Resting-State Functional Connectivity and Scholastic Performance in Preadolescent Children: A Data-Driven Multivoxel Pattern Analysis (MVPA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. SES
2.3. IQ and Scholastic Performance
2.4. MRI Acquisition
2.5. MRI Preprocessing
2.6. Multi-Voxel Pattern Analysis
3. Results
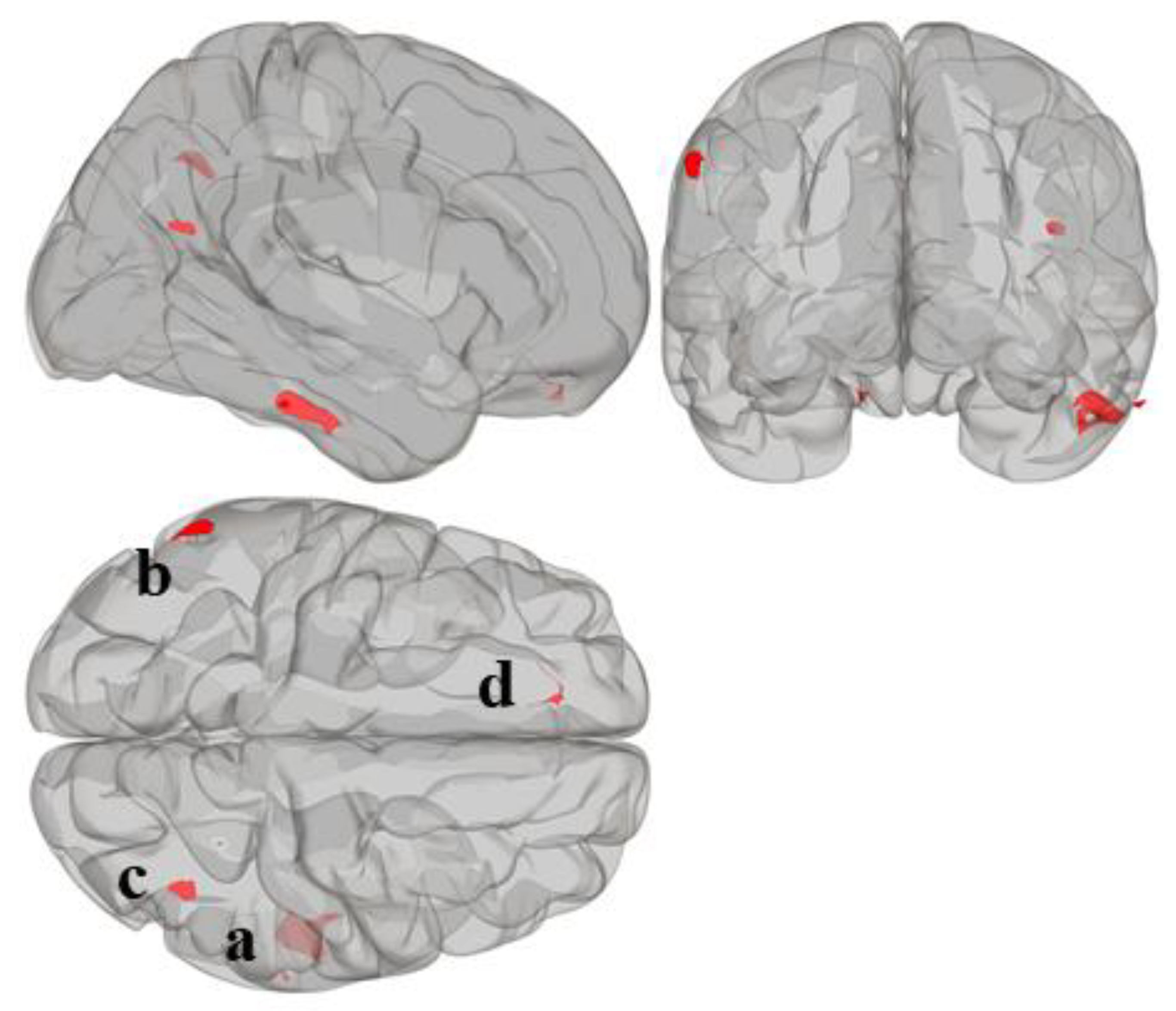
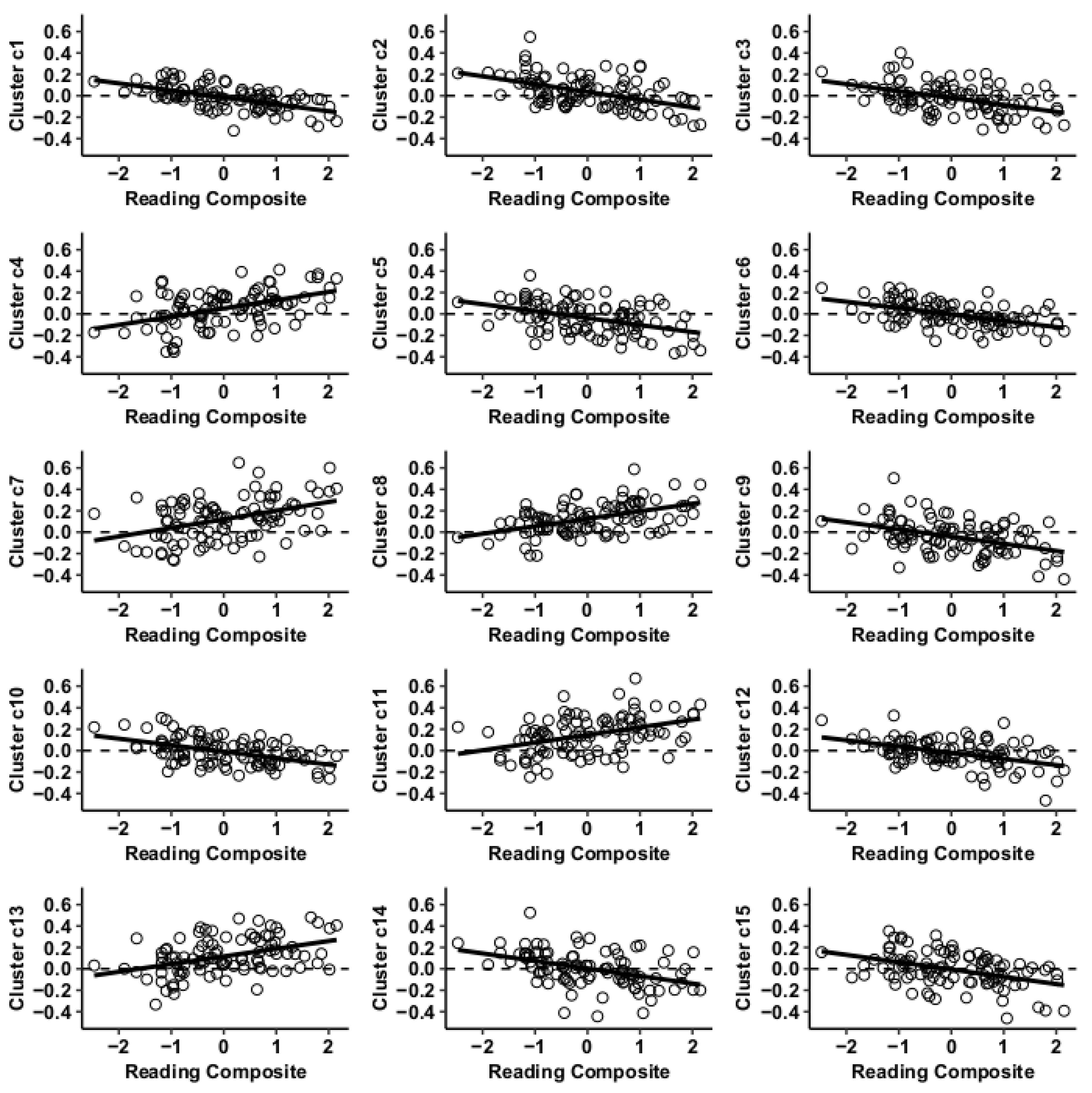
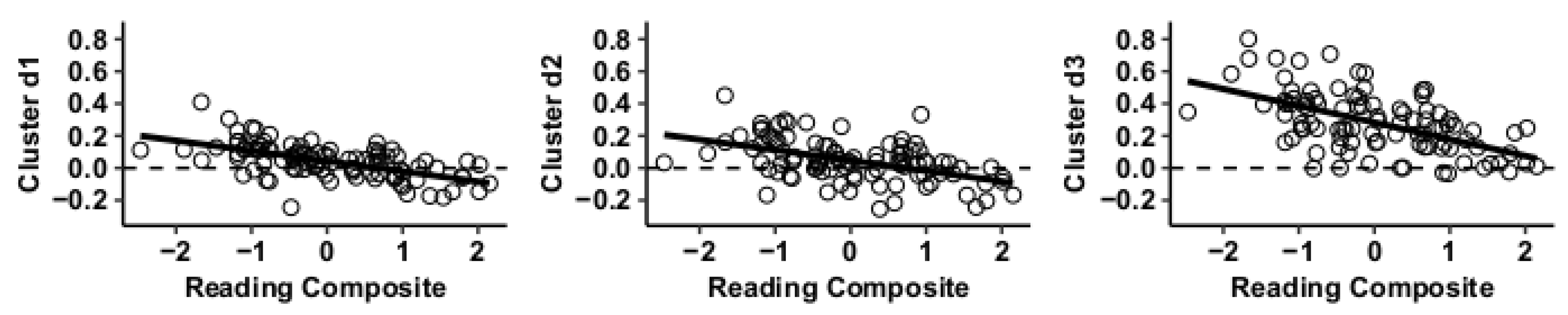
3.1. MVPA Results
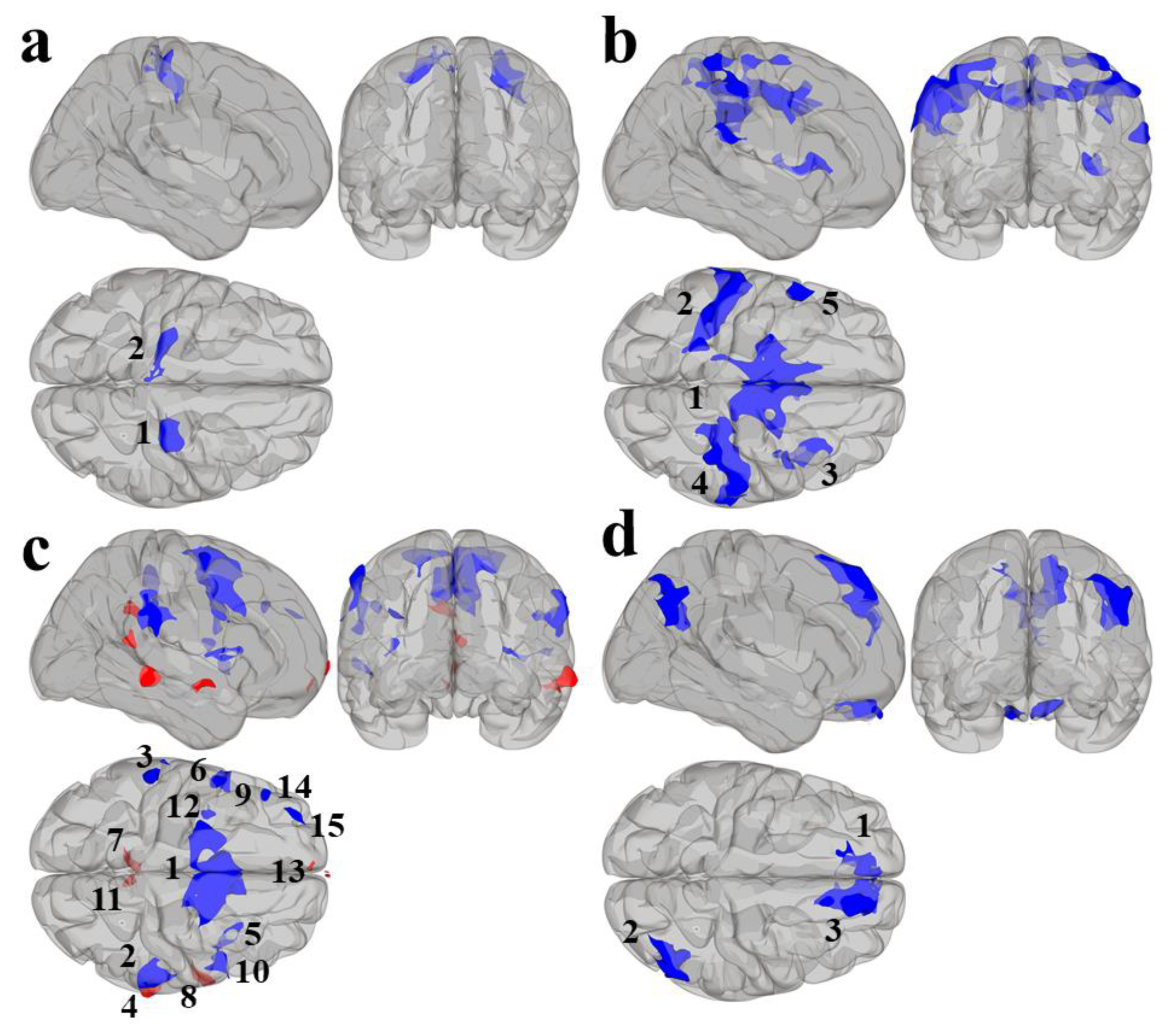
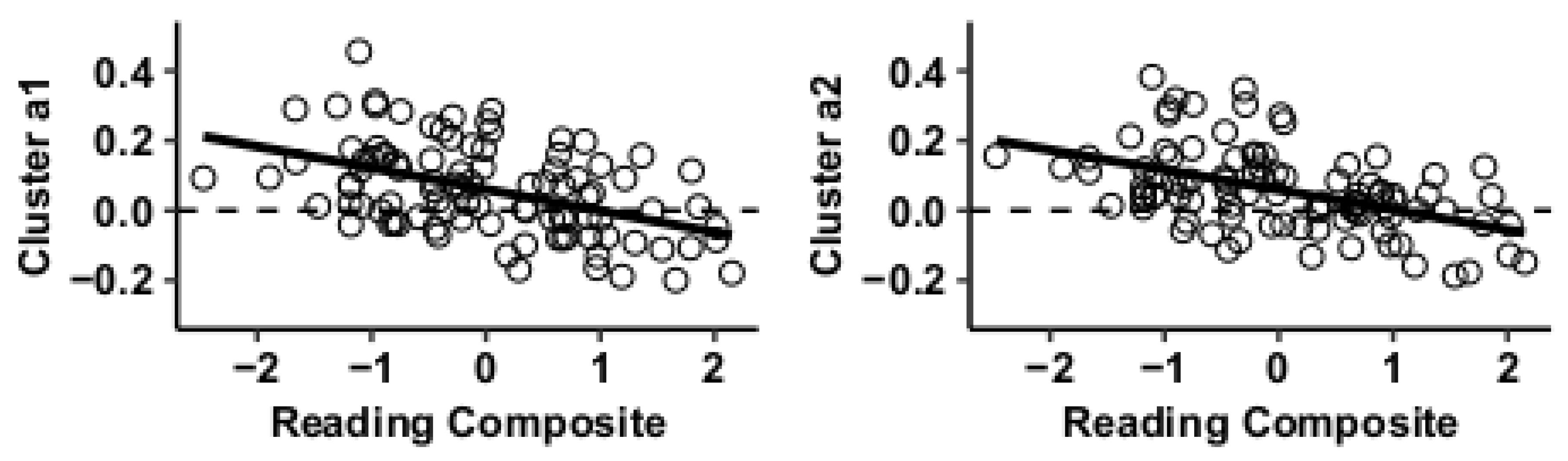
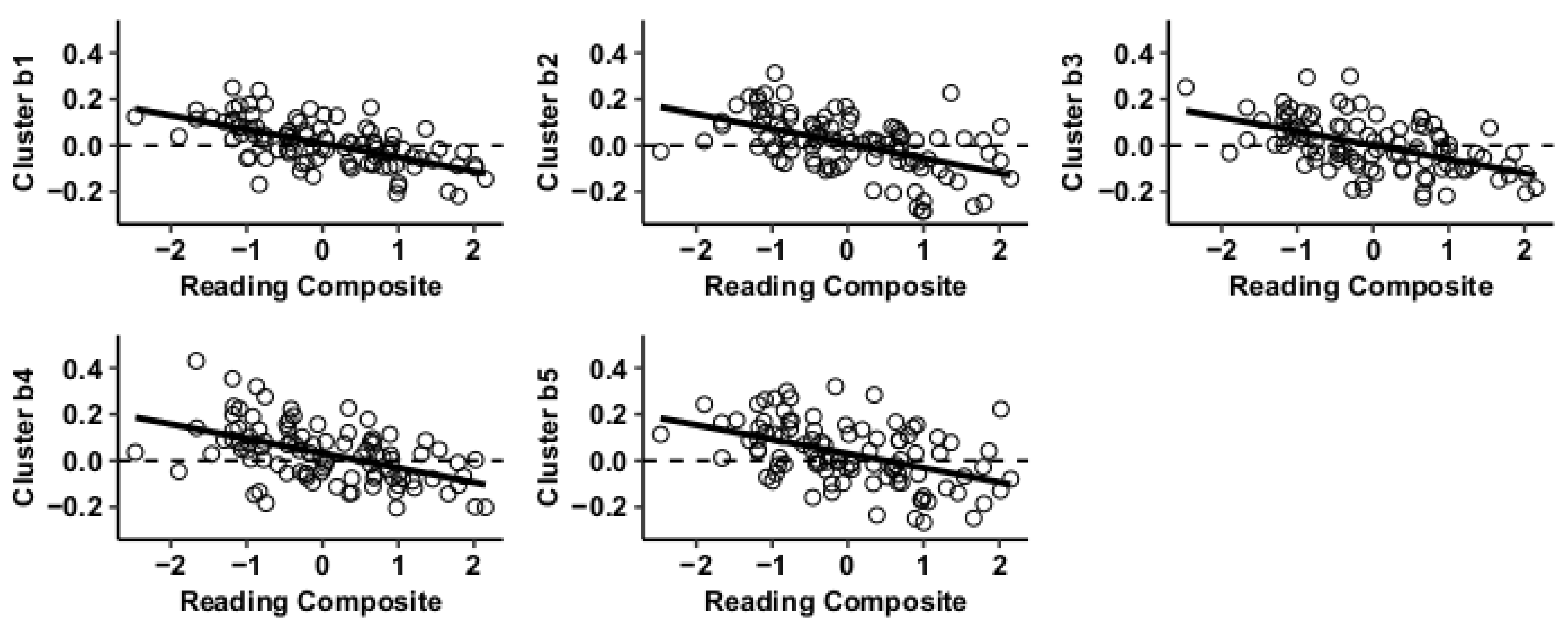
3.2. Post Hoc Seed-to-Voxel Analysis of MVPA-Derived Clusters of Interest
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kuncel, N.R.; Hezlett, S.A. Fact and Fiction in Cognitive Ability Testing for Admissions and Hiring Decisions. Curr. Dir. Psychol. Sci. 2010, 19, 339–345. [Google Scholar] [CrossRef]
- Kuncel, N.R.; Hezlett, S.A. Standardized Tests Predict Graduate Students’ Success. Science 2007, 315, 1080–1081. [Google Scholar] [CrossRef]
- Reback, R.; Rockoff, J.; Schwartz, H.L. Under Pressure: Job Security, Resource Allocation, and Productivity in Schools under No Child Left Behind. Am. Econ. J. Econ. Policy 2014, 6, 207–241. [Google Scholar] [CrossRef]
- Biswal, B.; Yetkin, F.Z.; Haughton, V.M.; Hyde, J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn. Reson. Med. 1995, 34, 537–541. [Google Scholar] [CrossRef]
- van den Heuvel, M.P.; Pol, H.E.H. Exploring the brain network: A review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 2010, 20, 519–534. [Google Scholar] [CrossRef]
- Damoiseaux, J.S.; Rombouts, S.A.R.B.; Barkhof, F.; Scheltens, P.; Stam, C.J.; Smith, S.M.; Beckmann, C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. USA 2006, 103, 13848–13853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehzad, Z.; Kelly, C.; Reiss, P.T.; Gee, D.G.; Gotimer, K.; Uddin, L.Q.; Lee, S.H.; Margulies, D.S.; Roy, A.K.; Biswal, B.B.; et al. The Resting Brain: Unconstrained yet Reliable. Cereb. Cortex 2009, 19, 2209–2229. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.-N.; Kelly, C.; Adelstein, J.S.; Klein, D.F.; Castellanos, F.X.; Milham, M.P. Reliable intrinsic connectivity networks: Test–retest evaluation using ICA and dual regression approach. NeuroImage 2010, 49, 2163–2177. [Google Scholar] [CrossRef] [Green Version]
- Hoff, G.E.A.-J.; Heuvel, M.V.D.; Benders, M.J.; Kersbergen, K.J.; De Vries, L.S. On development of functional brain connectivity in the young brain. Front. Hum. Neurosci. 2013, 7, 650. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.K.; Regina, A.C.B.; Kovacevic, N.; Martin, M.D.G.; Santos, P.P.; Carneiro, C.D.G.; Kerr, D.S.; Amaro, J.E.; McIntosh, A.R.; Busatto, G.F. Aging Effects on Whole-Brain Functional Connectivity in Adults Free of Cognitive and Psychiatric Disorders. Cereb. Cortex 2015, 26, 3851–3865. [Google Scholar] [CrossRef] [Green Version]
- Whitfield-Gabrieli, S.; Ford, J.M. Default Mode Network Activity and Connectivity in Psychopathology. Annu. Rev. Clin. Psychol. 2012, 8, 49–76. [Google Scholar] [CrossRef] [PubMed]
- García-García, I.; Jurado, M.M.M.A.; Garolera, M.; Segura, B.; Sala-Llonch, R.; Marques-Iturria, I.; Pueyo, R.; Sender-Palacios, M.J.; Vernet-Vernet, M.; Narberhaus, A.; et al. Alterations of the salience network in obesity: A resting-state fMRI study. Hum. Brain Mapp. 2012, 34, 2786–2797. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.W.; Weng, T.B.; Burzynska, A.Z.; Wong, C.N.; Cooke, G.E.; Clark, R.; Fanning, J.; Awick, E.; Gothe, N.P.; Olson, E.A.; et al. Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging. NeuroImage 2016, 131, 113–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.; Uddin, L.Q.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Competition between functional brain networks mediates behavioral variability. NeuroImage 2008, 39, 527–537. [Google Scholar] [CrossRef]
- Hampson, M.; Driesen, N.; Roth, J.K.; Gore, J.C.; Constable, R.T. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn. Reson. Imaging 2010, 28, 1051–1057. [Google Scholar] [CrossRef] [Green Version]
- Chaddock-Heyman, L.; Weng, T.B.; Kienzler, C.; Erickson, K.I.; Voss, M.W.; Drollette, E.S.; Raine, L.B.; Kao, S.; Hillman, C.H.; Kramer, A.F. Scholastic performance and functional connectivity of brain networks in children. PLoS ONE 2018, 13, e0190073. [Google Scholar] [CrossRef] [Green Version]
- Koyama, M.S.; Di Martino, A.; Zuo, X.-N.; Kelly, C.; Mennes, M.; Jutagir, D.R.; Castellanos, F.X.; Milham, M.P. Resting-state functional connectivity indexes reading competence in children and adults. J. Neurosci. 2011, 31, 8617–8624. [Google Scholar] [CrossRef] [Green Version]
- Benischek, A.; Long, X.; Rohr, C.S.; Bray, S.; Dewey, D.; Lebel, C. Pre-reading language abilities and the brain’s functional reading network in young children. NeuroImage 2020, 217, 116903. [Google Scholar] [CrossRef]
- Houdeé, O.; Rossi, S.; Lubin, A.; Joliot, M. Mapping numerical processing, reading, and executive functions in the developing brain: An fMRI meta-analysis of 52 studies including 842 children. Dev. Sci. 2010, 13, 876–885. [Google Scholar] [CrossRef]
- Dixon, M.L.; De La Vega, A.; Mills, C.; Andrews-Hanna, J.; Spreng, R.N.; Cole, M.W.; Christoff, K. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc. Natl. Acad. Sci. USA 2018, 115, 1598–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, X.J.; Ofen, N.; Gabrieli, J.D.E.; Whitfield-Gabrieli, S. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. J. Cogn. Neurosci. 2013, 26, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, K.R.A.; Hedden, T.; Venkataraman, A.; Evans, K.C.; Lazar, S.; Buckner, R.L. Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. J. Neurophysiol. 2009, 103, 297–321. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, A.S.; Lytle, L.A.; Murray, D.M.; Story, M.; Perry, C.L.; Boutelle, K.N. Survey development for assessing correlates of young adolescents’ eating. Am. J. Health Behav. 2002, 26, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, R.W. The Woodcock-Johnson Tests of Cognitive Ability-Revised; Houghton Mifflin Harcourt: Boston, MA, USA, 1997. [Google Scholar]
- Kaufman, A.S.; Kaufman, N.L. Kaufman Test of Educational Achievement, 2nd ed.; Ktea, I.I., Ed.; American Guidance Service: Circle Pines, MN, USA, 2004.
- Whitfield-Gabrieli, S.; Nieto-Castañón, A. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef] [Green Version]
- Ciric, R.; Wolf, D.H.; Power, J.D.; Roalf, D.R.; Baum, G.L.; Ruparel, K.; Shinohara, R.T.; Elliott, M.A.; Eickhoff, S.B.; Davatzikos, C.; et al. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. NeuroImage 2017, 154, 174–187. [Google Scholar] [CrossRef]
- Chai, X.J.; Castañón, A.N.; Öngür, D.; Whitfield-Gabrieli, S. Anticorrelations in resting state networks without global signal regression. NeuroImage 2012, 59, 1420–1428. [Google Scholar] [CrossRef] [Green Version]
- Durston, S.; Tottenham, N.T.; Thomas, K.M.; Davidson, M.C.; Eigsti, I.-M.; Yang, Y.; Uluğ, A.M.; Casey, B.J. Differential patterns of striatal activation in young children with and without ADHD. Biol. Psychiatry 2003, 53, 871–878. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, K.R.A.; Sabuncu, M.R.; Buckner, R.L. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage 2012, 59, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Guell, X.; Anteraper, S.A.; Gardner, A.J.; Whitfield-Gabrieli, S.; Kay-Lambkin, F.J.; Iverson, G.L.; Gabrieli, J.; Stanwell, P. Functional Connectivity Changes in Retired Rugby League Players: A Data-Driven Functional Magnetic Resonance Imaging Study. J. Neurotrauma 2020, 37, 1788–1796. [Google Scholar] [CrossRef]
- Anteraper, S.A.; Collin, G.; Guell, X.; Schneidert, T.; Molokotos, E.; Henriksen, M.T.; Mesholam-Gately, R.; Thermenos, H.W.; Seidman, L.J.; Keshavan, M.S.; et al. Altered resting-state functional connectivity in young children at familial high risk for psychotic illness: A preliminary study. Schizophr. Res. 2020, 216, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Anteraper, S.A.; Guell, X.; D’Mello, A.; Joshi, N.; Whitfield-Gabrieli, S.; Joshi, G. Disrupted Cerebrocerebellar Intrinsic Functional Connectivity in Young Adults with High-Functioning Autism Spectrum Disorder: A Data-Driven, Whole-Brain, High-Temporal Resolution Functional Magnetic Resonance Imaging Study. Brain Connect. 2019, 9, 48–59. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Ghosh, S.S.; Nieto-Castanon, A.; Saygin, Z.; Doehrmann, O.; Chai, X.J.; Reynolds, G.O.; Hofmann, S.G.; Pollack, M.H.; Gabrieli, J.D.E. Brain connectomics predict response to treatment in social anxiety disorder. Mol. Psychiatry 2015, 21, 680–685. [Google Scholar] [CrossRef]
- Eklund, A.; Nichols, T.E.; Knutsson, H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. USA 2016, 113, 7900–7905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wechsler, D. Individual Achievement Test-II (WIAT-II); The Psychological Corporation: San Antonio, TX, USA, 2001. [Google Scholar]
- Fair, D.A.; Cohen, A.L.; Power, J.D.; Dosenbach, N.U.F.; Church, J.A.; Miezin, F.M.; Schlaggar, B.L.; Petersen, S.E. Functional Brain Networks Develop from a “Local to Distributed” Organization. PLoS Comput. Biol. 2009, 5, e1000381. [Google Scholar] [CrossRef] [Green Version]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Zabelina, D.L.; Andrews-Hanna, J.R. Dynamic network interactions supporting internally-oriented cognition. Curr. Opin. Neurobiol. 2016, 40, 86–93. [Google Scholar] [CrossRef]
| Measure | Mean |
|---|---|
| n | 97 (53 female) |
| Age (years) | 8.7 ± 0.6 (range: 7.6–9.8) |
| IQ | 108.7 ± 13.0 |
| SES | 1.9 ± 0.8 |
| Reading Composite | 111.9 ± 13.7 |
| Mathematics Composite | 109.5 ± 15.1 |
| FC Regions | Peak Coordinates (MNI) | Peak Coordinate Brain Region | BA | Voxels per Cluster (k) | Total k | T-Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visual | Somatomotor | Dorsal Attention | Ventral Attention | Limbic | Frontoparietal | Default | ||||||
| MVPA a | +52 −34 −28 | Inferior temporal gyrus (r) | BA20 | 0 | 0 | 7 | 0 | 114 | 4 | 0 | 212 | - |
| Cluster 1 | +24 −26 +52 | Precentral gyrus (r) | BA3 | 0 | 298 | 0 | 0 | 0 | 0 | 0 | 488 | −5.12 |
| Cluster 2 | −26 −26 +54 | Precentral gyrus (l) | BA3 | 0 | 259 | 6 | 0 | 0 | 0 | 0 | 372 | −5.25 |
| MVPA b | −58 −52 +36 | Supramarginal gyrus (l) | BA40 | 0 | 0 | 0 | 0 | 0 | 0 | 62 | 80 | - |
| Cluster 1 | −12 −04 +42 | Juxtapositional Lobule Cortex (l) | BA6 | 0 | 1186 | 660 | 687 | 0 | 100 | 2 | 3295 | −7.59 |
| Cluster 2 | −56 −30 +36 | Supramarginal gyrus, anterior division (l) | BA2 | 0 | 247 | 766 | 301 | 0 | 94 | 1 | 1556 | −5.99 |
| Cluster 3 | +38 +16 +04 | Insular cortex (r) | BA48 | 0 | 43 | 0 | 305 | 0 | 2 | 0 | 368 | −6.07 |
| Cluster 4 | +60 −34 +20 | Planum temporale (r) | BA42 | 0 | 16 | 0 | 145 | 0 | 0 | 0 | 183 | −5.80 |
| Cluster 5 | −52 +08 +36 | Precentral gyrus (l) | BA44 | 0 | 10 | 93 | 0 | 0 | 22 | 25 | 175 | −5.32 |
| MVPA c | +40 −64 +22 | Lateral occipital cortex, superior division (r) | BA39 | 0 | 0 | 18 | 0 | 0 | 0 | 20 | 53 | - |
| Cluster 1 | +10 +04 +64 | Superior frontal gyrus (r) | BA6 | 0 | 422 | 365 | 1103 | 0 | 128 | 8 | 2352 | −7.16 |
| Cluster 2 | +60 −30 +28 | Supramarginal gyrus, anterior division (r) | BA48 | 0 | 16 | 3 | 389 | 0 | 47 | 0 | 464 | −5.62 |
| Cluster 3 | −56 −34 +56 | Supramarginal gyrus, anterior division (l) | BA2 | 0 | 10 | 149 | 65 | 0 | 19 | 0 | 253 | −5.07 |
| Cluster 4 | +72 −34 −10 | Middle temporal gyrus, posterior division (r) | BA21 | 0 | 0 | 0 | 0 | 0 | 85 | 99 | 219 | 4.85 |
| Cluster 5 | +56 +00 +06 | Central opercular cortex (r) | BA48 | 0 | 11 | 0 | 189 | 0 | 0 | 0 | 212 | −4.82 |
| Cluster 6 | −54 +08 +32 | Precentral gyrus (l) | BA44 | 0 | 26 | 125 | 42 | 0 | 0 | 0 | 200 | −6.11 |
| Cluster 7 | −02 −40 +30 | Cingulate gyrus, posterior division (l) | BA23 | 0 | 0 | 0 | 0 | 0 | 0 | 137 | 138 | 4.32 |
| Cluster 8 | +64 +04 −12 | Superior temporal gyrus, anterior division (r) | BA21 | 0 | 25 | 0 | 0 | 0 | 0 | 74 | 132 | 5.46 |
| Cluster 9 | −56 +10 +00 | Inferior frontal gyrus, pars opercularis (l) | BA48 | 0 | 15 | 0 | 80 | 0 | 2 | 0 | 130 | −4.77 |
| Cluster 10 | +48 +08 +28 | Precentral gyrus (r) | BA44 | 0 | 0 | 88 | 2 | 0 | 0 | 0 | 106 | −5.52 |
| Cluster 11 | +04 −44 +12 | Cingulate gyrus, posterior division (r) | BA29 | 0 | 0 | 0 | 0 | 0 | 0 | 52 | 93 | 4.33 |
| Cluster 12 | −34 +02 +12 | Insular cortex (l) | BA48 | 0 | 2 | 0 | 66 | 0 | 0 | 0 | 90 | −5.20 |
| Cluster 13 | −02 +60 −12 | Frontal pole (l) | BA11 | 0 | 0 | 0 | 0 | 8 | 0 | 35 | 89 | 4.57 |
| Cluster 14 | −46 +34 +34 | Middle frontal gyrus (l) | BA45 | 0 | 0 | 0 | 0 | 0 | 63 | 0 | 86 | −4.77 |
| Cluster 15 | −36 +48 +30 | Frontal pole (l) | BA46 | 0 | 0 | 0 | 56 | 0 | 5 | 5 | 77 | −4.39 |
| MVPA d | −12 +44 −24 | Frontal pole (l) | BA11 | 0 | 0 | 0 | 0 | 39 | 2 | 0 | 50 | - |
| Cluster 1 | −08 +46 +40 | Frontal pole (l) | BA9 | 0 | 0 | 0 | 0 | 0 | 46 | 1057 | 1347 | −7.01 |
| Cluster 2 | +50 −64 +30 | Lateral occipital cortex, superior division (r) | BA39 | 0 | 0 | 10 | 0 | 0 | 114 | 490 | 688 | −5.43 |
| Cluster 3 | +08 +48 −22 | Frontal medial cortex (r) | BA11 | 0 | 0 | 0 | 0 | 375 | 0 | 3 | 532 | −6.41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westfall, D.R.; Anteraper, S.A.; Chaddock-Heyman, L.; Drollette, E.S.; Raine, L.B.; Whitfield-Gabrieli, S.; Kramer, A.F.; Hillman, C.H. Resting-State Functional Connectivity and Scholastic Performance in Preadolescent Children: A Data-Driven Multivoxel Pattern Analysis (MVPA). J. Clin. Med. 2020, 9, 3198. https://doi.org/10.3390/jcm9103198
Westfall DR, Anteraper SA, Chaddock-Heyman L, Drollette ES, Raine LB, Whitfield-Gabrieli S, Kramer AF, Hillman CH. Resting-State Functional Connectivity and Scholastic Performance in Preadolescent Children: A Data-Driven Multivoxel Pattern Analysis (MVPA). Journal of Clinical Medicine. 2020; 9(10):3198. https://doi.org/10.3390/jcm9103198
Chicago/Turabian StyleWestfall, Daniel R., Sheeba A. Anteraper, Laura Chaddock-Heyman, Eric S. Drollette, Lauren B. Raine, Susan Whitfield-Gabrieli, Arthur F. Kramer, and Charles H. Hillman. 2020. "Resting-State Functional Connectivity and Scholastic Performance in Preadolescent Children: A Data-Driven Multivoxel Pattern Analysis (MVPA)" Journal of Clinical Medicine 9, no. 10: 3198. https://doi.org/10.3390/jcm9103198





