Effect of Flowable Thrombin-Containing Collagen-Based Hemostatic Matrix for Preventing Pancreatic Fistula after Pancreatectomy: A Randomized Clinical Trial
Abstract
1. Introduction
2. Methods
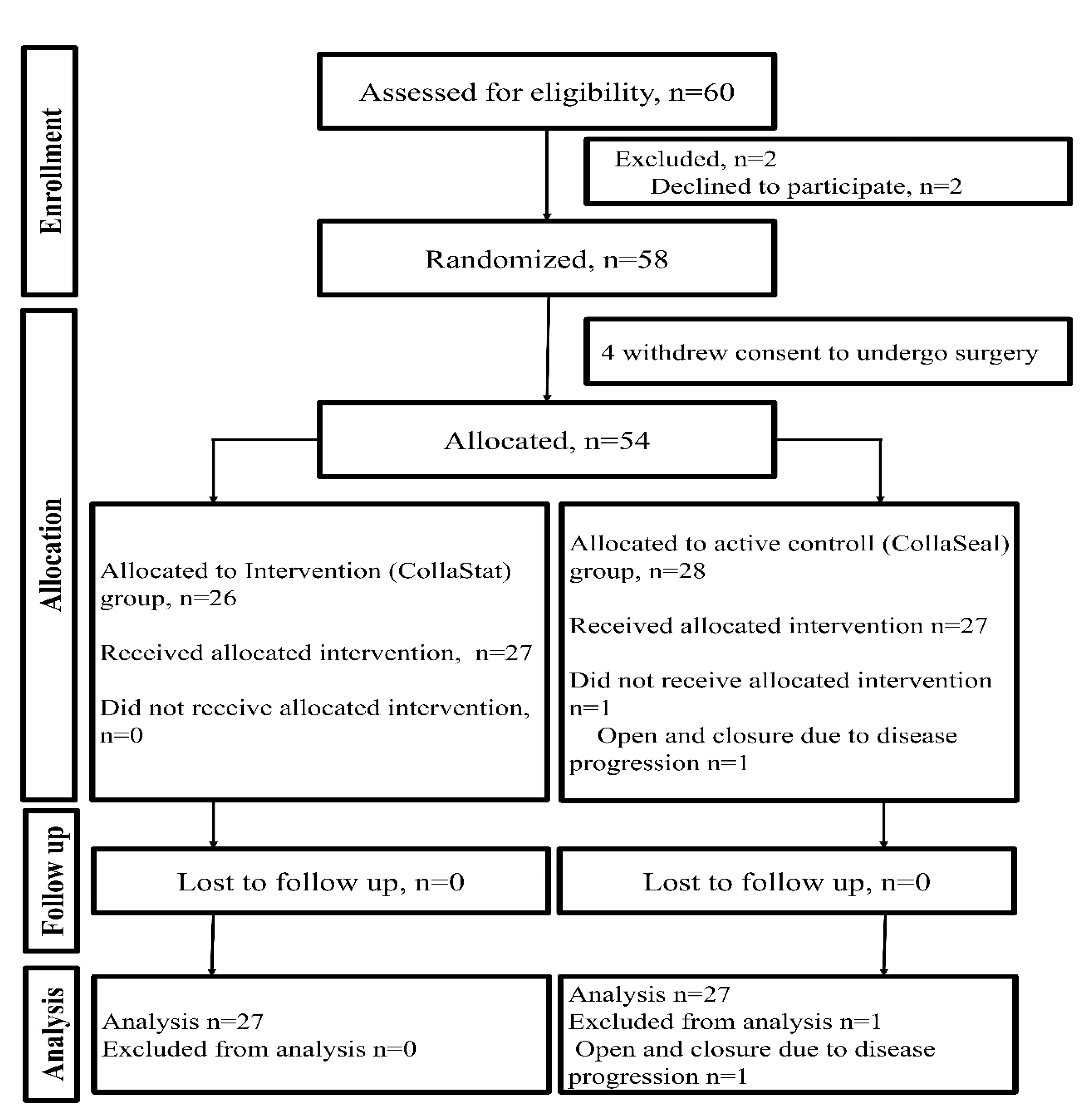
2.1. Trial Design
2.2. Inclusion and Exclusion Criteria
2.3. Surgical Technique and Study Protocol
2.4. Application of Thrombin-Containing Collagen Hemostatic Matrix (T-C Matrix) in the Intervention Group
2.5. Application of Thrombin-Coated L-Dopa-Containing Collagen Patch (T-CD Patch) in the Control Group
2.6. Outcome Measures
2.7. Sample Size
2.8. Randomization
2.9. Statistical Analysis
3. Results
3.1. Primary Outcomes
3.2. Secondary Outcomes
3.3. Sub-Analysis of Patients Who Underwent Distal Pancreatectomy
3.4. Risk Factors for POPF and Clinically Relevant POPF
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kimura, W.; Miyata, H.; Gotoh, M.; Hirai, I.; Kenjo, A.; Kitagawa, Y.; Shimada, M.; Baba, H.; Tomita, N.; Nakagoe, T.; et al. A Pancreaticoduodenectomy Risk Model Derived From 8575 Cases From a National Single-Race Population (Japanese) Using a Web-Based Data Entry System The 30-Day and In-hospital Mortality Rates for Pancreaticoduodenectomy. Ann. Surg. 2014, 259, 773–780. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Nakamura, S.; Suzuki, S.; Kojima, Y.; Tsuchiya, Y.; Konno, H.; Nakaoka, J.; Nishiyama, R. Marginal ulceration after pylorus-preserving pancreaticoduodenectomy. J. Hepatobiliary Pancreat. Surg. 2000, 7, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Lillemoe, K.D.; Kaushal, S.; Cameron, J.L.; Sohn, T.A.; Pitt, H.A.; Yeo, C.J. Distal pancreatectomy: Indications and outcomes in 235 patients. Ann. Surg. 1999, 229, 693–698; discussion 698–700. [Google Scholar] [CrossRef] [PubMed]
- Song, K.B.; Kim, S.C.; Hwang, D.W.; Lee, J.H.; Lee, D.J.; Lee, J.W.; Park, K.M.; Lee, Y.J. Matched Case-Control Analysis Comparing Laparoscopic and Open Pylorus-preserving Pancreaticoduodenectomy in Patients With Periampullary Tumors. Ann. Surg. 2015, 262, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Knaebel, H.P.; Diener, M.K.; Wente, M.N.; Buchler, M.W.; Seiler, C.M. Systematic review and meta-analysis of technique for closure of the pancreatic remnant after distal pancreatectomy. Br. J. Surg. 2005, 92, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Song, K.B.; Kim, S.C.; Park, J.B.; Kim, Y.H.; Jung, Y.S.; Kim, M.H.; Lee, S.K.; Seo, D.W.; Lee, S.S.; Park, D.H.; et al. Single-center experience of laparoscopic left pancreatic resection in 359 consecutive patients: Changing the surgical paradigm of left pancreatic resection. Surg. Endosc. 2011, 25, 3364–3372. [Google Scholar] [CrossRef]
- Montorsi, M.; Zerbi, A.; Bassi, C.; Capussotti, L.; Coppola, R.; Sacchi, M.; Italian Tachosil Study, G. Efficacy of an absorbable fibrin sealant patch (TachoSil) after distal pancreatectomy: A multicenter, randomized, controlled trial. Ann. Surg. 2012, 256, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Reeh, M.; Nentwich, M.F.; Bogoevski, D.; Koenig, A.M.; Gebauer, F.; Tachezy, M.; Izbicki, J.R.; Bockhorn, M. High surgical morbidity following distal pancreatectomy: Still an unsolved problem. World J. Surg. 2011, 35, 1110–1117. [Google Scholar] [CrossRef]
- Ecker, B.L.; McMillan, M.T.; Allegrini, V.; Bassi, C.; Beane, J.D.; Beckman, R.M.; Behrman, S.W.; Dickson, E.J.; Callery, M.P.; Christein, J.D.; et al. Risk Factors and Mitigation Strategies for Pancreatic Fistula After Distal Pancreatectomy: Analysis of 2026 Resections From the International, Multi-institutional Distal Pancreatectomy Study Group. Ann. Surg. 2019, 269, 143–149. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, D.-H.; Jang, J.-Y.; Han, Y.; Yoon, D.-S.; Kim, J.K.; Han, H.-S.; Yoon, Y.; Hwang, D.W.; Kim, K.S.; et al. Use of TachoSil® patches to prevent pancreatic leaks after distal pancreatectomy: A prospective, multicenter, randomized controlled study. J. Hepatobiliary Pancreat. Sci. 2016, 23, 110–117. [Google Scholar] [CrossRef]
- Menahem, B.; Guittet, L.; Mulliri, A.; Alves, A.; Lubrano, J. Pancreaticogastrostomy Is Superior to Pancreaticojejunostomy for Prevention of Pancreatic Fistula after Pancreaticoduodenectomy: An updated meta-analysis of randomized controlled trials. Ann. Surg. 2015, 261, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Mita, K.; Ito, H.; Fukumoto, M.; Murabayashi, R.; Koizumi, K.; Hayashi, T.; Kikuchi, H. Pancreaticojejunostomy using a Fibrin Adhesive Sealant (TachoComb (R)) for the Prevention of Pancreatic Fistula after Pancreaticoduodenectomy. Hepato-Gastroenterology 2011, 58, 187–191. [Google Scholar] [PubMed]
- Mita, K.; Ito, H.; Fukumoto, M.; Murabayashi, R.; Koizumi, K.; Hayashi, T.; Kikuchi, H.; Kagaya, T. A fibrin adhesive sealing method for the prevention of pancreatic fistula following distal pancreatectomy. Hepato-Gastroenterology 2011, 58, 604–608. [Google Scholar]
- Silvestri, S.; Franchello, A.; Gonella, F.; Deiro, G.; Campra, D.; Cassine, D.; Fiore, A.; Ostuni, E.; Garino, M.; Resegotti, A.; et al. Role of TachoSil® in distal pancreatectomy: A single center experience. Minerva Chir. 2015, 70, 175–180. [Google Scholar] [PubMed]
- Marangos, I.P.; Rosok, B.I.; Kazaryan, A.M.; Rosseland, A.R.; Edwin, B. Effect of TachoSil Patch in Prevention of Postoperative Pancreatic Fistula. J. Gastrointest. Surg. 2011, 15, 1625–1629. [Google Scholar] [CrossRef]
- Kwon, H.E.; Seo, H.I.; Yun, S.P. Use of Neoveil or TachoSil to prevent pancreatic fistula following pancreaticoduodenectomy: A retrospective study. Medicine 2019, 98, e15293. [Google Scholar] [CrossRef]
- Kwon, J.; Shin, S.H.; Lee, S.; Park, G.; Park, Y.; Lee, S.J.; Lee, W.; Song, K.B.; Hwang, D.W.; Kim, S.C.; et al. The Effect of Fibrinogen/Thrombin-Coated Collagen Patch (TachoSil®) Application in Pancreaticojejunostomy for Prevention of Pancreatic Fistula after Pancreaticoduodenectomy: A Randomized Clinical Trial. World J. Surg. 2019, 43, 3128–3137. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.H.; Jeon, C.S.; Ko, J.H.; Park, S.N.; Lee, Y.T. Evaluation of a novel collagen hemostatic matrix in a porcine heart and cardiac vessel injury model. J. Thorac. Dis. 2019, 11, 2722–2729. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.H.; Kim, J.S.; Choe, J.H. Evaluation of a Novel Collagen Hemostatic Matrix: Comparison of Two Hemostatic Matrices in a Rabbits Jejunal Artery Injury Model. J. Surg. Res. 2019, 243, 553–559. [Google Scholar] [CrossRef]
- Stein, C.; Kuchler, S. Targeting inflammation and wound healing by opioids. Trends Pharm. Sci. 2013, 34, 303–312. [Google Scholar] [CrossRef]
- Ruszczak, Z. Effect of collagen matrices on dermal wound healing. Adv. Drug Deliv. Rev. 2003, 55, 1595–1611. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Hüttner, F.J.; Mihaljevic, A.L.; Hackert, T.; Ulrich, A.; Büchler, M.W.; Diener, M.K. Effectiveness of Tachosil® in the prevention of postoperative pancreatic fistula after distal pancreatectomy: A systematic review and meta-analysis. Langenbecks Arch. Surg. 2016, 401, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gotzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012, 10, 28–55. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Kim, S.C.; Song, K.B.; Hwang, D.W.; Lee, J.H.; Lee, D.; Lee, J.W.; Jun, E.; Park, K.M.; Lee, Y.J. A comparative study of laparoscopic vs. open distal pancreatectomy for left-sided ductal adenocarcinoma: A propensity score-matched analysis. J. Am. Coll. Surg. 2015, 220, 177–185. [Google Scholar] [CrossRef]
- Park, Y.; Hwang, D.W.; Lee, J.H.; Song, K.B.; Jun, E.; Lee, W.; Kwon, J.; Kim, S.C. Analysis of Symptomatic Marginal Ulcers in Patients Who Underwent Pancreaticoduodenectomy for Periampullary Tumors. Pancreas 2020, 49, 208–215. [Google Scholar] [CrossRef]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Chirletti, P.; Caronna, R.; Fanello, G.; Schiratti, M.; Stagnitti, F.; Peparini, N.; Benedetti, M.; Martino, G. Pancreaticojejunostomy with Applicationof Fibrinogen/Thrombin-Coated Collagen Patch (TachoSil®) in Roux-en-Y Reconstruction after Pancreaticoduodenectomy. J. Gastrointest. Surg. 2009, 13, 1396–1398. [Google Scholar] [CrossRef]
- Schindl, M.; Fugger, R.; Gotzinger, P.; Langle, F.; Zitt, M.; Stattner, S.; Kornprat, P.; Sahora, K.; Hlauschek, D.; Gnant, M. Randomized clinical trial of the effect of a fibrin sealant patch on pancreatic fistula formation after pancreatoduodenectomy. Br. J. Surg. 2018, 105, 811–819. [Google Scholar] [CrossRef]
- Cunha, A.S.; Carrere, N.; Meunier, B.; Fabre, J.-M.; Sauvanet, A.; Pessaux, P.; Ortega-Deballon, P.; Fingerhut, A.; Lacaine, F. Stump closure reinforcement with absorbable fibrin collagen sealant sponge (TachoSil) does not prevent pancreatic fistula after distal pancreatectomy: The FIABLE multicenter controlled randomized study. Am. J. Surg. 2015, 210, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Diener, M.K.; Seiler, C.M.; Rossion, I.; Kleeff, J.; Glanemann, M.; Butturini, G.; Tomazic, A.; Bruns, C.J.; Busch, O.R.; Farkas, S.; et al. Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): A randomised, controlled multicentre trial. Lancet 2011, 377, 1514–1522. [Google Scholar] [CrossRef]
- Nathan, H.; Cameron, J.L.; Goodwin, C.R.; Seth, A.K.; Edil, B.H.; Wolfgang, C.L.; Pawlik, T.M.; Schulick, R.D.; Choti, M.A. Risk factors for pancreatic leak after distal pancreatectomy. Ann. Surg. 2009, 250, 277–281. [Google Scholar] [CrossRef] [PubMed]
| Variable | . | Intervention (n = 26) | Control (n = 27) | p Value 1 |
|---|---|---|---|---|
| Age (years) | Median, IQR | 59 (56~63) | 63 (52~70) | 0.849 |
| Sex, n (%) | Female | 14 (53.8%) | 12 (44.4%) | 0.494 |
| Male | 12 (46.2%) | 15 (55.6%) | ||
| Diabetes Mellitus | Yes | 2 (7.7%) | 7 (25.9%) | 0.077 |
| Preoperative Fasting glucose (mg/dL) | Yes | 9 (34.6%) | 13 (48.1%) | 0.406 |
| BMI (kg/m2) | Mean, SD | 24.2 ± 3.2 | 22.4 ± 2.4 | 0.026 |
| ASA classification, n (%) | <3 | 26 (100.0%) | 26 (96.3%) | >0.999 |
| ≥3 | 0 (0.0%) | 1 (3.7%) | ||
| Charlson comorbidity score, n (%) | <4 | 13 (50.0%) | 9 (33.3%) | 0.218 |
| ≥4 | 13 (50.0%) | 18 (66.7%) | ||
| Operative type, n (%) | Laparotomy | 13 (50.0%) | 12 (44.4%) | 0.685 |
| Minimal invasive | 13 (50.0%) | 15 (55.6%) | ||
| Operative name, n (%) | Whipple’s operation | 14 (53.8%) | 14 (51.9%) | >0.999 |
| Distal pancreatectomy | 12 (46.2%) | 13 (48.1%) | ||
| Additional resection, n (%) | Yes | 6 (22.2%) | 3 (11.1%) | 0.467 |
| Gallbladder | 0 | 1 | ||
| SMV or PV | 3 | 2 | ||
| Total gastrectomy/celiac axis | 1 | 0 | ||
| Right hemicolectomy | 1 | 0 | ||
| Liver | 1 | 0 | ||
| Operative time, min | Mean, SD | 293.5 ± 108.3 | 280.0 ± 110.9 | 0.656 |
| Estimated blood loss, n (%) | ≤400 mL | 24 (92.4%) | 25 (92.6%) | >0.999 |
| 401~700 mL | 1 (3.8%) | 1 (3.7%) | ||
| ≥701 mL | 1 (3.8%) | 1 (3.7%) | ||
| Pathological diagnosis, n (%) | Malignancy | 15 (57.7%) | 11 (40.7%) | 0.217 |
| PDAC | 14 (53.8%) | 9 (33.3%) | ||
| Benign | 11 (42.3%) | 16 (59.3%) | ||
| Pancreas texture, n (%) | Soft | 12 (46.2%) | 15 (55.6%) | 0.494 |
| Firm or hard | 14 (53.8%) | 12 (44.4%) | ||
| Mass size, cm | Mean, SD | 3.4 ± 2.1 | 3.3 ± 1.7 | 0.793 |
| Pancreatic duct size, mm | Mean, SD | 3.1 ± 1.9 | 3.0 ± 1.7 | 0.816 |
| Neoadjuvant therapy, n (%) | Yes | 4 (15.4%) | 1 (3.7%) | 0.192 |
| Alternative fistula risk score, n (%) 2 | Low | 4 (14.8%) | 8 (29.6%) | 0.264 |
| Intermediate | 15 (55.6%) | 15 (55.6%) | ||
| High | 8 (29.6%) | 4 (14.8%) |
| Variable | . | Intervention (n = 26) | Control (n = 27) | p Value 1 |
|---|---|---|---|---|
| Drain removal, days | Median, IQR | 4 (3~5) | 5 (3~6) | 0.241 |
| POPF, n (%) 2 | Yes | 8 (30.8%) | 16 (59.3%) | 0.037 |
| POPF grade, n (%) 2 | BL | 4 (15.4%) | 8 (29.6%) | 0.438 |
| B | 4 (15.4%) | 7 (25.9%) | ||
| C | 0 (0.0%) | 1 (3.7%) | ||
| Clinically relevant POPF, n (%) 2 | Yes | 4 (15.4%) | 8 (29.6%) | 0.409 |
| Postoperative complication, n (%) 2 | Yes | 9 (34.6%) | 17 (63.0%) | 0.039 |
| Complication grade, n (%) 2 | ≥Grade III | 5 (19.2%) | 3 (11.1%) | 0.444 |
| Length of hospital stay, days | Median, IQR | 8 (7~11) | 9 (7~14) | 0.284 |
| 90-day mortality, n (%) | Yes | 0 (0.0%) | 0 (0.0%) | >0.999 |
| Readmission, n (%) | Yes | 5 (19.2%) | 3 (11.1%) | 0.409 |
| Variable | . | Intervention (n = 12) | Control (n = 13) | p Value 1 |
|---|---|---|---|---|
| Drain removal, days | Median, IQR | 4 (3~5) | 4 (3~6) | 0.167 |
| POPF, n (%) 2 | Yes | 4 (33.3%) | 12 (92.3%) | 0.004 |
| POPF grade, n (%) 2 | BL | 3 (25.0%) | 6 (46.2%) | 0.018 |
| B | 1 (8.3%) | 5 (38.5%) | ||
| C | 0 (0.0%) | 1 (7.7%) | ||
| Clinically relevant POPF, n (%) 2 | Yes | 1 (8.3%) | 6 (46.2%) | 0.027 |
| Postoperative complication, n (%) 2 | Yes | 4 (33.3%) | 12 (92.3%) | 0.004 |
| Complication grade, n (%) 2 | ≥Grade III | 1 (8.3%) | 3 (23.1%) | 0.593 |
| Length of hospital stay, days | Median, IQR | 7 (6–9) | 7 (6–14) | 0.274 |
| 90-day mortality, n (%) | Yes | 0 (0.0%) | 0 (0.0%) | >0.999 |
| Readmission, n (%) | Yes | 1 (8.3%) | 1 (7.7%) | >0.999 |
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR 1 | 95% CI | p Value 2 | OR 1 | 95% CI | p Value 2 | |
| All patients (n = 53) | ||||||
| Intervention | 0.031 | 0.029 | ||||
| Control | 3.455 | 1.119~10.669 | 4.744 | 1.172~19.210 | ||
| Pancreatic duct size, mm | 0.012 | 0.018 | ||||
| ≥3 | ||||||
| <3 | 6.125 | 1.501~24.997 | 7.120 | 1.399~36.241 | ||
| Pancreatic texture | 0.002 | 0.007 | ||||
| Firm or hard | ||||||
| Soft | 7.000 | 2.088~23.468 | 6.525 | 1.668~25.529 | ||
| Patients who underwent distal pancreatectomy (n = 25) | ||||||
| Intervention | 0.006 | 0.024 | ||||
| Control | 27.000 | 2.561~284.696 | 17.379 | 1.453~207.870 | ||
| Pancreatic texture | 0.011 | 0.096 | ||||
| Firm or hard | ||||||
| Soft | 12.000 | 1.762~81.745 | 6.666 | 0.712~62.374 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.; Ko, J.H.; Kang, D.R.; Lee, J.H.; Hwang, D.W.; Lee, J.H.; Lee, W.; Kwon, J.; Park, S.-N.; Song, K.-B.; et al. Effect of Flowable Thrombin-Containing Collagen-Based Hemostatic Matrix for Preventing Pancreatic Fistula after Pancreatectomy: A Randomized Clinical Trial. J. Clin. Med. 2020, 9, 3085. https://doi.org/10.3390/jcm9103085
Park Y, Ko JH, Kang DR, Lee JH, Hwang DW, Lee JH, Lee W, Kwon J, Park S-N, Song K-B, et al. Effect of Flowable Thrombin-Containing Collagen-Based Hemostatic Matrix for Preventing Pancreatic Fistula after Pancreatectomy: A Randomized Clinical Trial. Journal of Clinical Medicine. 2020; 9(10):3085. https://doi.org/10.3390/jcm9103085
Chicago/Turabian StylePark, Yejong, Jae Hyung Ko, Dae Ryong Kang, Jun Hyeok Lee, Dae Wook Hwang, Jae Hoon Lee, Woohyung Lee, Jaewoo Kwon, Si-Nae Park, Ki-Byung Song, and et al. 2020. "Effect of Flowable Thrombin-Containing Collagen-Based Hemostatic Matrix for Preventing Pancreatic Fistula after Pancreatectomy: A Randomized Clinical Trial" Journal of Clinical Medicine 9, no. 10: 3085. https://doi.org/10.3390/jcm9103085
APA StylePark, Y., Ko, J. H., Kang, D. R., Lee, J. H., Hwang, D. W., Lee, J. H., Lee, W., Kwon, J., Park, S.-N., Song, K.-B., & Kim, S. C. (2020). Effect of Flowable Thrombin-Containing Collagen-Based Hemostatic Matrix for Preventing Pancreatic Fistula after Pancreatectomy: A Randomized Clinical Trial. Journal of Clinical Medicine, 9(10), 3085. https://doi.org/10.3390/jcm9103085






