Effects of a High Fat Meal Associated with Water, Juice, or Champagne Consumption on Endothelial Function and Markers of Oxidative Stress and Inflammation in Young, Healthy Subjects
Abstract
:1. Introduction
2. Population and Methods
2.1. Population
2.2. Study Design
2.3. Parameters Determined
2.3.1. Hemodynamic Parameters
2.3.2. Biological Parameters
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Subjects
3.2. Effects of the High-Fat Meal on the Entire Population Drinking Water
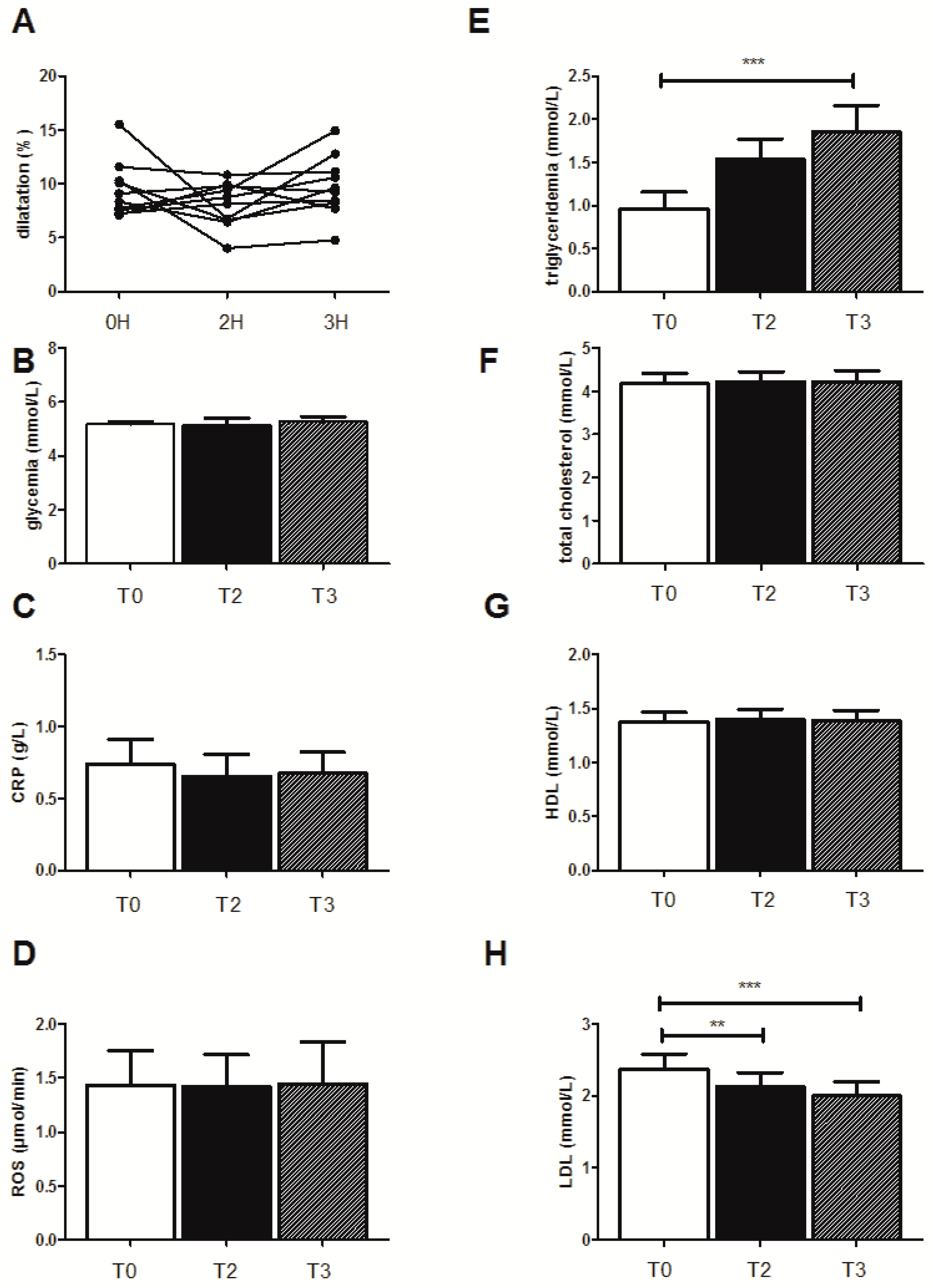
3.2.1. FMD Evolution
3.2.2. Biological Effects
3.3. Effects of the High-Fat Meal on the Selected Ten Subjects Showing Decreased FMD When Drinking Water
3.3.1. FMD Evolution
3.3.2. Biological Effects
3.4. Effects of the High-Fat Meal on the Selected Subjects Drinking the Fruit Juice
3.4.1. FMD Evolution
3.4.2. Biological Effects
3.5. Effects of the High-Fat Meal on the Selected Patients Drinking Champagne
3.5.1. FMD Evolution
3.5.2. Biological Effects
4. Discussion
4.1. Effects of the High-Fat Meal When Drinking Water
4.2. Effects of Fruit Juice and Champagne Wine on the Postprandial Endothelial Function after the High-Fat Meal
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oak, M.H.; Auger, C.; Belcastro, E.; Park, S.H.; Lee, H.H.; Schini-Kerth, V.B. Potential mechanisms underlying cardiovascular protection by polyphenols: Role of the endothelium. Free Radic. Biol. Med. 2018, 122, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Charakida, M.; Masi, S.; Lüscher, T.F.; Kastelein, J.J.; Deanfield, J.E. Assessment of atherosclerosis: The role of flow-mediated dilatation. Eur. Heart J. 2010, 31, 2854–2861. [Google Scholar] [CrossRef] [PubMed]
- Leardini-Tristao, M.; Charles, A.L.; Lejay, A.; Pizzimenti, M.; Meyer, A.; Estato, V.; Tibiriçá, E.; Andres, E.; Geny, B. Beneficial Effect of Exercise on Cognitive Function during Peripheral Arterial Disease: Potential Involvement of Myokines and Microglial Anti-Inflammatory Phenotype Enhancement. J. Clin. Med. 2019, 8, 653. [Google Scholar] [CrossRef]
- Areas, G.P.T.; Mazzuco, A.; Caruso, F.R.; Jaenisch, R.B.; Cabiddu, R.; Phillips, S.A.; Arena, R.; Borghi-Silva, A. Flow-mediated dilation and heart failure: A review with implications to physical rehabilitation. Heart Fail Rev. 2019, 24, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Talha, S.; Rouyer, O.; Doutreleau, S.; Di Marco, A.P.; Kindo, M.; Richard, R.; Piquard, F.; Geny, B. Exercise-induced increase in brain natriuretic peptide is related to vascular endothelial function after heart transplantation. J. Heart Lung Transplant. 2007, 26, 1075–1076. [Google Scholar] [CrossRef] [PubMed]
- Rouyer, O.; Talha, S.; Di Marco, P.; Ellero, B.; Doutreleau, S.; Diemunsch, P.; Piquard, F.; Geny, B. Lack of endothelial dysfunction in patients under tacrolimus after orthotopic liver transplantation. Clin. Transplant. 2009, 23, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, D.H.; Black, M.A.; Pyke, K.E.; Padilla, J.; Atkinson, G.; Harris, R.A.; Parker, B.; Widlansky, M.E.; Tschakovsky, M.E.; Green, D.J. Assessment of flow-mediated dilation in humans: A methodological and physiological guideline. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2–H12. [Google Scholar] [CrossRef]
- Anderson, T.J.; Phillips, S.A. Assessment and prognosis of peripheral artery measures of vascular function. Prog. Cardiovasc. Dis. 2015, 57, 497–509. [Google Scholar] [CrossRef]
- Ong, P.J.; Dean, T.S.; Hayward, C.S.; Della Monica, P.L.; Sanders, T.A.; Collins, P. Effect of fat and carbohydrate consumption on endothelial function. Lancet 1999, 354, 2134. [Google Scholar] [CrossRef]
- Steer, P.; Sarabi, D.M.; Karlström, B.; Basu, S.; Berne, C.; Vessby, B.; Lind, L. The effect of a mixed meal on endothelium-dependent vasodilation is dependent on fat content in healthy humans. Clin. Sci. (Lond.) 2003, 105, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, K.G.; Armah, C.K.; Minihane, A.M. Meal fatty acids and postprandial vascular reactivity. Biochem. Soc. Trans. 2007, 35, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-H.; Bassenge, E.; Kim, K.-B.; Kim, Y.-N.; Kim, K.-S.; Lee, H.-J.; Moon, K.-C.; Lee, M.-S.; Park, K.-Y.; Schwemmer, M. Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress. Atherosclerosis 2001, 155, 517–523. [Google Scholar] [CrossRef]
- Durrer, C.; Lewis, N.; Wan, Z.; Ainslie, P.; Jenkins, N.T.; Little, J.P. Short-Term Low-Carbohydrate High-Fat Diet in Healthy Young Males Renders the Endothelium Susceptible to Hyperglycemia-Induced Damage, An Exploratory Analysis. Nutrients 2019, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, D.F.; Hirschfield, S.L.; Coffey, R.G. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am. J. Physiol. 1993, 265, H774–H778. [Google Scholar] [CrossRef] [PubMed]
- Sarr, M.; Chataigneau, M.; Martins, S.; Schott, C.; El Bedoui, J.; Oak, M.H.; Muller, B.; Chataigneau, T.; Schini-Kerth, V.B. Red wine polyphenols prevent angiotensin II-induced hypertension and endothelial dysfunction in rats: Role of NADPH oxidase. Cardiovasc. Res. 2006, 71, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.L.; Meyer, A.; Dal-Ros, S.; Auger, C.; Keller, N.; Ramamoorthy, T.G.; Zoll, J.; Metzger, D.; Schini-Kerth, V.; Geny, B. Polyphenols prevent ageing-related impairment in skeletal muscle mitochondrial function through decreased reactive oxygen species production. Exp. Physiol. 2013, 98, 536–545. [Google Scholar]
- Dal-Ros, S.; Zoll, J.; Lang, A.L.; Auger, C.; Keller, N.; Bronner, C.; Geny, B.; Schini-Kerth, V.B. Chronic intake of red wine polyphenols by young rats prevents aging-induced endothelial dysfunction and decline in physical performance: Role of NADPH oxidase. Biochem. Biophys. Res. Commun. 2011, 404, 743–749. [Google Scholar] [CrossRef]
- Hashimoto, M.; Kim, S.; Eto, M.; Iijima, K.; Ako, J.; Yoshizumi, M.; Akishita, M.; Kondo, K.; Itakura, H.; Hosoda, K.; et al. Effect of acute intake of red wine on flow-mediated vasodilatation of the brachial artery. Am. J. Cardiol. 2001, 88, 1457–1460. [Google Scholar] [CrossRef]
- Whelan, A.P.; Sutherland, W.H.; McCormick, M.P.; Yeoman, D.J.; de Jong, S.A.; Williams, M.J. Effects of white and red wine on endothelial function in subjects with coronary artery disease. Intern. Med. J. 2004, 34, 224–228. [Google Scholar] [CrossRef]
- Stein, J.H.; Keevil, J.G.; Wiebe, D.A.; Aeschlimann, S.; Folts, J.D. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation 1999, 100, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-uribe, C.; Schmitz, H.H.; Kelm, M. Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 24, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, M.; Penumathsa, S.V.; Samuel, S.M.; Akita, Y.; Zhan, L.; Bertelli, A.A.E.; Maulik, G.; Maulik, N. White wine induced cardioprotection against ischemia-reperfusion injury is mediated by life extending Akt/FOXO3a/NFkB survival pathway. J. Agric. Food Chem. 2008, 56, 6733–6739. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, S.; Alexander, B.; Baranchuk, A. Wine and Cardiovascular Health: A Comprehensive Review. Circulation 2017, 136, 1434–1448. [Google Scholar] [CrossRef] [PubMed]
- Marchandot, B.; Kibler, M.; Charles, A.L.; Trinh, A.; Eisenmann, H.P.; Zeyons, F.; Von Hunolstein, J.J.; Reydel, A.; Matsushita, K.; Kindo, M.; et al. Does Transcatheter Aortic Valve Replacement Modulate the Kinetic of Superoxide Anion Generation? Antioxid. Redox Signal. 2019. [Google Scholar] [CrossRef]
- Liu, X.; Hill, A.M.; West, S.G.; Gabauer, R.M.; McCrea, C.E.; Fleming, J.A.; Kris-Etherton, P.M. Acute Peanut Consumption Alters Postprandial Lipids and Vascular Responses in Healthy Overweight or Obese Men. J. Nutr. 2017, 147, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Kobayasi, R.; Akamine, E.H.; Davel, A.P.; Rodrigues, M.A.; Carvalho, C.R.; Rossoni, L.V. Oxidative stress and inflammatory mediators contribute to endothelial dysfunction in high-fat diet-induced obesity in mice. J. Hypertens. 2010, 28, 2111–2119. [Google Scholar] [CrossRef]
- Cuevas, A.M.; Guasch, V.; Castillo, O.; Irribarra, V.; Mizon, C.; San Martin, A.; Strobel, P.; Perez, D.; Germain, A.M.; Leighton, F. A high-fat diet induces and red wine counteracts endothelial dysfunction in human volunteers. Lipids 2000, 35, 143–148. [Google Scholar] [CrossRef]
- Bae, J.H.; Schwemmer, M.; Lee, I.K.; Lee, H.J.; Park, K.R.; Kim, K.Y.; Bassenge, E. Postprandial hypertriglyceridemia-induced endothelial dysfunction induced in healthy subjects is independent of lipid oxyidation. Int. J. Cardiol. 2003, 87, 259–267. [Google Scholar] [CrossRef]
- Kawano, H.; Motoyama, T.; Hirashima, O.; Hirai, N.; Miyao, Y.; Sakamoto, T.; Kugiyama, K.; Ogawa, H.; Yasue, H. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J. Am. Coll. Cardiol. 1999, 34, 146–154. [Google Scholar] [CrossRef]
- Mah, E.; Noh, S.K.; Ballard, K.D.; Matos, M.E.; Volek, J.S.; Bruno, R.S. Postprandial hyperglycemia impairs vascular endothelial function in healthy men by inducing lipid peroxidation and increasing asymmetric dimethylarginine:arginine. J. Nutr. 2011, 141, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Esposito, K.; Piconi, L.; Ihnat, M.; Thorpe, J.; Testa, R.; Bonfigli, A.R.; Giugliano, D. Glucose “peak” and glucose “spike”: Impact on endothelial function and oxidative stress. Diabetes Res. Clin. Pract. 2008, 82, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.A.; Corretti, M.C.; Plotnick, G.D. Effect of a single high-fat meal on endothelial function in healthy subjects. Am. J. Cardiol. 1997, 79, 350–354. [Google Scholar] [CrossRef]
- Marchesi, S.; Lupattelli, G.; Siepi, D.; Roscini, A.R.; Vaudo, G.; Sinzinger, H.; Mannarino, E. Oral L-arginine administration attenuates postprandial endothelial dysfunction in young healthy males. J. Clin. Pharm. Ther. 2001, 26, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Dalbeni, A.; Treggiari, D.; Tagetti, A.; Bevilaqua, M.; Bonafini, S.; Montagnana, M.; Scaturro, G.; Minuz, P.; Fava, C. Positive Effects of Tomato Paste on Vascular Function After a Fat Meal in Male Healthy Subjects. Nutrients 2018, 10, 1310. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimétrie des phénols totaux avec des réactifs phosphomolybdic-phosphotungstiques. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Auger, C.; Kim, J.H.; Trinh, S.; Chataigneau, T.; Popken, A.M.; Schini-Kerth, V.B. Fruit juice induced endothelium-dependent relaxations in isolated porcine coronary arteries: Evaluation of different fruit juice and pures and optimization of a red fruit juice blend. Food Funct. 2011, 2, 245–250. [Google Scholar] [CrossRef]
- Vauzour, D. Study of the Biological Properties of the Constituents of Champagne Wines. Ph.D. Thesis, University of Montpellier I, Montpellier, France, 2004. [Google Scholar]
- Thom, N.J.; Early, A.R.; Hunt, B.E.; Harris, R.A.; Herring, M.P. Eating and arterial endothelial function: A meta-analysis of the acute effects of meal consumption on flow-mediated dilation. Obes. Rev. 2016, 17, 1080–1090. [Google Scholar] [CrossRef]
- Green, D.J.; Jones, H.; Thijssen, D.; Cable, N.T.; Atkinson, G. Flow-mediated dilation and cardiovascular event prediction: Does nitric oxide matter? Hypertension 2011, 57, 363–369. [Google Scholar] [CrossRef]



| Clinical Characteristics | |
| Age (years) | 24.6 ± 0.9 |
| Weight (kg) | 76.2 ± 2.3 |
| BMI (kg/m2) | 23.6 ± 0.8 |
| MAP (mmHg) | 95.8 ± 1.7 |
| HR (beats/min) | 68 ± 2 |
| FMD (%) | 9.69 ± 0.86 |
| Biological characteristics | |
| Triglyceridemia (mmol/L) | 0.76 ± 0.07 |
| Total cholesterol (mmol/L) | 4.01 ± 0.19 |
| HDL (mmol/L) | 1.32 ± 0.07 |
| LDL (mmol/L) | 2.33 ± 0.15 |
| Glycemia (mmol/L) | 5.12 ± 0.11 |
| usCRP (g/L) | 0.68 ± 0.18 |
| ROS (µmol/min) | 1.42 ± 0.32 |
| Biological Parameters | 10 Patient-Group | 7 Patient-Group | ||||
|---|---|---|---|---|---|---|
| Water | Juice | Champagne | Water | Juice | Champagne | |
| MAP (mmHg) | 95.3 ± 2.3 | 96.3 ± 2.3 | ||||
| HR (beats/min) | 67.5± 2.4 | 73.7 ± 2.5 | ||||
| FMD (%) | 10.73 ± 0.95 | 8.17 ± 0.92 * | 9.45 ± 0.82 | 8.22 ± 1.49 | 8.31 ± 1.46 | 8.28 ± 0.98 |
| Triglyceridemia (mmol/L) | 0.81 ± 0.11 | 0.77 ± 0.11 | 0.96 ± 0.2 | 0.70 ± 0.06 | 0.78 ±0.06 | 0.73 ± 0.09 |
| Total cholesterol (mmol/L) | 4.23 ± 0.3 | 4.24 ± 0.26 | 4.18 ± 0.23 | 3.69 ± 0.11 | 3.74 ± 0.11 | 3.71 ± 0.08 |
| HDL (mmol/L) | 1.43 ± 0.11 | 1.41 ± 0.1 | 1.38 ± 0.09 | 1.18 ±0.05 | 1.19 ± 0.05 | 1.19 ± 0.06 |
| LDL (mmol/L) | 2.43 ± 0.25 | 2.49 ± 0.21 | 2.36 ± 0.22 | 2.19 ± 0.13 | 2.20 ± 0.16 | 2.19 ± 0.09 |
| Glycemia (mmol/L) | 5.11 ± 0.17 | 5.21 ± 0.1 | 5.19 ± 0.08 | 5.13 ± 0.12 | 5.14 ± 0.14 | 5.09 ± 0.11 |
| usCRP (g/L) | 0.72 ± 0.2 | 0.71 ± 0.18 | 0.74 ± 0.17 | 0.63 ± 0.35 | 0.3 ± 0.08 | 0.56 ± 0.24 |
| ROS (µmol/min) | 1.42 ± 0.32 | 1.11 ± 0.11 | 1.43 ± 0.32 | 0.85 ± 0.13 | 1 ± 0.07 | 0.99 ± 0.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouyer, O.; Auger, C.; Charles, A.-L.; Talha, S.; Meyer, A.; Piquard, F.; Andres, E.; Schini-Kerth, V.; Geny, B. Effects of a High Fat Meal Associated with Water, Juice, or Champagne Consumption on Endothelial Function and Markers of Oxidative Stress and Inflammation in Young, Healthy Subjects. J. Clin. Med. 2019, 8, 859. https://doi.org/10.3390/jcm8060859
Rouyer O, Auger C, Charles A-L, Talha S, Meyer A, Piquard F, Andres E, Schini-Kerth V, Geny B. Effects of a High Fat Meal Associated with Water, Juice, or Champagne Consumption on Endothelial Function and Markers of Oxidative Stress and Inflammation in Young, Healthy Subjects. Journal of Clinical Medicine. 2019; 8(6):859. https://doi.org/10.3390/jcm8060859
Chicago/Turabian StyleRouyer, Olivier, Cyril Auger, Anne-Laure Charles, Samy Talha, Alain Meyer, Francois Piquard, Emmanuel Andres, Valerie Schini-Kerth, and Bernard Geny. 2019. "Effects of a High Fat Meal Associated with Water, Juice, or Champagne Consumption on Endothelial Function and Markers of Oxidative Stress and Inflammation in Young, Healthy Subjects" Journal of Clinical Medicine 8, no. 6: 859. https://doi.org/10.3390/jcm8060859
APA StyleRouyer, O., Auger, C., Charles, A.-L., Talha, S., Meyer, A., Piquard, F., Andres, E., Schini-Kerth, V., & Geny, B. (2019). Effects of a High Fat Meal Associated with Water, Juice, or Champagne Consumption on Endothelial Function and Markers of Oxidative Stress and Inflammation in Young, Healthy Subjects. Journal of Clinical Medicine, 8(6), 859. https://doi.org/10.3390/jcm8060859






