. 2005, 41, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Groeber, F.; Engelhardt, L.; Lange, J.; Kurdyn, S.; Schmid, F.F.; Rücker, C.; Mielke, S.; Walles, H.; Hansmann, J. A first vascularized skin equivalent as an alternative to animal experimentation. ALTEX-Altern. Anim. Exp. 2016, 33, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Norton, D.; Haycock, J.W.; Ryan, A.J.; MacNeil, S. Development of a Closed Bioreactor System for Culture of Tissue-Engineered Skin at an Air—Liquid Interface. Tissue Eng. 2005, 11, 1824–1831. [Google Scholar] [CrossRef]
- Hartmann-Fritsch, F. About ATMPs, SOPs and GMP: The Hurdles to Produce novel skin grafts for clinical use. Transfus. Med. Hemother. 2016, 43, 344–352. [Google Scholar] [CrossRef]
- Griffin, J.W.; Mcarthur, J.C.; Polydefkis, M. Assessment of cutaneous innervation by skin biopsies. Curr. Opin. Neurol. 2001, 14, 655–659. [Google Scholar] [CrossRef]
- Casale, C.; Imparato, G.; Urciuolo, F.; Netti, P. Endogenous human skin equivalent promotes in vitro morphogenesis of follicle-like structures. Biomaterials 2016, 101, 86–95. [Google Scholar] [CrossRef]
- Vader, D.; Kabla, A.; Weitz, D.; Mahadevan, L. Strain-Induced Alignment in Collagen Gels. PLoS ONE 2009, 4, e5902. [Google Scholar] [CrossRef]
- Imparato, G.; Urciuolo, F.; Casale, C.; Netti, P.A.; Imparato, G.; Urciuolo, F.; Casale, C.; Netti, P.A. The role of microscaffold properties in controlling the collagen assembly in 3D dermis equivalent using modular tissue engineering. Biomaterials 2013, 34, 7851–7861. [Google Scholar] [CrossRef] [PubMed]
- Varkey, M.; Ding, J.; Tredget, E.E. Advances in Skin Substitutes—Potential of Tissue Engineered Skin for Facilitating Anti-Fibrotic Healing. J. Funct. Biomater. 2015, 6, 547–563. [Google Scholar] [CrossRef] [PubMed]
- van Zuijlen, P.P.; Ruurda, J.J.; van Veen, H.A.; van Marle, J.; van Trier, A.J.; Groenevelt, F.; Kreis, R.W.; Middelkoop, E. Collagen morphology in human skin and scar tissue: No adaptations in response to mechanical loading at joints. Burns 2003, 29, 423–431. [Google Scholar] [CrossRef]
- Sidgwick, G.P.; McGeorge, D.; Bayat, A. A comprehensive evidence-based review on the role of topicals and dressings in the management of skin scarring. Arch. Dermatol. Res. 2015, 307, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Khademhosseini, A.; Nichol, J.W. Modular tissue engineering: Engineering biological tissues from the bottom up. Soft Matter 2009, 5, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yamato, M.; Kohno, C.; Nishimoto, A. Cell sheet engineering: Recreating tissues without biodegradable scaffolds $. Biomaterials 2005, 26, 6415–6422. [Google Scholar] [CrossRef]
- Mironov, V.; Visconti, R.P.; Kasyanov, V.; Forgacs, G.; Drake, C.J.; Markwald, R.R. Organ printing: Tissue spheroids as building blocks. Biomaterials 2009, 30, 2164–2174. [Google Scholar] [CrossRef]
- Urciuolo, F.; Garziano, A.; Imparato, G.; Panzetta, V.; Fusco, S.; Casale, C.; Netti, P.A. Biophysical properties of dermal building-blocks affect extra cellular matrix assembly in 3D endogenous macrotissue. Biofabrication 2016, 8, 015010. [Google Scholar] [CrossRef]
- Leong, W.; Wang, D. Cell-laden Polymeric Microspheres for Biomedical Applications. Trends Biotechnol. 2015, 33, 653–666. [Google Scholar] [CrossRef]
- Matsunaga, Y.T.; Morimoto, Y.; Takeuchi, S. Molding Cell Beads for Rapid Construction of Macroscopic 3D Tissue Architecture. Adv. Healthc. Mater. 2011, 23, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Onoe, H.; Okitsu, T.; Itou, A.; Kato-Negishi, M.; Gojo, R.; Kiriya, D.; Sato, K.; Miura, S.; Iwanaga, S.; Kuribayashi-Shigetomi, K.; et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 2013, 12, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Larouche, D.; Cantin-warren, L.; Moulin, J.; Germain, L. Improved Methods to Produce Tissue-Engineered Skin Substitutes Suitable for the Permanent Closure of Full-Thickness Skin Injuries. Biores. Open Access 2016, 5, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, B.; Kim, H.N.; Bang, S.; Yang, H.S.; Kang, S.M.; Suh, K.Y.; Park, S.H. 3D tissue formation by stacking detachable cell sheets formed on nanofiber mesh tissues and organs. Biofabrication 2017, 9, 15029. [Google Scholar] [CrossRef]
- Toma, C.C.; Corato, R.; Di Rinaldi, R. Microfluidics and BIO-encapsulation for drug-and cell-therapy. In Proceedings of the Organic Sensors and Bioelectronics X—International Society for Optics and Photonics 2017, San Diego, CA, USA, 6–7 August 2017. [Google Scholar]
- Jiang, W.; Li, M.; Chen, Z.; Leong, K.W. Cell-laden microfluidic microgels for tissue regeneration. Lab Chip 2016, 16, 4482–4506. [Google Scholar] [CrossRef]
- Palmiero, C.; Imparato, G.; Urciuolo, F.; Netti, P.A. Engineered dermal equivalent tissue in vitro by assembly of microtissue precursors. Acta Biomater. 2010, 6, 2548–2553. [Google Scholar] [CrossRef]
- Urciuolo, F.; Imparato, G.; Totaro, A.; Netti, P.A. Building a Tissue In Vitro from the Bottom Up: Implications in Regenerative Medicine. DeBakey Cardiovasc. J. 2013, 9, 213–217. [Google Scholar] [CrossRef]
- Urciuolo, F.; Imparato, G.; Palmiero, C.; Trilli, A.; Netti, P.A. Effect of Process Conditions on the Growth of Three-Dimensional Dermal-Equivalent Tissue Obtained by Microtissue Precursor Assembly. Tissue Eng. Part C 2011, 17, 155–164. [Google Scholar] [CrossRef]
- Urciuolo, F. Lab on a Chip A micro-perfusion bioreactor for on line hydrodynamic and biochemical stimulation. Lab Chip 2016, 16, 855–867. [Google Scholar]
- Jakab, K.; Norotte, C.; Damon, B.; Marga, F.; Neagu, A.; Besch-Williford, C.L.; Kachurin, A.; Church, K.H.; Park, H.; Mironov, V.; et al. Tissue Engineering by Self-Assembly of Cells Printed into Topologically Defined Structures. Tissue Eng. Part A 2008, 14, 413–421. [Google Scholar] [CrossRef]
- Moroni, L.; Boland, T.; Burdick, J.A.; De Maria, C.; Derby, B.; Forgacs, G.; Mota, C. Biofabrication: A guide to technology and terminology. Trends Biotechnol. 2018, 36, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Boland, T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 2009, 30, 6221–6227. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Young, V. A Review of 3D Printing Techniques and the Future in Biofabrication of Bioprinted Tissue. Cell Biochem. Biophys. 2016, 74, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Fetah, K.; Tebon, P.; Goudie, M.J.; Eichenbaum, J.; Ren, L.; Barros, N.; Nasiri, R.; Ahadian, S.; Ashammakhi, N.; Dokmeci, M.R.; et al. The emergence of 3D bioprinting in organ-on-chip systems. Reports Prog. Biomed. Eng. 2019, 1, 012001. [Google Scholar] [CrossRef]
- Pourchet, L.J.; Thepot, A.; Albouy, M.; Courtial, E.J.; Boher, A.; Blum, L.J.; Marquette, C.A. Human Skin 3D Bioprinting Using Scaffold-Free Approach. Adv. Healthc. Mater. 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Tarassoli, S.P.; Jessop, Z.M.; Al-Sabah, A.; Gao, N.; Whitaker, S.; Doak, S.; Whitaker, I.S. Skin tissue engineering using 3D bioprinting: An evolving research field. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 615–623. [Google Scholar] [CrossRef]
- Cubo, N.; Garcia, M.; Cañizo, J.F.; Velasco, D.; Jorcano, J.L. 3D bioprinting of functional human skin: Production and in vivo analysis 3D bioprinting of functional human skin: Production and in vivo analysis. Biofabrication 2017, 9, 1–12. [Google Scholar]
- Lee, W.; Debasitis, J.C.; Lee, V.K.; Lee, J.H.; Fischer, K.; Edminster, K.; Park, J.K.; Yoo, S.S. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 2009, 30, 1587–1595. [Google Scholar] [CrossRef]
- Yanez, M.; Rincon, J.; Dones, A.; De Maria, C.; Gonzales, R.; Boland, T. In Vivo Assessment of Printed Microvasculature in a Bilayer Skin Graft to Treat Full-Thickness Wounds. Tissue Eng. Part A 2015, 21, 224–233. [Google Scholar] [CrossRef]
- De Angelis, B.; Orlandi, F.; Fernandes Lopes Morais D’Autilio, M.; Scioli, M.G.; Orlandi, A.; Cervelli, V.; Gentile, P. Long-term follow-up comparison of two different bi-layer dermal substitutes in tissue regeneration: Clinical outcomes and histological findings. Int. Wound J. 2018, 15, 695–706. [Google Scholar] [CrossRef]
- Shukla, A.; Dey, N.; Nandi, P. Acellular Dermis as a Dermal Matrix of Tissue Engineered Skin Substitute for Burns Treatment. Ann. Public Heal. Res. 2015, 2, 1023. [Google Scholar]
- Wainwright, D.J. Use of an acellular allograft dermal matrix in the management of full-thickness burns. Burns 1995, 21, 243–248. [Google Scholar] [CrossRef]
- Troy, J.; Karlnoski, R.; Downes, K. The Use of EZ Derm R in Partial-Thickness Burns: An Institutional Review of 157 Patients. Eplasty 2013, 13, 108–119. [Google Scholar]
- Baus, A.; Combes, F.; Lakhel, A.; Pradier, J.P.; Brachet, M.; Duhoux, A.; Duhamel, P.; Fossat, S.; Bey, E. Chirurgia delle ustioni gravi in fase acuta. EMC-Tec. Chir.-Chir. Plast. Ricostr. Estet. 2017, 15, 1–25. [Google Scholar] [CrossRef]
- Costa-almeida, R.; Soares, R.; Granja, P.L. Fibroblasts as maestros orchestrating tissue regeneration. J. Tissue Eng. Regen. Med. 2018, 12, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R.A.; Ong, J.F.; Junker, J.P.E. Tissue Engineering of Skin. J. Am. Coll. Surg. 2013, 217, 533–555. [Google Scholar] [CrossRef]
- Philandrianos, C.; Andrac-Meyer, L.; Mordon, S.; Feuerstein, J.M.; Sabatier, F.; Veran, J.; Magalon, G.; Casanova, D. Comparison of five dermal substitutes in full-thickness skin wound healing in a porcine model. Burns 2012, 38, 820–829. [Google Scholar] [CrossRef]
- Hansbrough, J.F.; Mozingo, D.W.; Kealey, G.P.; Davis, M.; Gidner, A.; Gentzkow, G.D. Clinical trials of a biosynthetic temporary skin replacement DermagraftTC compared with cryoperserved human cadaver skin for temporary coverage of excised burn. J. Burn Care Rehabil. 1997, 18, 43–51. [Google Scholar] [CrossRef]
- Hart, C.E.; Loewen-rodriguez, A.; Lessem, J. Wound care products dermagraft: Use in the Treatment of Chronic Wounds. Adv. Wound Care 2012, 1, 6–18. [Google Scholar] [CrossRef]
- Demling, R.H.; Desanti, L. Management of partial thickness facial burns (comparison of topical antibiotics and bio-engineered skin substitutes) p. Burns 1999, 25, 256–261. [Google Scholar] [CrossRef]
- Kumar, R.J.; Kimble, R.M.; Boots, R.; Pegg, S.P. Treatment of partial thicness burns: A prospective, randomized trial using TranscyteTM. ANZ J. Surg. 2004, 74, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Santema, T.B.K.; Poyck, P.P.C.; Ubbink, D.T. Systematic review and meta-analysis of skin substitutes in the treatment of diabetic foot ulcers: Highlights of a Cochrane systematic review. Wound Repair Regen. 2016, 24, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Sabolinski, M. A bilayered living skin construct (APLIGRAF ®) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen. 1999, 7, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.; Ojeh, N.; Res, M.; Livingstone, R.; Price, R. Survival of Apligraf in Acute Human Wounds. Tissue Eng. 2004, 10, 1180–1195. [Google Scholar] [CrossRef] [PubMed]
- Kirsner, R.S.; Sabolinski, M.L.; Parsons, N.B.; Skornicki, M.; Marston, W.A. Comparative effectiveness of a bioengineered living cellular construct vs. a dehydrated human amniotic membrane allograft for the treatment of diabetic foot ulcers in a real world setting. Wound Repair Regen. 2015, 23, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Falabella, A.F.; Valencia, I.C.; Eaglstein, W.H.; Schachner, L.A. Tissue-Engineered Skin (Apligraf) in the Healing of Patients with Epidermolysis Bullosa Wounds. Arch. Dermatol. 2000, 136, 1225–1230. [Google Scholar] [CrossRef]
- Efanov, J.I.; Tchiloemba, B.; Duong, A.; Bélisle, A.; Izadpanah, A.; Coeugniet, E.; Danino, M.A. Use of bilaminar grafts as life-saving interventions for severe burns: A single-center experience. Burns 2018, 44, 1336–1345. [Google Scholar] [CrossRef]
- Forbes-Duchart, L.; Marshall, S.; Strock, A.; Cooper, J.E. Determination of Inter-Rater Reliability in Pediatric Burn Scar Assessment Using a Modified Version of the Vancouver Scar Scale. J. Burn Care Res. 2007, 28, 460–467. [Google Scholar] [CrossRef]
- Fearmonti, R.; Bond, J.; Erdmann, D. A Review of Scar Scales and Scar Measuring. Eplasty 2010, 10, 354–363. [Google Scholar]
- Draaijers, L.J.; Tempelman, F.R.; Botman, Y.A.; Tuinebreijer, W.E.; Middelkoop, E.; Kreis, R.W.; van Zuijlen, P.P. The Patient and Observer Scar Assessment Scale: A Reliable and Feasible Tool for Scar Evaluation. Plast. Reconstr. Surg. 2003, 113, 1960–1965. [Google Scholar] [CrossRef]
- Tyack, Z.; Simons, M.; Spinks, A.; Wasiak, J. A systematic review of the quality of burn scar rating scales for clinical and research use. Burns 2011, 38, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.D.; Kessler, M.; Meyer, A.A.; Bonham Morris, P.A. A Trial of the Effectiveness of Artificial Dermis in the Treatment of Patients with Burns Greater Than 45% Total Body Surface Area. J. Trauma 1988, 39, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, D.A.; Luterman, A.R.; Burke, J.O.; Cram, A.L.; Herndon, D.A.; Hunt, J.O.; Jordan, M.A.; McMANUS, W.I.; Solem, L.Y.; Warden, G.L. Artificial dermis for major burns. A multi-center randomized clinical trial. Ann. Surg. 1988, 208, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Rennekampff, H.O.; Pfau, M.; Schaller, H.E. Acellular allograft dermal matrix: Immediate or delayed epidermal coverage? Burns 2001, 27, 150–153. [Google Scholar] [CrossRef]
- Bloemen, M.C.; van der Wal, M.B.; Verhaegen, P.D.; Nieuwenhuis, M.K.; van Baar, M.E.; van Zuijlen, P.P.; Middelkoop, E. Clinical effectiveness of dermal substitution in burns by topical negative pressure: A multicenter randomized controlled trial. Wound Repair Regen. 2012, 20, 797–805. [Google Scholar] [CrossRef]
- Wall, I.B.; Moseley, R.; Baird, D.M.; Kipling, D.; Giles, P.; Laffafian, I.; Price, P.E.; Thomas, D.W.; Stephens, P. Fibroblast dysfunction is a key factor in the non-healing of chronic venous leg ulcers. J. Investig. Dermatol. 2008, 128, 2526–2540. [Google Scholar] [CrossRef]
- Harding, K.; Sumner, M.; Cardinal, M. A prospective, multicentre, randomised controlled study of human fibroblast-derived dermal substitute (Dermagraft) in patients with venous leg ulcers. Int. Wound J. 2013, 10, 132–137. [Google Scholar] [CrossRef]
- Hanft, J.R.; Surprenant, M.S. Healing of chronic foot ulcers in diabetic patients treated with a human fibroblast-derived dermis. J. Foot Ankle Surg. 2002, 41, 291–299. [Google Scholar] [CrossRef]
- Omar, A.A.; Mavor, A.I.D.; Jones, A.M.; Homer-Vanniasinkam, S. Treatment of venous leg ulcers with Dermagraft. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 666–672. [Google Scholar] [CrossRef]
- ClinicalTraials. Phase I Study for Autologous Dermal Substitutes and Dermo-epidermal Skin Substitutes for Treatment of Skin Defects. 2014. Available online: https://clinicaltrials.gov/ct2/show/NCT02145130 (accessed on 10 September 2019).
- Braziulis, E.; Diezi, M.; Biedermann, T.; Pontiggia, L.; Schmucki, M.; Hartmann-Fritsch, F.; Luginbühl, J.; Schiestl, C.; Meuli, M.; Reichmann, E. Modified plastic compression of collagen hydrogels provides an ideal matrix for clinically applicable skin substitutes. Tissue Eng. Part C Methods 2012, 18, 464–474. [Google Scholar] [CrossRef]
- Suzuki, M.; Yakushiji, N.; Nakada, Y.; Satoh, A. Limb Regeneration in Xenopus laevis Froglet. Sci. World J. 2006, 6, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials. Available online: https://clinicaltrials.gov/ (accessed on 10 September 2019).
- FDA. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/ (accessed on 10 September 2019).
- Frueh, F.S.; Später, T.; Körbel, C.; Scheuer, C.; Simson, A.C.; Lindenblatt, N.; Giovanoli, P.; Menger, M.D.; Laschke, M.W. Prevascularization of dermal substitutes with adipose tissue-derived microvascular fragments enhances early skin grafting. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]

| Product | Composition | Indications | FDA Status |
|---|---|---|---|
| ALLODERM® | Acellular human dermis– non crosslinked | Repair or replacement of damaged or inadequate integument tissue | HCT/P |
| DERMACELL® | Acellular human dermis– non crosslinked | Chronic non-healing wounds | HCT/P |
| DERMAMATRIX® | Acellular human dermis– non crosslinked | Soft tissue replacement Breast Reconstruction | Available through the Musculoskeletal Transplant Foundation which meets and exceeds the standards and regulations of the American Association of Tissue Banks (AATB) and the Food and Drug Administration (FDA) |
| SUREDERM® | Acellular human dermis– non crosslinked | Soft tissue replacement | HCT/P |
| OASIS® | Porcine acellular lyophilized small intestine submucosa– non crosslinked | Acute, chronic and burns wounds. It delivers growth factors to stimulate and cell migration angiogenesis | 510(k) |
| PERMACOLL® | Porcine acellular diisocyanite -crosslinked | Full-thickness defects such as burns and for soft tissue reconstruction such as hernia repair | 510(k) |
| EZ-DERM® | Porcine aldehyde cross-linked reconstituted dermal collagen | Partial-thickness burns | 510(k) |
| INTEGRA® | Acellular Bovine type I collagen and chondroitin- 6-sulfate copolymer coated with a thin silicone elastomer - crosslinked | Deep partial thickness and full thickness burns | PMA (1996) 510(k) (2002) |
| BIOBRANE® | Ultrathin silicone as epidermal analog film and 3D nylon filament as dermal analog with type I collagen peptides | Partial-thickness burns in children; toxic epidermal necrolysis, paraneoplastic pemphigus and chronic wounds | 510(k) |
| MATRIDERM® | Bovine non-crosslinked lyophilized dermis, coated with α-elastin hydrolysate | Full-thickness burns | 510(k) |
| HYALOMATRIX® | Acellular non-woven pad of benzyl ester of hyaluronic acid and a silicone membrane– non crosslinked | Burns, chronic wounds. | 510(k) |
| Product | Composition | Indications | Status |
|---|---|---|---|
| DERMAGRAFT® (d) | Human cultured neonatal fibroblasts seeded on polyglactin scaffold | Treatment of diabetic foot ulcers, epidermolysisbullosa | PMA (2001) |
| TRANSCYTE® (d) | Nylon mesh coated with bovine collagen and seeded with allogenic neonatal human foreskin fibroblasts | Full and partial thickness burns | PMA (1998) |
| ICX-SKN® (d) | A fibrin matrix seeded with neonatal human fibroblasts | Deep dermal wounds | - |
| DENOVODERM® (d) | Autologous fibroblasts in collagen hydrogel | Deep defect of the skin | In development, under clinical trials |
| HYALOGRAFT3D® (d) | Based on estherified hyaluronic acid derivate with cultured fibroblasts and covered by a silicone membrane | Use in diabetic ulcer therapy has been reported | 510(k) |
| APLIGRAFT® (ft) | Bovine collagen matrix seeded with neonatal foreskin fibroblasts and keratinocytes | Treatment of various forms of epidermolysisbullosa Diabetic and venous ulcers | PMA Commercially available in the USA |
| TISSUETECH® (ft) | Hyaluronic acid with cultured autologous keratinocytes and fibroblasts (Hyalograft 3D® + Laserskin®) | Ulcers | - |
| PERMADERM® (ft) | Autologous fibroblasts and keratinocytes in culture with bovine collagen and GAG substrates | Sever Burns | - |
| ORCEL® (ft) | Type I bovine collagen matrix seeded with allogenic neonatal foreskin fibroblasts and keratinocyte | Donor sites in Epidermolysis Bullosa Fresh, clean split thickness donor site wounds in burn patients | PMA (2001) |
| DENOVOSKIN® (ft) | Autologous fibroblasts in collagen hydrogel and autologous keratinocytes | Deep defect of the skin | In development, under clinical trials |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urciuolo, F.; Casale, C.; Imparato, G.; Netti, P.A. Bioengineered Skin Substitutes: The Role of Extracellular Matrix and Vascularization in the Healing of Deep Wounds. J. Clin. Med. 2019, 8, 2083. https://doi.org/10.3390/jcm8122083
Urciuolo F, Casale C, Imparato G, Netti PA. Bioengineered Skin Substitutes: The Role of Extracellular Matrix and Vascularization in the Healing of Deep Wounds. Journal of Clinical Medicine. 2019; 8(12):2083. https://doi.org/10.3390/jcm8122083
Chicago/Turabian StyleUrciuolo, Francesco, Costantino Casale, Giorgia Imparato, and Paolo A. Netti. 2019. "Bioengineered Skin Substitutes: The Role of Extracellular Matrix and Vascularization in the Healing of Deep Wounds" Journal of Clinical Medicine 8, no. 12: 2083. https://doi.org/10.3390/jcm8122083
APA StyleUrciuolo, F., Casale, C., Imparato, G., & Netti, P. A. (2019). Bioengineered Skin Substitutes: The Role of Extracellular Matrix and Vascularization in the Healing of Deep Wounds. Journal of Clinical Medicine, 8(12), 2083. https://doi.org/10.3390/jcm8122083




