Interval Cancer Rate and Diagnostic Performance of Fecal Immunochemical Test According to Family History of Colorectal Cancer
Abstract
:1. Introduction
2. Materials and Methods
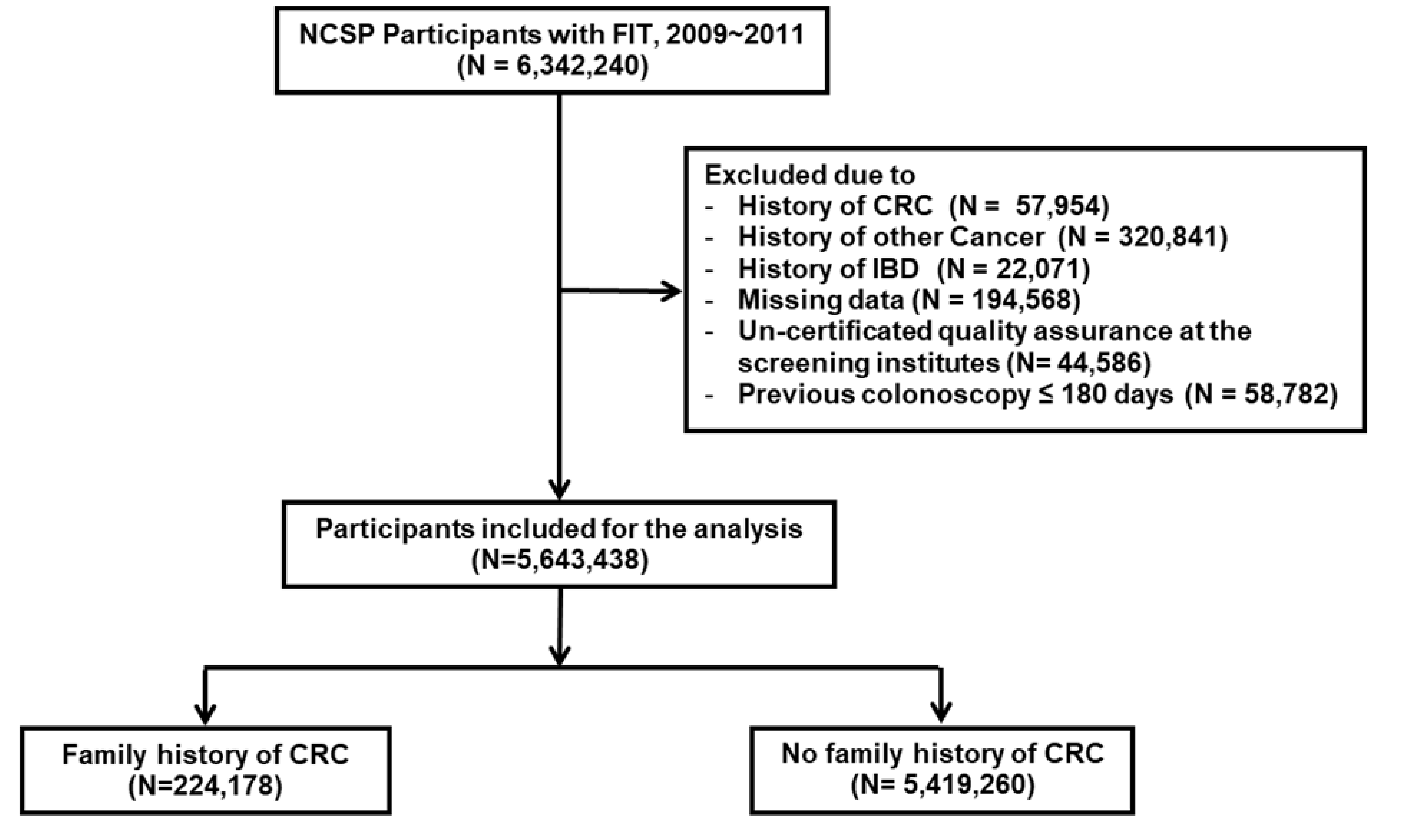
2.1. Study Population
2.2. Fecal Immunochemical Test
2.3. Definition of CRC Status and Variables of Interest
2.4. Statistical Analysis
3. Results
3.1. Participants and Baseline Characteristics
3.2. FIT Positivity Rate, CDR, and ICR
3.3. Diagnostic Performance of FIT
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butterworth, A.S.; Higgins, J.P.; Pharoah, P. Relative and absolute risk of colorectal cancer for individuals with a family history: A meta-analysis. Eur. J. Cancer 2006, 42, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.; Ding, H.; Wang, J.; Chan, P.S.; Huang, J. Prevalence and risk factors of colorectal cancer in asia. Intest. Res. 2019, 17, 317–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johns, L.E.; Houlston, R.S. A systematic review and meta-analysis of familial colorectal cancer risk. Am. J. Gastroenterol. 2001, 96, 2992–3003. [Google Scholar] [CrossRef]
- Rex, D.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Levin, T.R.; Lieberman, D.; Robertson, D.J. Colorectal cancer screening: Recommendations for physicians and patients from the u.S. Multi-society task force on colorectal cancer. Am. J. Gastroenterol. 2017, 112, 1016–1030. [Google Scholar] [CrossRef] [PubMed]
- Leddin, D.; Lieberman, D.A.; Tse, F.; Barkun, A.N.; Abou-Setta, A.M.; Marshall, J.K.; Samadder, N.J.; Singh, H.; Telford, J.J.; Tinmouth, J.; et al. Clinical practice guideline on screening for colorectal cancer in individuals with a family history of nonhereditary colorectal cancer or adenoma: The canadian association of gastroenterology banff consensus. Gastroenterology 2018, 155, 1325–1347.e1323. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, M.A.; Ait Ouakrim, D.; Boussioutas, A.; Hopper, J.L.; Ee, H.C.; Emery, J.D.; Macrae, F.A.; Chetcuti, A.; Wuellner, L.; St John, D.J.B. Revised australian national guidelines for colorectal cancer screening: Family history. Med. J. Aust. 2018, 209, 455–460. [Google Scholar] [CrossRef]
- Quintero, E.; Gimeno-García, A.Z. Colorectal cancer screening in the non-syndromic familial risk population: Is it time to revise the clinical guidelines? Am. J. Gastroenterol. 2017, 112, 1774–1776. [Google Scholar] [CrossRef]
- Castro, I.; Cubiella, J.; Rivera, C.; González-Mao, C.; Vega, P.; Soto, S.; Hernandez, V.; Iglesias, F.; Teresa Alves, M.; Bujanda, L.; et al. Fecal immunochemical test accuracy in familial risk colorectal cancer screening. Int. J. Cancer 2014, 134, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Otero-Estévez, O.; De Chiara, L.; Rodríguez-Berrocal, F.J.; Páez de la Cadena, M.; Cubiella, J.; Castro, I.; Gonzalez-Mao, C.; Hernandez, V.; Martínez-Zorzano, V.S. Serum scd26 for colorectal cancer screening in family-risk individuals: Comparison with faecal immunochemical test. Br. J. Cancer 2015, 112, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Quintero, E.; Carrillo, M.; Gimeno-García, A.Z.; Hernández-Guerra, M.; Nicolás-Pérez, D.; Alonso-Abreu, I.; Díez-Fuentes, M.L.; Abraira, V. Equivalency of fecal immunochemical tests and colonoscopy in familial colorectal cancer screening. Gastroenterology 2014, 147, 1021–1030.e1021, quiz e1016–e1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, A.; Choi, K.S.; Jun, J.K.; Noh, D.K.; Suh, M.; Jung, K.W.; Kim, B.C.; Oh, J.H.; Park, E.C. Validity of fecal occult blood test in the national cancer screening program, korea. PLoS ONE 2013, 8, e79292. [Google Scholar] [CrossRef] [Green Version]
- Cha, J.M.; Suh, M.; Kwak, M.S.; Sung, N.Y.; Choi, K.S.; Park, B.; Jun, J.K.; Hwang, S.H.; Lee, D.H.; Kim, B.C.; et al. Risk of interval cancer in fecal immunochemical test screening significantly higher during the summer months: Results from the national cancer screening program in korea. Am. J. Gastroenterol. 2018, 113, 611–621. [Google Scholar] [CrossRef]
- Almendingen, K.; Hofstad, B.; Vatn, M.H. Does a family history of cancer increase the risk of occurrence, growth, and recurrence of colorectal adenomas? Gut 2003, 52, 747–751. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, G.; Liljegren, A.; Jaramillo, E.; Rubio, C.; Lindblom, A. Adenoma prevalence and cancer risk in familial non-polyposis colorectal cancer. Gut 2002, 50, 228–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imperiale, T.F.; Gruber, R.N.; Stump, T.E.; Emmett, T.W.; Monahan, P.O. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: A systematic review and meta-analysis. Ann. Intern. Med. 2019, 170, 319–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niedermaier, T.; Balavarca, Y.; Brenner, H. Stage-specific sensitivity of fecal immunochemical tests for detecting colorectal cancer: Systematic review and meta-analysis. Am. J. Gastroenterol. 2020, 115, 56–69. [Google Scholar] [CrossRef] [Green Version]
- Bujanda, L.; Sarasqueta, C.; Zubiaurre, L.; Cosme, A.; Muñoz, C.; Sánchez, A.; Martín, C.; Tito, L.; Piñol, V.; Castells, A.; et al. Low adherence to colonoscopy in the screening of first-degree relatives of patients with colorectal cancer. Gut 2007, 56, 1714–1718. [Google Scholar] [CrossRef] [Green Version]
- Puente Gutiérrez, J.J.; Marín Moreno, M.A.; Domínguez Jiménez, J.L.; Bernal Blanco, E.; Díaz Iglesias, J.M. Effectiveness of a colonoscopic screening programme in first-degree relatives of patients with colorectal cancer. Colorectal. Dis. 2011, 13, e145–e153. [Google Scholar] [CrossRef]
- Ng, S.C.; Ching, J.Y.; Chan, V.; Wong, M.C.; Suen, B.Y.; Hirai, H.W.; Lam, T.Y.; Lau, J.Y.; Ng, S.S.; Wu, J.C.; et al. Diagnostic accuracy of faecal immunochemical test for screening individuals with a family history of colorectal cancer. Aliment. Pharm. 2013, 38, 835–841. [Google Scholar] [CrossRef]
- Katsoula, A.; Paschos, P.; Haidich, A.B.; Tsapas, A.; Giouleme, O. Diagnostic accuracy of fecal immunochemical test in patients at increased risk for colorectal cancer: A meta-analysis. JAMA Intern. Med. 2017, 177, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Liles, E.G.; Bent, S.; Levin, T.R.; Corley, D.A. Accuracy of fecal immunochemical tests for colorectal cancer: Systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 171. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.; Song, S.; Cho, H.N.; Park, B.; Jun, J.K.; Choi, E.; Kim, Y.; Choi, K.S. Trends in participation rates for the national cancer screening program in korea, 2002–2012. Cancer Res. Treat. 2017, 49, 798–806. [Google Scholar] [CrossRef] [PubMed]

| Total N = 5,643,438 | Family History of CRC n = 224,178 | No Family History of CRC n = 5,419,260 | p-value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 2,465,771 (43.7) | 95,298 (42.5) | 2,370,473 (43.7) | <0.001 |
| Female | 3,177,667 (56.3) | 128,880 (57.5) | 3,048,787 (56.3) | |
| Age (years) | 60.6 ± 8.2 | 59.6 ± 7.8 | 60.7 ± 8.2 | <0.001 |
| 50–59 | 2,796,297 (49.5) | 123,579 (55.1) | 2,672,718 (49.3) | <0.001 |
| 60–69 | 1,878,505 (33.3) | 70,750 (31.6) | 1,807,755 (33.4) | |
| 70–79 | 857,530 (15.2) | 26,805 (12.0) | 830,725 (15.3) | |
| ≥80 | 111,106 (2.0) | 3,044 (1.4) | 108,062 (2.0) | |
| History of colonoscopy within past 5 years | ||||
| No | 4,773,868 (84.6) | 179,259 (80.0) | 4,594,609 (84.8) | <0.001 |
| Yes | 869,570 (15.4) | 44,919 (20.0) | 824,651 (15.2) | |
| History of polypectomy | 186,437 (3.3) | 9,887 (4.4) | 176,550 (3.3) | <0.001 |
| FIT positive | 334,195 (5.9) | 14,385 (6.4) | 319,810 (5.9) | <0.001 |
| CRC detection * | 16,363 (0.29) | 845 (0.38) | 15,518 (0.29) | <0.001 |
| Interval cancer ** | 5,865/5,309,243 (0.11) | 301/209,793 (0.14) | 5,564/5,099,450 (0.11) | <0.001 |
| FIT Positive Rate | Cancer Detection Rate | Interval Cancer Rate | |||||||
|---|---|---|---|---|---|---|---|---|---|
| % (95% CI) | aRR (95% CI) | p-value | Per 1000 * (95% CI) | aRR (95% CI) | p-value | Per 1000 ** (95% CI) | aRR (95% CI) | p-value | |
| All participants | |||||||||
| FHx of CRC | |||||||||
| No | 5.9 (5.9–5.9) | 1.00 (ref) | 2.9 (2.8–2.9) | 1.00 (ref) | 1.1 (1.1–1.1) | 1.00 (ref) | |||
| Yes | 6.4 (6.3–6.5) | 1.11 (1.09–1.13) | <0.001 | 3.8 (3.5–4.0) | 1.43 (1.33–1.53) | <0.001 | 1.4 (1.3–1.6) | 1.43 (1.27–1.60) | <0.001 |
| Without previous colonoscopy ≤5 years | |||||||||
| FHx of CRC | |||||||||
| No | 6.0 (5.9–6.0) | 1.00 (ref) | 3.2 (3.2–3.3) | 1.00 (ref) | 1.2 (1.2–1.2) | 1.00 (ref) | |||
| Yes | 6.6 (6.5–6.7) | 1.12 (1.10–1.14) | <0.001 | 4.4 (4.1–4.7) | 1.48 (1.38–1.59) | <0.001 | 1.6 (1.4–1.8) | 1.46 (1.29–1.65) | <0.001 |
| With previous colonoscopy ≤5 years | |||||||||
| FHx of CRC | |||||||||
| No | 5.5 (5.5–5.6) | 1.00 (ref) | 0.9 (0.9–1.0) | 1.00 (ref) | 0.6 (0.5–0.6) | 1.00 (ref) | |||
| Yes | 5.8 (5.6–6.0) | 1.07 (1.03–1.11) | <0.001 | 1.2 (0.9–1.6) | 1.50 (1.15–1.97) | 0.003 | 0.8 (0.5–1.0) | 1.51 (1.06–2.15) | 0.023 |
| Sensitivity | Specificity | PPV | NPV | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % (95% CI) | aRR (95% CI) | p-value | % (95% CI) | aRR (95% CI) | p-value | % (95% CI) | aRR | p-value | % (95% CI) | aRR | p-value | |
| Total | ||||||||||||
| FHx of CRC | ||||||||||||
| No | 64.1 (63.4–64.9) | 1.00 (ref) | 94.3 (94.2–94.3) | 1.00 (ref) | 3.1 (3.1–3.2) | 1.00 (ref) | 99.9 (99.9–99.9) | 1.00 (ref) | ||||
| Yes | 64.4 (61.2–67.6) | 1.01 (0.92–1.10) | 0.903 | 93.8 (93.7–93.9) | 0.994 (0.990–0.999) | 0.008 | 3.8 (3.5–4.1) | 1.29 (1.18–1.41) | <0.001 | 99.9 (99.8–99.9) | 1.000 (0.995–1.004) | 0.849 |
| Without previous colonoscopy ≤5 years | ||||||||||||
| FHx of CRC | ||||||||||||
| No | 65.3 (64.6–66.1) | 1.00 (ref) | 94.2 (94.2–94.2) | 1.00 (ref) | 3.5 (3.5–3.6) | 1.00 (ref) | 99.9 (99.9–99.9) | 1.00 (ref) | ||||
| Yes | 66.0 (62.7–69.3) | 1.01 (0.93–1.11) | 0.785 | 93.7 (93.6–93.8) | 0.993 (0.989–0.998) | 0.008 | 4.4 (4.1–4.8) | 1.33 (1.22–1.45) | <0.001 | 99.8 (99.8–99.9) | 1.000 (0.995–1.004) | 0.841 |
| With previous colonoscopy ≤5 years | ||||||||||||
| FHx of CRC | ||||||||||||
| No | 41.4 (37.9–44.9) | 1.00 (ref) | 94.5 (94.4–94.5) | 1.00 (ref) | 0.7 (0.6–0.8) | 1.00 (ref) | 99.9 (99.9–99.9) | 1.00 (ref) | ||||
| Yes | 41.1 (28.2–54.0) | 1.00 (0.66–1.53) | 0.990 | 94.2 (94.0–94.4) | 0.996 (0.986–1.006) | 0.435 | 0.9 (0.5–1.2) | 1.41 (0.92–2.15) | 0.114 | 99.9 (99.9–99.9) | 1.00 (0.99–1.01) | 0.958 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, Y.S.; Lee, J.; Lee, H.A.; Moon, C.M. Interval Cancer Rate and Diagnostic Performance of Fecal Immunochemical Test According to Family History of Colorectal Cancer. J. Clin. Med. 2020, 9, 3302. https://doi.org/10.3390/jcm9103302
Jung YS, Lee J, Lee HA, Moon CM. Interval Cancer Rate and Diagnostic Performance of Fecal Immunochemical Test According to Family History of Colorectal Cancer. Journal of Clinical Medicine. 2020; 9(10):3302. https://doi.org/10.3390/jcm9103302
Chicago/Turabian StyleJung, Yoon Suk, Jinhee Lee, Hye Ah Lee, and Chang Mo Moon. 2020. "Interval Cancer Rate and Diagnostic Performance of Fecal Immunochemical Test According to Family History of Colorectal Cancer" Journal of Clinical Medicine 9, no. 10: 3302. https://doi.org/10.3390/jcm9103302
APA StyleJung, Y. S., Lee, J., Lee, H. A., & Moon, C. M. (2020). Interval Cancer Rate and Diagnostic Performance of Fecal Immunochemical Test According to Family History of Colorectal Cancer. Journal of Clinical Medicine, 9(10), 3302. https://doi.org/10.3390/jcm9103302





