Measurements of Plasma-Free Metanephrines by Immunoassay Versus Urinary Metanephrines and Catecholamines by Liquid Chromatography with Amperometric Detection for the Diagnosis of Pheochromocytoma/Paraganglioma
Abstract
:1. Introduction
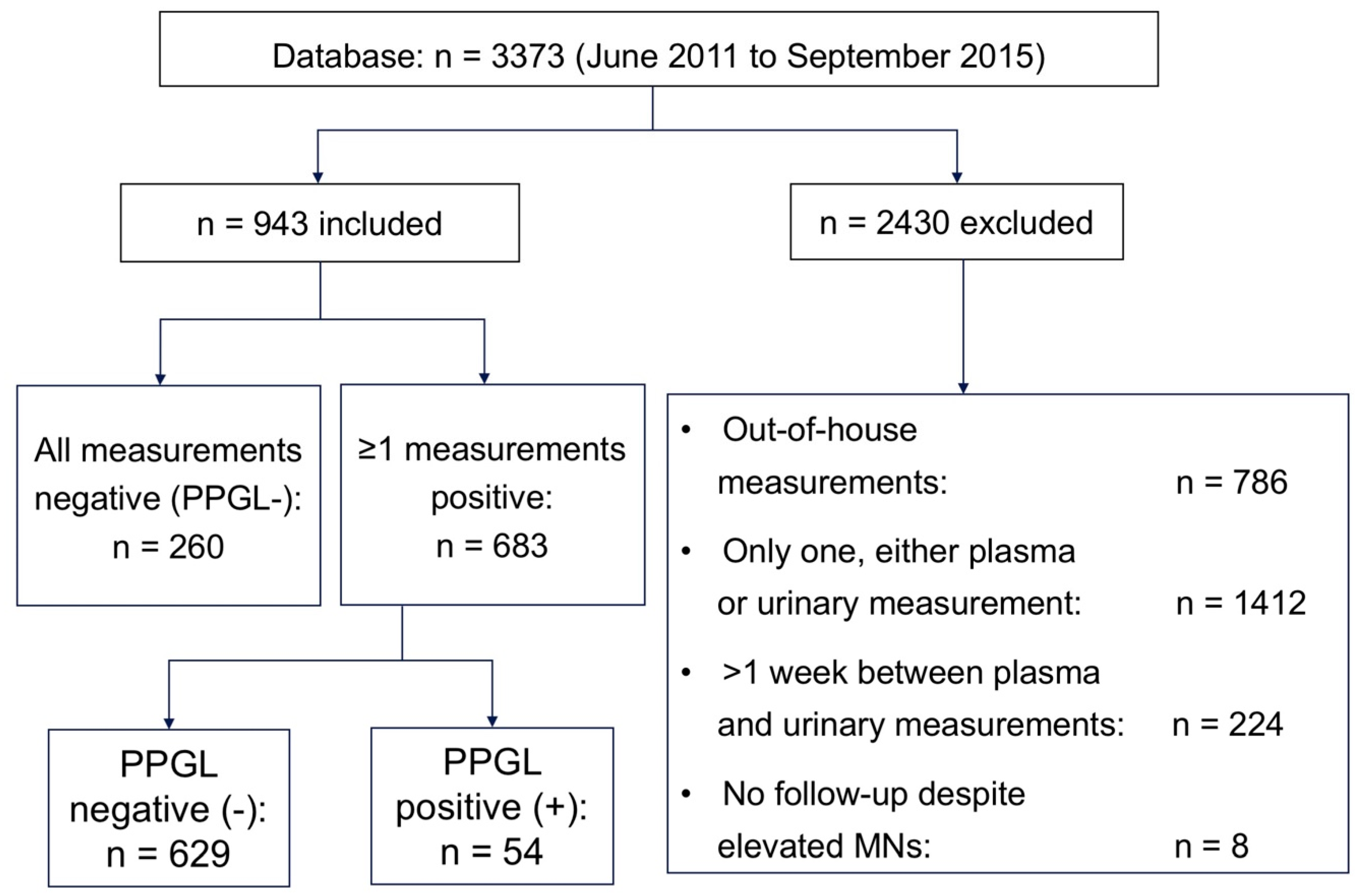
2. Subjects and Methods
2.1. Subjects
2.2. Methods
2.3. Statistics
3. Results
3.1. Baseline Data
3.2. Sensitivity and Specificity
3.2.1. Sensitivity and Specificity of Single Parameters
3.2.2. Sensitivity and Specificity of Parameters Combined
3.3. ROC Curves and Comparison of AUCs
3.4. Post-Test Probability of PPGL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pacak, K.; Tella, S.H. Pheochromocytoma and Paraganglioma. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000–2018; (last update: 4 January 2018). Available online: https://www.ncbi.nlm.nih.gov/books/NBK481899/ (accessed on 7 June 2020).
- Chen, H.; Sippel, R.S.; O’Dorisio, M.S.; Vinik, A.I.; Lloyd, R.V.; Pacak, K. The North American Neuroendocrine Tumor Society Consensus Guideline for the diagnosis and management of neuroendocrine tumors: Pheochromocytoma, Paraganglioma, and Medullary Thyroid Cancer. Pancreas 2010, 39, 775–783. [Google Scholar] [CrossRef] [Green Version]
- Omura, M.; Saito, J.; Yamaguchi, K.; Kakuta, Y.; Nishikawa, T. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res. 2004, 27, 193–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brain, K.L.; Kay, J.; Shine, B. Measurement of urinary metanephrines to screen for pheochromocytoma in an unselected hospital referral population. Clin. Chem. 2006, 52, 2060–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, F.C.; Sánchez, M.; Alvarez, A.; Díaz, J.; Pascual, R.; Pérez, M.; Tovar, I.; Martínez, P. A Five-Year Report on Experience in the Detection of Pheochromocytoma. Clin. Biochem. 2000, 33, 649–655. [Google Scholar] [CrossRef]
- Václavík, J.; Stejskal, D.; Lacnák, B.; Lazárová, M.; Jedelský, L.; Kadalová, L.; Janosová, M.; Frysák, Z.; Vlcek, P. Free plasma metanephrines as a screening test for pheochromocytoma in low-risk patients. J. Hypertens. 2007, 25, 1427–1431. [Google Scholar]
- Fassnacht, M.; Arlt, W.; Bancos, I.; Dralle, H.; Newell-Price, J.; Sahdev, A.; Tabarin, A.; Terzolo, M.; Tsagarakis, S.; Dekkers, O.M. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2016, 175, G1–G34. [Google Scholar] [CrossRef] [Green Version]
- Amar, L.; Fassnacht, M.; Gimenez-Roqueplo, A.-P.; Januszewicz, A.; Prejbisz, A.; Timmers, H.; Plouin, P.-F. Long-term Postoperative Follow-up in Patients with Apparently Benign Pheochromocytoma and Paraganglioma. Horm. Metab. Res. 2012, 44, 385–389. [Google Scholar] [CrossRef]
- Lenders, J.W.; Eisenhofer, G.; Mannelli, M.; Pacak, K. Phaeochromocytoma. Lancet 2005, 366, 665–675. [Google Scholar] [CrossRef]
- Manger, W.M.; Gifford, R.W., Jr. Pheochromocytoma: A clinical overview. In Textbook of Hypertension; Swales, J.D., Ed.; Blackwell Scientific Publications: Oxford, UK, 1994; pp. 941–958. [Google Scholar]
- Manger, W.M. An Overview of Pheochromocytoma: History, Current Concepts, Vagaries, and Diagnostic Challenges. Ann. N. Y. Acad. Sci. 2006, 1073, 1–20. [Google Scholar] [CrossRef]
- Riester, A.; Weismann, D.; Quinkler, M.; Lichtenauer, U.D.; Sommerey, S.; Halbritter, R.; Penning, R.; Spitzweg, C.; Schopohl, J.; Beuschlein, F.; et al. Life-threatening events in patients with pheochromocytoma. Eur. J. Endocrinol. 2015, 173, 757–764. [Google Scholar] [CrossRef]
- Weismann, D.; Liu, D.; Bergen, T.; Peitzsch, M.; Raida, A.; Wagner, M.; Fassnacht, M.; Weidemann, F.; Allolio, B. Hypertension and hypertensive cardiomyopathy in patients with a relapse-free history of phaeochromocytoma. Clin. Endocrinol. 2014, 82, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; EVist, G.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenders, J.; Duh, Q.-Y.; Eisenhofer, G.; Gimenez-Roqueplo, A.-P.; Grebe, S.K.G.; Murad, M.; Naruse, M.; Pacak, K.; Young, W.F. Pheochromocytoma and Paraganglioma: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2014, 99, 1915–1942. [Google Scholar] [CrossRef] [PubMed]
- Därr, R.; Kuhn, M.; Bode, C.; Bornstein, S.R.; Pacak, K.; Lenders, J.W.; Eisenhofer, G. Accuracy of recommended sampling and assay methods for the determination of plasma-free and urinary fractionated metanephrines in the diagnosis of pheochromocytoma and paraganglioma: A systematic review. Endocrine 2017, 56, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Weismann, D.; Peitzsch, M.; Raida, A.; Prejbisz, A.; Gosk, M.; Riester, A.; Willenberg, H.S.; Klemm, R.; Manz, G.; Deutschbein, T.; et al. Measurements of plasma metanephrines by immunoassay vs liquid chromatography with tandem mass spectrometry for diagnosis of pheochromocytoma. Eur. J. Endocrinol. 2015, 172, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Labor Diagnostika Nord, Nordhorn, Germany (LDN). Instruction for Use. 2-MET Plasma ELISA Fast Track. Available online: https://ldn.de/product/2-met-plasma-elisa-fast-track/ (accessed on 7 June 2020).
- Pussard, E.; Neveux, M.; Guigueno, N. Reference intervals for urinary catecholamines and metabolites from birth to adulthood. Clin. Biochem. 2009, 42, 536–539. [Google Scholar] [CrossRef]
- Dawson-Saunders, B.; Trapp, R.G. Evaluating diagnostic procedures. In Basic and Clinical Biostatics, 2nd ed.; Appleton & Lange: New York City, NY, USA, 1994; pp. 232–248. [Google Scholar]
- Wilson, E.B. Probable inference, the law of succession, and statistical inference. J. Am. Stat. Ass. 1927, 22, 209–212. [Google Scholar] [CrossRef]
- Newcombe, R.G. Two-sided confidence intervals for the single proportion: Comparison of seven methods. Stat. Med. 1998, 17, 857–872. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef] [Green Version]
- Fagan, T.J. Nomogram for Bayes’s Theorem. N. Engl. J. Med. 1975, 293, 257. [Google Scholar]
- Caraguel, C.G.; Vanderstichel, R. The two-step Fagan’s nomogram: Ad hoc interpretation of a diagnostic test result without calculation. Evid.-Based Med. 2013, 18, 125–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, A. Diagnostic Test Calculator-Version 2010042101: Available Under the Clarified Artistic License. 2006. Available online: http://araw.mede.uic.edu/cgi-bin/testcalc.pl (accessed on 7 June 2020).
- Manz, B.; Kuper, M.; Booltink, E.; Fischer-Brügge, U. Development of Enantioselective Immunoassays for Free Plasma Metanephrines. Ann. N. Y. Acad. Sci. 2004, 1018, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Pillai, D.; Ross, H.A.; Kratzsch, J.; Pedrosa, W.; Kema, I.; Hoad, K.; Rouaix, N.; Fathi, M.; Nader, H.; Mathian, B.; et al. Proficiency test of plasma free and total metanephrines: Report from a study group. Clin. Chem. Lab. Med. 2009, 47, 786–790. [Google Scholar] [CrossRef] [Green Version]
- Horowitz, G.L.; Boyd, J.C.; Ceriotti, F.; Garg, U.; Horn, P.; Pesce, A.; Sine, H.E.; Zakowski, J. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory: Approved Guideline, 3rd ed.; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2008; CLSI document c28-a3; ISBN 1-56238-682-4. [Google Scholar]
- Mullins, F.; O’Shea, P.; Fitzgerald, R.; Tormey, W. Enzyme-linked immunoassay for plasma-free metanephrines in the biochemical diagnosis of phaeochromocytoma in adults is not ideal. Clin. Chem. Lab. Med. 2012, 50, 105–110. [Google Scholar] [CrossRef]
- Lenders, J.; Willemsen, J.J.; Eisenhofer, G.; Ross, H.A.; Pacak, K.; Timmers, H.J.L.M.; Sweep, F.C.G.J. Is Supine Rest Necessary before Blood Sampling for Plasma Metanephrines? Clin. Chem. 2007, 53, 352–354. [Google Scholar] [CrossRef] [Green Version]
- Därr, R.; Pamporaki, C.; Peitzsch, M.; Miehle, K.; Prejbisz, A.; Peczkowska, M.; Weismann, D.; Beuschlein, F.; Sinnott, R.; Bornstein, S.R.; et al. Biochemical diagnosis of phaeochromocytoma using plasma-free normetanephrine, metanephrine and methoxytyramine: Importance of supine sampling under fasting conditions. Clin. Endocrinol. 2013, 80, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Eisenhofer, G. Editorial: Biochemical diagnosis of pheochromocytoma—Is it time to switch to plasma free metanephrines? J. Clin. Endocrinol. Metab. 2003, 88, 550–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deutschbein, T.; Unger, N.; Jaeger, A.; Broecker-Preuss, M.; Mann, K.; Petersen, S. Influence of various confounding varaibles and storage conditions on metanephrine and normetanephrine levels in plasma. Clin. Endocrinol. 2010, 73, 153–160. [Google Scholar]
- Raber, W.; Raffesberg, W.; Bischof, M.; Scheuba, C.; Niederle, B.; Gasic, S.; Waldhäusl, W.; Roden, M. Diagnostic efficacy of unconjugated plasma metanephrines for the detection of pheochromocytoma. Arch. Intern. Med. 2000, 160, 2957–2963. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Lattke, P.; Herberg, M.; Siegert, G.; Qin, N.; Därr, R.; Hoyer, J.; Villringer, A.; Prejbisz, A.; Januszewicz, A.; et al. Reference intervals for plasma free metanephrines with an age adjustment for normetanephrine for optimized laboratory testing of phaeochromocytoma. Ann. Clin. Biochem. Int. J. Lab. Med. 2012, 50, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Sawka, A.M.; Jaeschke, R.; Singh, R.J.; Young, W.F., Jr. A Comparison of Biochemical Tests for Pheochromocytoma: Measurement of Fractionated Plasma Metanephrines Compared with the Combination of 24-Hour Urinary Metanephrines and Catecholamines. J. Clin. Endocrinol. Metab. 2003, 88, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Procopiou, M.; Finney, H.; Akker, S.A.; Chew, S.L.; Drake, W.M.; Burrin, J.; Grossman, A.B. Evaluation of an enzyme immunoassay for plasma-free metanephrines in the diagnosis of catecholamine-secreting tumors. Eur. J. Endocrinol. 2009, 161, 131–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, T.T.; Frystyk, J.; Poulsen, P.L. Comparison of plasma metanephrines measured by a commercial immunoassay and urinary catecholamines in the diagnosis of pheochromocytoma. Scand. J. Clin. Lab. Investig. 2011, 71, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Isobe, K.; Ma, E.; Imai, T.; Kikumori, T.; Matsuda, T.; Maeda, Y.; Sakurai, A.; Midorikawa, S.; Hataya, Y.; et al. Plasma free metanephrines in the diagnosis of pheochromocytoma: Diagnostic accuracy and strategies for Japanese patients. Endocr. J. 2014, 61, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Pussard, E.; Chaouch, A.; Said, T. Radioimmunoassay of free plasma metanephrines for the diagnosis of catecholamine-producing tumors. Clin. Chem. Lab. Med. 2014, 52, 437–444. [Google Scholar] [CrossRef]
- Bílek, R.; Zelinka, T.; Vlček, P.; Dušková, J.; Michalský, D.; Novák, K.; Václavíková, E.; Widimský, J. Radioimmunoassay of Chromogranin A and Free Metanephrines in Diagnosis of Pheochromocytoma. Physiol. Res. 2017, 66 (Suppl. 3), 397–408. [Google Scholar]
- Gao, Y.-C.; Lu, H.-K.; Luo, Q.-Y.; Chen, L.-B.; Ding, Y.; Zhu, R.-S. Comparison of free plasma metanephrines enzyme immunoassay with 131I-MIBG scan in diagnosis of pheochromocytoma. Clin. Exp. Med. 2008, 8, 87–91. [Google Scholar] [CrossRef]
- Unger, N.; Pitt, C.; Schmidt, I.L.; Walz, M.K.; Schmid, K.W.; Philipp, T.; Mann, K.; Petersenn, S. Diagnostic value of various biochemical parameters for the diagnosis of pheochromocytoma in patients with adrenal mass. Eur. J. Endocrinol. 2006, 154, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Plouin, P.F.; Amar, L.; Dekkers, O.M.; Fassnacht, M.; Gimenez-Roqueplo, A.-P.; Lenders, J.W.M.; Lussey-Lepoutre, C.; Steichen, O.; On behalf of the Guideline Working Group. European Society of Endocrinology Clinical Practice Guideline for long-term follow-up of patients operated on for a phaeochromocytoma or a paraganglioma. Eur. J. Endocrinol. 2016, 174, G1–G10. [Google Scholar] [CrossRef] [Green Version]
- Koratala, A.; Bhattacharya, D.; Wakefield, D.N. Benign-appearing pheochromocytoma with late recurrence. Am. J. Med Sci. 2017, 353, e13. [Google Scholar] [CrossRef]
- Lenders, J.W.M.; Pacak, K.; Walther, M.M.; Linehan, W.M.; Mannelli, M.; Friberg, P.; Keiser, H.R.; Goldstein, D.S.; Eisenhofer, G. Biochemical Diagnosis of Pheochromocytoma. JAMA 2002, 287, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Pacak, K.; Eisenhofer, G.; Ahlman, H.; Bornstein, S.R.; Gimenez-Roqueplo, A.-P.; Grossman, A.B.; Kimura, N.; Mannelli, M.; McNicol, A.M.; Tischler, A.S. Pheochromocytoma: Recommendations for clinical practice from the First International Symposium. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Grossman, A.; Pacak, K.; Sawka, A.; Lenders, J.W.M.; Harlander, D.; Peaston, R.T.; Reznek, R.; Sisson, J.; Eisenhofer, G. Biochemical Diagnosis and Localization of Pheochromocytoma: Can We Reach a Consensus? Ann. N. Y. Acad. Sci. 2006, 1073, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Mattingly, C. What is Clinical Reasoning? Am. J. Occup. Ther. 1991, 45, 979–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, T.H. Ten categories of statistical errors: A guide for research in endocrinology and metabolism. Am. J. Physiol. Metab. 2004, 286, E495–E501. [Google Scholar] [CrossRef]



| Biochemical Parameter | ULN |
|---|---|
| P-MN | 90 pg/mL |
| P-NMN | 180 pg/mL |
| P-MN * | 58 pg/mL |
| P-NMN * | 45–88 pg/mL |
| U-MN | 138 µg/24 h |
| U-NMN | 311 µg/24 h |
| U-A | 27 µg/24 h |
| U-NA | 97 µg/24 h |
| n | PPGL+ | N | PPGL− |
|---|---|---|---|
| 52 | histology+ | 2 | histology− |
| 2 | lab+ and J- MIBG-scintigraphy+ (1) and F-DOPA PET-CT+ (1) | 158 | lab and radiol FU− |
| 190 | lab and clinical FU− | ||
| 10 | alternative diagnosis | ||
| 269 | clinical FU− |
| Patients | n = 54 PPGL+ | n = 889 PPGL− | p-Value |
|---|---|---|---|
| Age (years) | 51 (18–76) | 55 (5–90) | 0.55 |
| Female sex, n (%) | 28 (52) | 537 (60) | 0.80 |
| Genetic syndrome, n (%) | 14 (26) | 0 | |
| P-MN (pg/mL) | 159 (17–2815) | 30 (17–248) | 0.001 |
| P-NMN (pg/mL) | 663 (45–35,832) | 48 (23–290) | 0.001 |
| U-MN (μg/24 h) | 814 (78–22,248) | 118 (5–672) | 0.001 |
| U-NMN (μg/24 h) | 3426 (340–203,009) | 321 (33–1388) | 0.001 |
| U-A (μg/24 h) | 23 (2–480) | 4 (1–47) | 0.001 |
| U-NA (μg/24 h) | 166 (18–34,987) | 46 (6–314) | 0.001 |
| Comparison EIA vs. HPLC | EIA (Plasma) Sensitivity (95% CI); ULN | HPLC (24 h-Urine) Sensitivity (95% CI); ULN | p-Value |
| P-MN + P-NMN/U-MN + U-NMN | 94.4% (84.9–98.5); 90 and 180 pg/ml | 100% (93.4–100); 138 and 311 μg/24 h | 0.25 |
| P-MN + P-NMN/U-A + U-NA | 94.4% (84.9–98.5); 90 and 180 pg/mL | 77.8% (65.1–86.8); 27 and 97 μg/24 h | 0.02 |
| P-MN * + P-NMN */U-MN + U-NMN | 98.1% (90.2–99.9); 58 and 45-88 pg/mL | 100% (93.4–100); 138 and 311 μg/24 h | 0.88 |
| P-MN * + P-NMN */U-A + U-NA | 98.1% (90.2–99.9); 58 and 45-88 pg/mL | 77.8% (65.1–86.8); 27 and 97 μg/24 h | 0.008 |
| Comparison EIA vs. HPLC | EIA (Plasma) Specificity (95% CI); ULN | HPLC (24 h-Urine) Specificity (95% CI); ULN | p-Value |
| P-MN + P-NMN/U-MN + U-NMN | 100% (99.6–100); 90 and 180 pg/mL | 73.6% (70.6–76.2); 138 and 311 μg/24 h | 0.001 |
| P-MN + P-NMN/U-A + U-NA | 100% (99.6–100); 90 and 180 pg/mL | 99.8% (99.2–100); 27 and 97 μg/24 h | 0.36 |
| P-MN *+ P-NMN */U-MN + U-NMN | 94.8% (93.2–96.1); 58 and 45–88 pg/mL | 73.6% (70.6–76.2); 138 and 311 μg/24 h | 0.004 |
| P-MN * + P-NMN */U-A + U-NA | 94.8% (93.2–96.1); 58 and 45–88 pg/mL | 99.8% (99.2–100); 27 and 97 μg/24 h | 0.01 |
| EIA (Plasma)/HPLC (24 h-Urine) | EIA (Plasma) AUC (95% CI) | HPLC (24 h-Urine) AUC (95% CI) | z-Value | p-Value |
| P-MN/U-MN | 0.882 (0.824–0.940) | 0.908 (0.856–0.960) | 0.64 | 0.52 |
| P-MN/U-NMN | 0.882 (0.824–0.940) | 0.960 (0.934–0.986) | 2.39 | 0.02 |
| P-MN/U-A | 0.882 (0.824–0.940) | 0.879 (0.817–0.940) | 0.07 | 0.94 |
| P-MN/U-NA | 0.882 (0.824–0.940) | 0.869 (0.812–0.927) | 0.31 | 0.76 |
| P-NMN/U-NMN | 0.978 (0.954–1.000) | 0.960 (0.934–0.986) | 1.02 | 0.31 |
| P-NMN/U-MN | 0.978 (0.954–1.000) | 0.908 (0.856–0.960) | 2.37 | 0.02 |
| P-NMN/U-A | 0.978 (0.954–1.000) | 0.879 (0.817–0.940) | 2.90 | 0.004 |
| P-NMN/U-NA | 0.978 (0.954–1.000) | 0.869 (0.812–0.927) | 3.47 | <0.0001 |
| EIA (Plasma)/HPLC (24 h-Urine) | EIA (Plasma)AUC (95% CI) | HPLC (24 h-Urine) AUC (95% CI) | z-Value | p-Value |
| P-MNs/U-MNs | 0.989 (0.972–1.000) | 0.995 (0.980–1.000) | 1.02 | 0.31 |
| P-MNs/U-CATs | 0.989 (0.972–1.000) | 0.956 (0.930–0.975) | 1.97 | 0.04 |
| U-MNs/U-CATs | 0.995 (0.980–1.000) | 0.956 (0.930–0.982) | 2.12 | 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raber, W.; Kotal, H.; Marculescu, R.; Scheuba, C.; Niederle, M.B.; Kautzky-Willer, A.; Krebs, M. Measurements of Plasma-Free Metanephrines by Immunoassay Versus Urinary Metanephrines and Catecholamines by Liquid Chromatography with Amperometric Detection for the Diagnosis of Pheochromocytoma/Paraganglioma. J. Clin. Med. 2020, 9, 3108. https://doi.org/10.3390/jcm9103108
Raber W, Kotal H, Marculescu R, Scheuba C, Niederle MB, Kautzky-Willer A, Krebs M. Measurements of Plasma-Free Metanephrines by Immunoassay Versus Urinary Metanephrines and Catecholamines by Liquid Chromatography with Amperometric Detection for the Diagnosis of Pheochromocytoma/Paraganglioma. Journal of Clinical Medicine. 2020; 9(10):3108. https://doi.org/10.3390/jcm9103108
Chicago/Turabian StyleRaber, Wolfgang, Hans Kotal, Rodrig Marculescu, Christian Scheuba, Martin B. Niederle, Alexandra Kautzky-Willer, and Michael Krebs. 2020. "Measurements of Plasma-Free Metanephrines by Immunoassay Versus Urinary Metanephrines and Catecholamines by Liquid Chromatography with Amperometric Detection for the Diagnosis of Pheochromocytoma/Paraganglioma" Journal of Clinical Medicine 9, no. 10: 3108. https://doi.org/10.3390/jcm9103108
APA StyleRaber, W., Kotal, H., Marculescu, R., Scheuba, C., Niederle, M. B., Kautzky-Willer, A., & Krebs, M. (2020). Measurements of Plasma-Free Metanephrines by Immunoassay Versus Urinary Metanephrines and Catecholamines by Liquid Chromatography with Amperometric Detection for the Diagnosis of Pheochromocytoma/Paraganglioma. Journal of Clinical Medicine, 9(10), 3108. https://doi.org/10.3390/jcm9103108







