Switching between Three Types of Mesalazine Formulation and Sulfasalazine in Patients with Active Ulcerative Colitis Who Have Already Received High-Dose Treatment with These Agents
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Evaluated Outcomes
2.3. Statistical Analyses
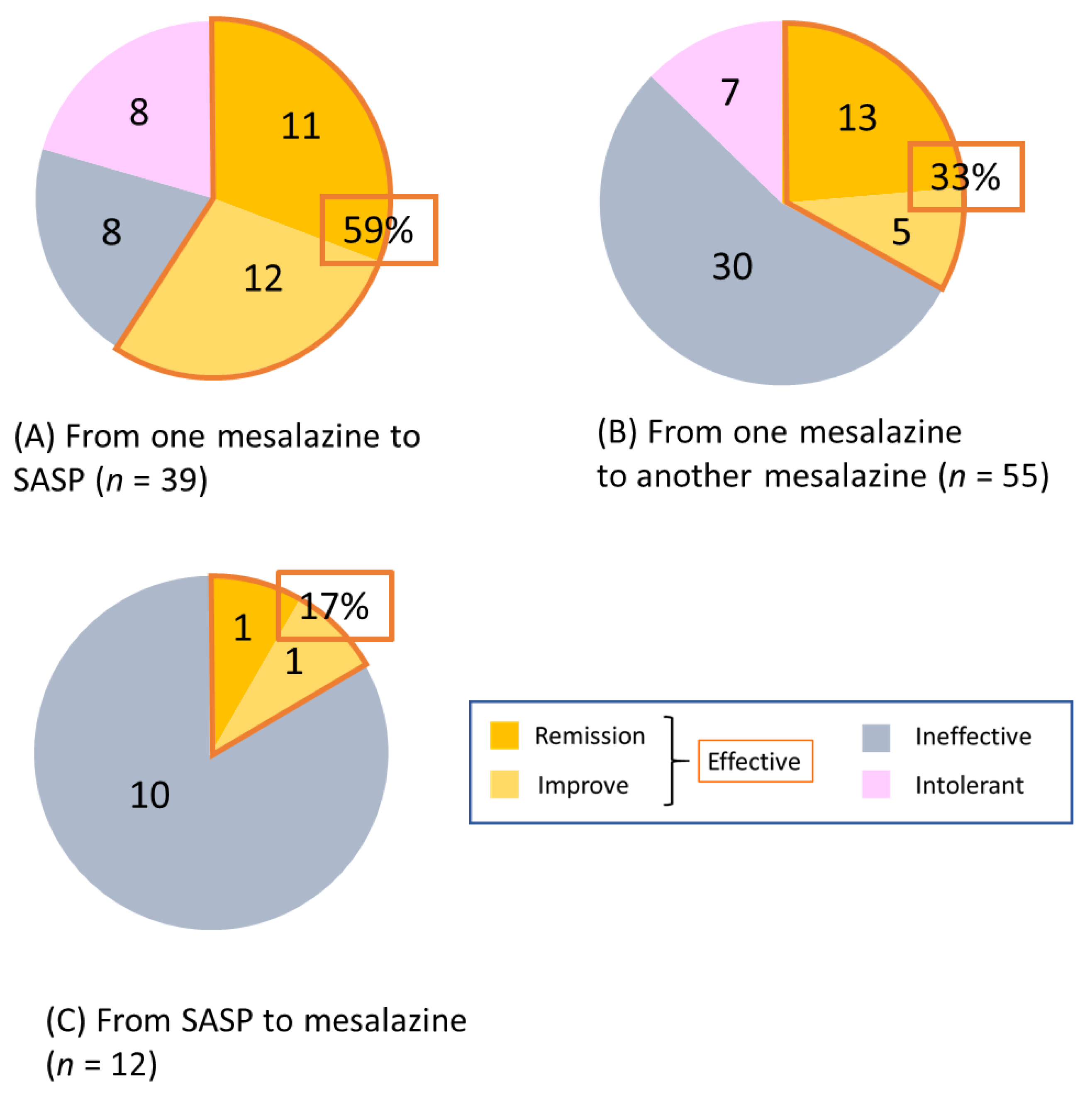
3. Results
3.1. Patient Characteristics
3.2. Short-Term Efficacy of Switching
3.3. Long-Term Outcomes of Switching
3.4. Achievement of Steroid-Free Remission after Switching between Mesalazine Formulations and SASP
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Podolsky, D.K. Inflammatory bowel disease. N. Engl. J. Med. 2002, 347, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Feuerstein, J.D.; Binion, D.G.; Tremaine, W.J. AGA Technical Review on the Management of Mild-to-Moderate Ulcerative Colitis. Gastroenterology 2019, 156, 769–808. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.L.; Sachar, D.B. Update on antitumor necrosis factor agents and other new drugs for inflammatory bowel disease. BMJ 2017, 357, j2505. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Parker, C.E.; Bhanji, T.; Feagan, B.G.; MacDonald, J.K. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2016, 4, CD000543. [Google Scholar] [CrossRef]
- Wang, Y.; Parker, C.E.; Feagan, B.G.; MacDonald, J.K. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2016, 5, CD000544. [Google Scholar] [CrossRef]
- Ye, B.; van Langenberg, D.R. Mesalazine preparations for the treatment of ulcerative colitis: Are all created equal? World J. Gastrointest. Pharmacol. Ther. 2015, 6, 137–144. [Google Scholar] [CrossRef]
- Schroeder, H.; Campbell, D.E.S. Absorption, metabolism, and excretion of salicylazosulfapyridine in man. Clin. Pharmacol. Ther. 1972, 13, 539–551. [Google Scholar] [CrossRef]
- Myers, B.; Evans, D.N.W.; Rhodes, J.; Evans, B.K.; Hughes, B.R.; Lee, M.G.; Richens, A.; Richards, D. Metabolism and urinary excretion of 5-aminosalicylic acid in healthy volunteers when given intravenously or released for absorption at different sites in the gastrointestinal tract. Gut 1987, 28, 196–200. [Google Scholar] [CrossRef]
- Gibson, P.R.; Fixa, B.; Pekárková, B.; Bátovský, M.; Radford-Smith, G.; Tibitanzl, J.; Gabalec, L.; Florin, T.H.; Greinwald, R. Comparison of the efficacy and safety of Eudragit-L-coated mesalazine tablets with ethylcellulose-coated mesalazine tablets in patients with mild to moderately active ulcerative colitis. Aliment. Pharmacol. Ther. 2006, 23, 1017–1026. [Google Scholar] [CrossRef]
- Ogata, H.; Yokoyama, T.; Mizushima, S.; Hagino, A.; Hibi, T. Comparison of efficacy of once-daily multimatrix mesalazine 2.4 g/day and 4.8 g/day with other 5-aminosalicylic acid preparation in active ulcerative colitis: A randomized, double-blind study. Intest. Res. 2018, 16, 255–266. [Google Scholar] [CrossRef]
- Yoshino, T.; Sono, M.; Yazumi, S. Usefulness of sulfasalazine for patients with refractory-ulcerative colits. BMJ Open Gastroenterol. 2016, 16, e000103. [Google Scholar] [CrossRef] [PubMed]
- Jairath, V.; Khanna, R.; Zou, G.Y.; Stitt, L.; Mosli, M.; Vandervoort, M.K.; D’Haens, G.; Sandborn, W.J.; Feagan, B.G.; Levesque, B.G. Development of interim patient-reported outcome measures for the assessment of ulcerative colitis disease activity in clinical trials. Aliment. Pharmacol. Ther. 2015, 42, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Rachmilewitz, D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: A randomised trial. BMJ 1989, 298, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Lichtiger, S.; Present, D.H.; Kornbluth, A.; Gelernt, I.; Bauer, J.; Galler, G.; Michelassi, F.; Hanauer, S. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N. Engl. J. Med. 1994, 330, 1841–1845. [Google Scholar] [CrossRef] [PubMed]
- Nakarai, A.; Kato, J.; Hiraoka, S.; Kuriyama, M.; Akita, M.; Hirakawa, T.; Okada, H.; Yamamoto, K. Evaluation of mucosal healing of ulcerative colitis by a quantitative fecal immunochemical test. Am. J. Gastroenterol. 2013, 108, 83–89. [Google Scholar] [CrossRef]
- Takashima, S.; Kato, J.; Hiraoka, S.; Nakarai, A.; Takei, D.; Inokuchi, T.; Sugihara, Y.; Takahara, M.; Harada, K.; Okada, H.; et al. Evaluation of Mucosal Healing in Ulcerative Colitis by Fecal Calprotectin vs. Fecal Immunochemical Test. Am. J. Gastroenterol. 2015, 110, 873–880. [Google Scholar] [CrossRef]
- Hiraoka, S.; Inokuchi, T.; Nakarai, A.; Takashima, S.; Takei, D.; Sugihara, Y.; Takahara, M.; Harada, K.; Okada, H.; Kato, J. Fecal immunochemical test and fecal calprotectin show different profiles in disease monitoring for ulcerative colitis. Gut Liver 2018, 12, 142–148. [Google Scholar] [CrossRef]
- Rasmussen, S.N.; Bondesen, S.; Hvidberg, E.F.; Hansen, S.H.; Binder, V.; Halskov, S.; Flachs, H. 5-aminosalicylic acid in a slow-release preparation: Bioavailability, plasma level, and excretion in humans. Gastroenterology 1982, 83, 1062–1070. [Google Scholar]
- Lakatos, P.L. Use of new once-daily 5-aminosalicylic acid preparations in the treatment of ulcerative colitis: Is there anything new under the sun? World J. Gastroenterol 2009, 15, 1799–1804. [Google Scholar] [CrossRef][Green Version]
- Ito, H.; Iida, M.; Matsumoto, T.; Suzuki, Y.; Sasaki, H.; Yoshida, T.; Takano, Y.; Hibi, T. Direct comparison of two different mesalamine formulations for the induction of remission in patients with ulcerative colitis: A double-blind, randomized study. Inflamm. Bowel. Dis. 2010, 16, 1567–1574. [Google Scholar] [CrossRef][Green Version]
- Prantera, C.; Kohn, A.; Campieri, M.; Caprilli, R.; Cottone, M.; Pallone, F.; Savarino, V.; Sturniolo, G.C.; Vecchi, M.; Ardia, A.; et al. Clinical trial: Ulcerative colitis maintenance treatment with 5-ASA: A 1-year, randomized multicentre study comparing MMX with Asacol. Aliment. Pharmacol. Ther. 2009, 30, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Ishihara, S.; Yuki, T.; Onishi, K.; Kushiyama, Y.; Fujishiro, H.; Miyaoka, Y.; Yuki, M.; Komazawa, Y.; Tanimura, T.; et al. Therapeutic efficacy of pH-dependent release formulation of mesalazine on active ulcerative colitis resistant to time-dependent release formulation: Analysis of fecal calprotectin concentration. BioMed Res. Int. 2014, 2014, 342751. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; Wei, S.C. Efficacy of Pentasa tablets for the treatment of inflammatory bowel disease. J. Formos. Med. Assoc. 2003, 102, 613–619. [Google Scholar] [PubMed]
- Martínez, G.J.; Busto, B.V.; Mesonero, G.F.; Parejo, C.S.; Garrido, E.; López, S.R.A. Rescue therapy with sulfasalazine prior to immunosuppressive or biological agents in ulcerative colitis poorly controlled with mesalazine. Gastroenterol. Hepatol. 2013, 36, 7–10. [Google Scholar]
- Svartz, N. Salazyopyrin, a new sulfanilamide preparation: A. Therapeutic results in rheumatic polyarthritis. B. Therapeutic results in ulcerative colitis. C. Toxic manifestations in treatment with sulfanilamide preparation. Acta Med. Scand. 1942, 110, 557–590. [Google Scholar]
- Misiewitz, J.J.; Lennard-Jones, J.E.; Connell, A.M.; Baron, J.H.; Jones, F.A. Controlled trial of sulfasalazine in maintenance therapy for ulcerative colitis. Lancet 1965, 1, 185–188. [Google Scholar] [CrossRef]
- Fujiwara, M.; Mitsui, K.; Yamamoto, I. Inhibition of proliferative response and interleukin 2 productions by salazosulpapyridine and its metabolites. Jpn. J. Pharmacol. 1990, 54, 121–131. [Google Scholar] [CrossRef]
- Fujiwara, M.; Mitsui, K.; Ishida, J.; Yamamoto, I. The effect of salazosulfapyridine on the in vitro antibody production in murine spleen cells. Immunopharmacology 1990, 19, 15–21. [Google Scholar] [CrossRef]
- Matasic, R.; Dietz, A.B.; Vuk-Pavlovic, S. Maturation of human dendritic cells as sulfasalazine target. Croat. Med. J. 2001, 42, 440–445. [Google Scholar]
- Rodenburg, R.J.T.; Ghnga, A.; van Lent, P.L.E.M.; Van De Putte, L.B.A.; Van Venrooij, W.J. The anti-inflammatory drug sulfasalazine inhibits tumor necrosis factor alpha expression in macrophages by inducing apoptosis. Arthritis Rheum. 2000, 43, 1941–1950. [Google Scholar] [CrossRef]
- Kruis, W.; Fric, P.; Pokrotnieks, J.; Lukáš, M.; Fixa, B.; Kaščák, M.; Kamm, M.A.; Weismueller, J.; Beglinger, C.; Stolte, M.; et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, G.; Sturniolo, G.C.; Bossa, F.; Cassinotti, A.; Di Sabatino, A.; Giuffrida, P.; Danese, S. A phase 2a, multicenter, randomized, double-blind, parallel-group, placebo-controlled trial of IBD98-M delayed-release capsules to induce remission in patients with active and mild to moderate ulcerative colitis. Cells 2019, 8, 523. [Google Scholar] [CrossRef] [PubMed]




| Patients | 88 |
|---|---|
| Gender | |
| Male/Female | 48/40 |
| Location of disease | |
| Pancolitis/Left-sided/Proctitis | 58/26/4 |
| Median (IQR) age at diagnosis, years | 30 (18–44) |
| Median (IQR) disease duration, years | 10 (7–16) |
| Median (IQR) age at the switching, years | 38 (26–50) |
| Switching of mesalazine/SASP formulations | 106 |
| Median (IQR) PRO2 | 2 (2–3) |
| Formulations of mesalazine or SASP before switch | |
| Time-dependent/pH-dependent/once-daily MMX/SASP | 46/48/0/12 |
| Formulations of mesalazine or SASP after switch | |
| Time-dependent/pH-dependent/once-daily MMX/SASP | 11/27/29/39 |
| Concomitant drugs | |
| Oral corticosteroid | 22 |
| Topical corticosteroid | 15 |
| Immun omodulator | 51 |
| Tacrolimus | 17 |
| Anti-TNFα agent | 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasutomi, E.; Hiraoka, S.; Yamamoto, S.; Oka, S.; Hirai, M.; Yamasaki, Y.; Inokuchi, T.; Kinugasa, H.; Takahara, M.; Harada, K.; et al. Switching between Three Types of Mesalazine Formulation and Sulfasalazine in Patients with Active Ulcerative Colitis Who Have Already Received High-Dose Treatment with These Agents. J. Clin. Med. 2019, 8, 2109. https://doi.org/10.3390/jcm8122109
Yasutomi E, Hiraoka S, Yamamoto S, Oka S, Hirai M, Yamasaki Y, Inokuchi T, Kinugasa H, Takahara M, Harada K, et al. Switching between Three Types of Mesalazine Formulation and Sulfasalazine in Patients with Active Ulcerative Colitis Who Have Already Received High-Dose Treatment with These Agents. Journal of Clinical Medicine. 2019; 8(12):2109. https://doi.org/10.3390/jcm8122109
Chicago/Turabian StyleYasutomi, Eriko, Sakiko Hiraoka, Shumpei Yamamoto, Shohei Oka, Mami Hirai, Yasushi Yamasaki, Toshihiro Inokuchi, Hideaki Kinugasa, Masahiro Takahara, Keita Harada, and et al. 2019. "Switching between Three Types of Mesalazine Formulation and Sulfasalazine in Patients with Active Ulcerative Colitis Who Have Already Received High-Dose Treatment with These Agents" Journal of Clinical Medicine 8, no. 12: 2109. https://doi.org/10.3390/jcm8122109
APA StyleYasutomi, E., Hiraoka, S., Yamamoto, S., Oka, S., Hirai, M., Yamasaki, Y., Inokuchi, T., Kinugasa, H., Takahara, M., Harada, K., Kato, J., & Okada, H. (2019). Switching between Three Types of Mesalazine Formulation and Sulfasalazine in Patients with Active Ulcerative Colitis Who Have Already Received High-Dose Treatment with These Agents. Journal of Clinical Medicine, 8(12), 2109. https://doi.org/10.3390/jcm8122109




