Infrapopliteal Artery Occlusive Disease: An Overview of Vessel Preparation and Treatment Options
Abstract
:1. Introduction
1.1. Vessel Preparation (VP)
1.2. Cutting Balloon/Scoring Balloon
1.3. Atherectomy
1.4. Orbital Atherectomy (OA)
1.5. Rotational Atherectomy (RA)
1.6. Directional Atherectomy (DA)
1.7. Laser Atherectomy (LA)
1.8. Treatment
1.9. Percutaneous Transluminal Angioplasty (PTA)
1.10. Drug Coated Balloon (DCB)
1.11. Drug Eluting Stent (DES)
1.12. TACK Endovascular System
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hiatt, W.R. Medical treatment of peripheral arterial disease and claudication. N. Engl. J. Med. 2001, 344, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Sauguet, A.; Leger, P. Tools & techniques: Below the knee interventions. EuroIntervention 2012, 7, 1120–1123. [Google Scholar] [PubMed]
- Razavi, M. Infrapopliteal Arterial Interventions. In Handbook of Interventional Radiologic Procedures, 5th ed.; Kandarpa, K., Machan, L., Durham, J., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2016; pp. 172–177. [Google Scholar]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.; TASC II Working Group; Bell, K.; Caporusso, J.; Durand-Zaleski, I.; et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef] [Green Version]
- Spiliopoulos, S.; Katsanos, K.; Karnabatidis, D.; Diamantopoulos, A.; Kagadis, G.C.; Christeas, N.; Siablis, D. Cryoplasty versus conventional balloon angioplasty of the femoropopliteal artery in diabetic patients: Long-term results from a prospective randomized single-center controlled trial. Cardiovasc. Intervent. Radiol. 2010, 33, 929–938. [Google Scholar] [CrossRef] [Green Version]
- Graziani, L.; Silvestro, A.; Bertone, V.; Manara, E.; Andreini, R.; Sigala, A.; Mingardi, R.; De Giglio, R. Vascular involvement in diabetic subjects with ischemic foot ulcer: A new morphologic categorization of disease severity. Eur. J. Vasc EndoVasc. Surg. 2007, 33, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Chung, J. Endovascular Devices and Revascularization Techniques for Limb-Threatening Ischemia in Individuals with Diabetes. J. Diabetes Sci. Technol. 2017, 11, 904–913. [Google Scholar] [CrossRef] [Green Version]
- Lyden, S.P. Techniques and outcomes for endovascular treatment in the tibial arteries. J. Vasc. Surg. 2009, 50, 1219–1223. [Google Scholar] [CrossRef] [Green Version]
- Nehler, M.R.; Hiatt, W.R.; Taylor, L.M., Jr. Is revascularization and limb salvage always the best treatment for critical limb ischemia? J. Vasc. Surg. 2003, 37, 704–708. [Google Scholar] [CrossRef] [Green Version]
- DeRubertis, B.G.; Faries, P.L.; McKinsey, J.F.; Chaer, R.A.; Pierce, M.; Karwowski, J.; Weinberg, A.; Nowygrod, R.; Morrissey, N.J.; Bush, H.L.; et al. Shifting paradigms in the treatment of lower extremity vascular disease: A report of 1000 percutaneous interventions. Ann. Surg. 2007, 246, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Romiti, M.; Albers, M.; Brochado-Neto, F.C.; Durazzo, A.E.; Pereira, C.A.; De Luccia, N. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J. Vasc. Surg. 2008, 47, 975–981. [Google Scholar] [CrossRef]
- Ferraresi, R.; Centola, M.; Ferlini, M.; Da Ros, R.; Caravaggi, C.; Assaloni, R.; Sganzaroli, A.; Pomidossi, G.; Bonanomi, C.; Danzi, G.B.; et al. Long-term outcomes after angioplasty of isolated, below-the-knee arteries in diabetic patients with critical limb ischaemia. Eur. J. Vasc EndoVasc. Surg. 2009, 37, 336–342. [Google Scholar] [CrossRef] [Green Version]
- Park, S.W.; Kim, J.S.; Yun, I.J.; Hwang, J.J.; Lee, S.A.; Chee, H.K.; Chang, S.H.; Shin, D.H.; Jung, H.G.; Chang, I.S.; et al. Clinical outcomes of endovascular treatments for critical limb ischemia with chronic total occlusive lesions limited to below-the-knee arteries. Acta Radiol. 2013, 54, 785–789. [Google Scholar] [CrossRef]
- Van Overhagen, H.; Spiliopoulos, S.; Tsetis, D. Below-the-knee interventions. Cardiovasc. Intervent. Radiol. 2013, 36, 302–311. [Google Scholar] [CrossRef]
- Söderström, M.I.; Arvela, E.M.; Korhonen, M.; Halmesmäki, K.H.; Albäck, A.N.; Biancari, F.; Lepäntalo, M.J.; Venermo, M.A. Infrapopliteal percutaneous transluminal angioplasty versus bypass surgery as first-line strategies in critical leg ischemia: A propensity score analysis. Ann. Surg. 2010, 252, 765–773. [Google Scholar] [CrossRef]
- Kudo, T.; Chandra, F.A.; Kwun, W.H.; Haas, B.T.; Ahn, S.S. Changing pattern of surgical revascularization for critical limb ischemia over 12 years: Endovascular vs. open bypass surgery. J. Vasc. Surg. 2006, 44, 304–313. [Google Scholar] [CrossRef] [Green Version]
- Faglia, E.; Clerici, G.; Clerissi, J.; Gabrielli, L.; Losa, S.; Mantero, M.; Caminiti, M.; Curci, V.; Lupattelli, T.; Morabito, A.; et al. Early and five-year amputation and survival rate of diabetic patients with critical limb ischemia: Data of a cohort study of 564 patients. Eur. J. Vasc. Endovasc. Surg. 2006, 32, 484–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.Q.; Zhao, J.G.; Liu, F.; Wang, J.B.; Cheng, Y.S.; Li, M.H.; Wang, J.; Li, J. Subintimal angioplasty for below-the-ankle arterial occlusions in diabetic patients with chronic critical limb ischemia. J. Endovasc. Ther. 2009, 16, 604–612. [Google Scholar] [CrossRef]
- Faglia, E.; Dalla Paola, L.; Clerici, G.; Clerissi, J.; Graziani, L.; Fusaro, M.; Gabrielli, L.; Losa, S.; Stella, A.; Gargiulo, M.; et al. Peripheral angioplasty as the first-choice revascularization procedure in diabetic patients with critical limb ischemia: Prospective study of 993 consecutive patients hospitalized and followed between 1999 and 2003. Eur. J. Vasc Endovasc. Surg. 2005, 29, 620–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pernès, J.M.; Auguste, M.; Borie, H.; Kovarsky, S.; Bouchareb, A.; Despujole, C.; Coppé, G. Infrapopliteal arterial recanalization: A true advance for limb salvage in diabetics. Diagn. Interv. Imaging 2015, 96, 423–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langhoff, R. The Importance of Vessel Preparation. Endovasc. Today 2018, 6, 4–7. [Google Scholar]
- Barath, P.; Fishbein, M.C.; Vari, S.; Forrester, J.S. Cutting balloon: A novel approach to percutaneous angioplasty. Am. J. Cardiol. 1991, 68, 1249–1252. [Google Scholar] [CrossRef]
- Barath, P. Microsurgical dilatation concept: Animal data. J. Invasive Cardiol. 1996, 8 (Suppl. SA), 2A–5A. [Google Scholar] [PubMed]
- Ansel, G.M.; Sample, N.S.; Botti III, C.F., Jr.; Tracy, A.J.; Silver, M.J.; Marshall, B.J.; George, B.S. Cutting balloon angioplasty of the popliteal and infrapopliteal vessels for symptomatic limb ischemia. Catheter. Cardiovasc. Interv. 2004, 61, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, R.; Posa, A.; Santoro, M.; Nestola, M.; Contegiacomo, A.; Tinelli, G.; Paolini, A.; Flex, A.; Pitocco, D.; Snider, F.; et al. Cutting Balloon Angioplasty in the Treatment of Short Infrapopliteal Bifurcation Disease. J. Endovasc. Ther. 2015, 22, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Canaud, L.; Alric, P.; Berthet, J.P.; Marty-Ané, C.; Mercier, G.; Branchereau, P. Infrainguinal cutting balloon angioplasty in de novo arterial lesions. J. Vasc. Surg. 2008, 48, 1182–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsanos, K.; Spiliopoulos, S.; Reppas, L.; Karnabatidis, D. Debulking Atherectomy in the Peripheral Arteries: Is There a Role and what is the Evidence? Cardiovasc. Intervent. Radiol. 2017, 40, 964–977. [Google Scholar] [CrossRef] [Green Version]
- Safian, R.D.; Niazi, K.; Runyon, J.P.; Dulas, D.; Weinstock, B.; Ramaiah, V.; Heuser, R.; OASIS Investigators. Orbital atherectomy for infrapopliteal disease: Device concept and outcome data for the OASIS trial. Catheter. Cardiovasc. Interv. 2009, 73, 406–412. [Google Scholar] [CrossRef]
- Giannopoulos, S.; Secemsky, E.A.; Mustapha, J.A.; Adams, G.; Beasley, R.E.; Pliagas, G.; Armstrong, E.J. Three-Year Outcomes of Orbital Atherectomy for the Endovascular Treatment of Infrainguinal Claudication or Chronic Limb-Threatening Ischemia. J. Endovasc. Ther. 2020, 27, 714–725. [Google Scholar] [CrossRef]
- Lee, M.S.; Mustapha, J.; Beasley, R.; Chopra, P.; Das, T.; Adams, G.L. Impact of lesion location on procedural and acute angiographic outcomes in patients with critical limb ischemia treated for peripheral artery disease with orbital atherectomy: A CONFIRM registries subanalysis. Catheter. Cardiovasc. Interv. 2016, 87, 440–445. [Google Scholar] [CrossRef]
- Dorros, G.; Iyer, S.; Zaitoun, R.; Lewin, R.; Cooley, R.; Olson, K. Acute angiographic and clinical outcome of high speed percutaneous rotational atherectomy (Rotablator). Cathet. Cardiovasc. Diagn. 1991, 22, 157–166. [Google Scholar] [CrossRef]
- Zeller, T.; Krankenberg, H.; Steinkamp, H.; Rastan, A.; Sixt, S.; Schmidt, A.; Sievert, H.; Minar, E.; Bosiers, M.; Peeters, P.; et al. One-year outcome of percutaneous rotational atherectomy with aspiration in infrainguinal peripheral arterial occlusive disease: The multicenter pathway PVD trial. J. Endovasc. Ther. 2009, 16, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Sixt, S.; Scheinert, D.; Rastan, A.; Krankenberg, H.; Steinkamp, H.; Schmidt, A.; Sievert, H.; Minar, E.; Bosiers, M.; Peeters, P.; et al. One-Year Outcome After Percutaneous Rotational and Aspiration Atherectomy in Infrainguinal Arteries in Patient With and Without Type 2 Diabetes Mellitus. Ann. Vasc. Surg. 2011, 25, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Akkus, N.I.; Abdulbaki, A.; Jimenez, E.; Tandon, N. Atherectomy devices: Technology update. Med. Devices 2015, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cawich, I.; Paixao, A.R.; Marmagkiolis, K.; Lendel, V.; Rodriguez-Araujo, G.; Rollefson, W.A.; Mego, D.M.; Cilingiroglu, M. Immediate and intermediate-term results of optical coherence tomography guided atherectomy in the treatment of peripheral arterial disease: Initial results from the VISION trial. Cardiovasc. Revasc. Med. 2016, 17, 463–467. [Google Scholar] [CrossRef]
- Ramaiah, V.; Gammon, R.; Kiesz, S.; Cardenas, J.; Runyon, J.P.; Fail, P.; Walker, C.; Allie, D.E.; Chamberlin, J.; Solis, M.; et al. Midterm outcomes from the TALON Registry: Treating peripherals with SilverHawk: Outcomes collection. J. Endovasc. Ther. 2006, 13, 592–602. [Google Scholar] [CrossRef]
- Zeller, T.; Sixt, S.; Schwarzwälder, U.; Schwarz, T.; Frank, U.; Bürgelin, K.; Pochert, V.; Müller, C.; Noory, E.; Krankenberg, H.; et al. Two-year results after directional atherectomy of infrapopliteal arteries with the SilverHawk device. J. Endovasc. Ther. 2007, 14, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Rastan, A.; McKinsey, J.F.; Garcia, L.A.; Rocha-Singh, K.J.; Jaff, M.R.; Noory, E.; Zeller, T.; DEFINITIVE LE Investigators. One-Year Outcomes Following Directional Atherectomy of Infrapopliteal Artery Lesions: Subgroup Results of the Prospective, Multicenter DEFINITIVE LE Trial. J. Endovasc. Ther. 2015, 22, 839–846. [Google Scholar] [CrossRef]
- Gray, B.H.; Laird, J.R.; Ansel, G.M.; Shuck, J.W. Complex endovascular treatment for critical limb ischemia in poor surgical candidates: A pilot study. J. Endovasc. Ther. 2002, 9, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Laird, J.R.; Zeller, T.; Gray, B.H.; Scheinert, D.; Vranic, M.; Reiser, C.; Biamino, G. Limb Salvage Follow-ing Laser-Assisted Angioplasty for Critical Limb Ischemia:Results of the LACI Multicenter Trial. J. Endovasc. Ther. 2006, 13, 1–11. [Google Scholar] [CrossRef]
- Singh, T.; Kodenchery, M.; Artham, S.; Piyaskulkaew, C.; Szpunar, S.; Parvataneni, K.; Ballout, H.; Chugtai, H.; Stewart, D.; LaLonde, T.; et al. Laser in infra-popliteal and popliteal stenosis (LIPS): Retro-spective review of laser-assisted balloon angioplasty versus balloon angioplasty alone for below knee pe-ripheral arterial disease. Cardiovasc. Interv. Ther. 2013, 29, 109–116. [Google Scholar] [CrossRef]
- Mustapha, J.A.; Finton, S.M.; Diaz-Sandoval, L.J.; Saab, F.A.; Miller, L.E. Percutaneous Transluminal Angioplasty in Patients With Infrapopliteal Arterial Disease. Sys-tematic Review and Meta-Analysis. Circ. Cardiovasc. Interv. 2016, 9, e003468. [Google Scholar] [CrossRef] [PubMed]
- Klumb, C.; Lehmann, T.; Aschenbach, R.; Eckardt, N.; Teichgräber, U. Benefit and risk from paclitaxel-coated balloon angioplasty for the treatment of femoropopliteal artery disease: A systematic review and meta-analysis of randomised controlled trials. EClinicalMedicine 2019, 16, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Zeller, T.; Beschorner, U.; Pilger, E.; Bosiers, M.; Deloose, K.; Peeters, P.; Scheinert, D.; Schulte, K.L.; Rastan, A.; Brodmann, M. Paclitaxel-Coated Balloon in Infrapopliteal Arteries: 12-Month Results From the BIOLUX P-II Randomized Trial (BIOTRONIK’S-First in Man study of the Passeo-18 LUX drug releasing PTA Balloon Catheter vs. the uncoated Passeo-18 PTA balloon catheter in subjects requiring revascularization of infrapopliteal arteries). JACC Cardiovasc. Interv. 2015, 8, 1614–1622. [Google Scholar] [PubMed] [Green Version]
- Zeller, T.; Baumgartner, I.; Scheinert, D.; Brodmann, M.; Bosiers, M.; Micari, A.; Peeters, P.; Vermassen, F.; Landini, M.; Snead, D.B.; et al. Drug-eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia: 12-month results from the IN.PACT DEEP randomized trial. J. Am. Coll. Cardiol. 2014, 64, 1568–1576. [Google Scholar] [CrossRef]
- Katsanos, K.; Spiliopoulos, S.; Paraskevopoulos, I.; Diamantopoulos, A.; Karnabatidis, D. Systematic Review and Meta-analysis of Randomized Controlled Trials of Paclitaxel-Coated Balloon Angioplasty in the Femoropopliteal Arteries: Role of Paclitaxel Dose and Bioavailability. J. Endovasc. Ther. 2016, 23, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, J.A.; Brodmann, M.; Geraghty, P.J.; Saab, F.; Settlage, R.A.; Jaff, M.R.; Lutonix BTK Study Investigator. Drug-Coated vs. Uncoated Percutaneous Transluminal Angioplasty in Infrapopliteal Arteries: Six-Month Results of the Lutonix BTK Trial. J. Invasive Cardiol. 2019, 31, 205–211. [Google Scholar] [PubMed]
- Katsanos, K.; Spiliopoulos, S.; Kitrou, P.; Krokidis, M.; Paraskevopoulos, I.; Karnabatidis, D. Risk of Death and Amputation with Use of Paclitaxel-Coated Balloons in the Infrapopliteal Arteries for Treatment of Criti-cal Limb Ischemia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Vasc. Interv. Radiol. 2020, 31, 202–212. [Google Scholar] [CrossRef]
- Katsanos, K.; Kitrou, P.; Spiliopoulos, S.; Diamantopoulos, A.; Karnabatidis, D. Comparative Effectiveness of Plain Balloon Angioplasty, Bare Metal Stents, Drug-Coated Balloons, and Drug-Eluting Stents for the Treatment of Infrapopliteal Artery Disease: Systematic Review and Bayesian Network Meta-analysis of Randomized Controlled Trials. J. Endovasc. Ther. 2016, 23, 851–863. [Google Scholar]
- Varcoe, R.L.; Paravastu, S.C.; Thomas, S.D.; Bennett, M.H. The use of drug-eluting stents in infrapopliteal arteries: An updated systematic review and meta-analysis of randomized trials. Int. Angiol. 2019, 38, 121–135. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Identifier NCT03551496, The DES BTK Vascular Stent System vs. PTA in Subjects with Critical Limb Ischemia (SAVAL). June 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT03551496 (accessed on 27 September 2020).
- Giannopoulos, S.; Varcoe, R.L.; Lichtenberg, M.; Rundback, J.; Brodmann, M.; Zeller, T.; Schneider, P.A.; Armstrong, E.J. Balloon angioplasty of infrapopliteal arteries: A systematic review and proposed algorithm for optimal endovascular therapy. J. Endovasc. Ther. 2020, 27, 547–564. [Google Scholar] [CrossRef]
- Geraghty, P.J.; Adams, G.; Schmidt, A.; TOBA II BTK Investigators. 6-Month Pivotal Results of Tack Optimized Balloon Angioplasty using the Tack Endovascular System® in Below-the-Knee Arteries. J. Vasc. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
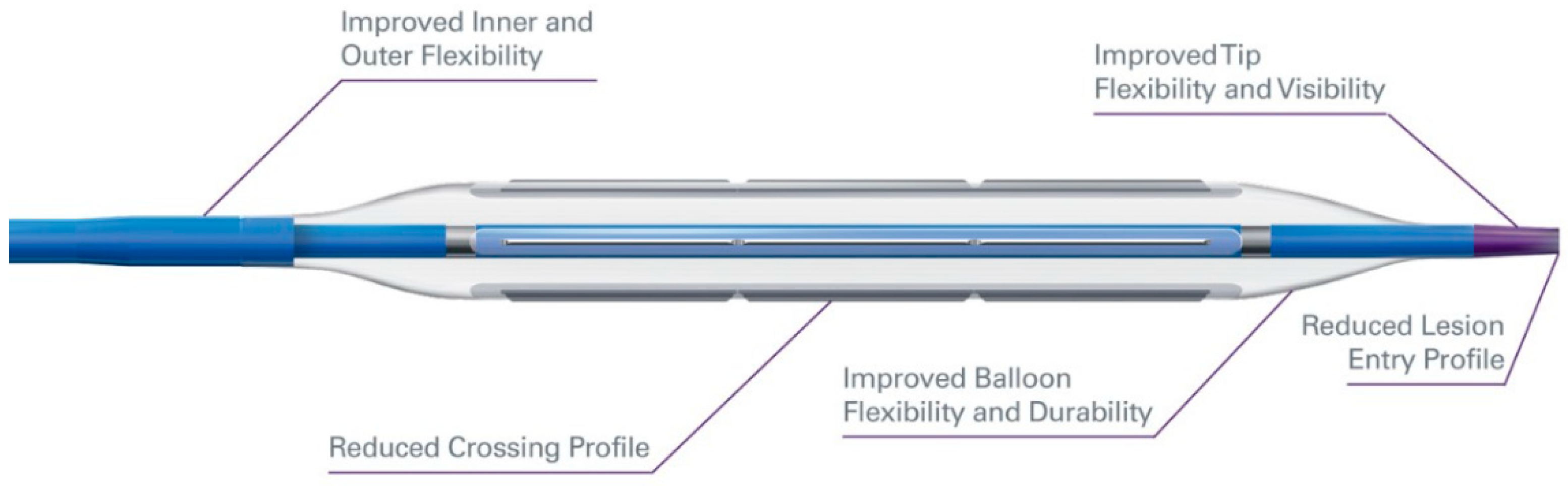





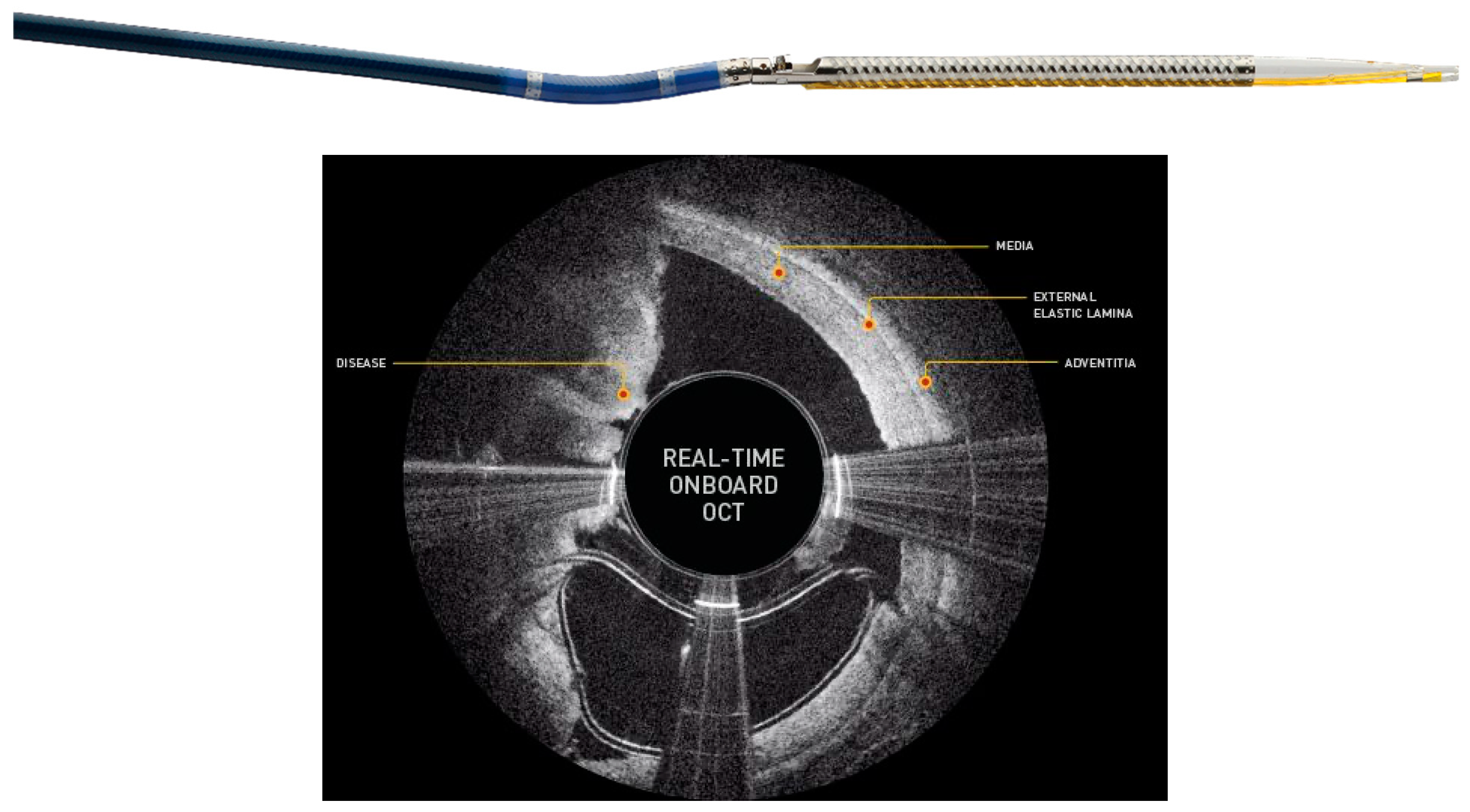


| Manufacturer | DCB | Drug | Dose (Micrograms/mm2) |
|---|---|---|---|
| BD Bard | Lutonix | Paclitaxel | 2.0 |
| Medtronic | In.PACT | Paclitaxel | 3.5 |
| Philips | Stellarex | Paclitaxel | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tummala, S.; Amin, A.; Mehta, A. Infrapopliteal Artery Occlusive Disease: An Overview of Vessel Preparation and Treatment Options. J. Clin. Med. 2020, 9, 3321. https://doi.org/10.3390/jcm9103321
Tummala S, Amin A, Mehta A. Infrapopliteal Artery Occlusive Disease: An Overview of Vessel Preparation and Treatment Options. Journal of Clinical Medicine. 2020; 9(10):3321. https://doi.org/10.3390/jcm9103321
Chicago/Turabian StyleTummala, Srini, Ayush Amin, and Ankit Mehta. 2020. "Infrapopliteal Artery Occlusive Disease: An Overview of Vessel Preparation and Treatment Options" Journal of Clinical Medicine 9, no. 10: 3321. https://doi.org/10.3390/jcm9103321




