Comparison of the Visibility of Fetal Tooth Buds on 1.5 and 3 Tesla MRI
Abstract
:1. Introduction
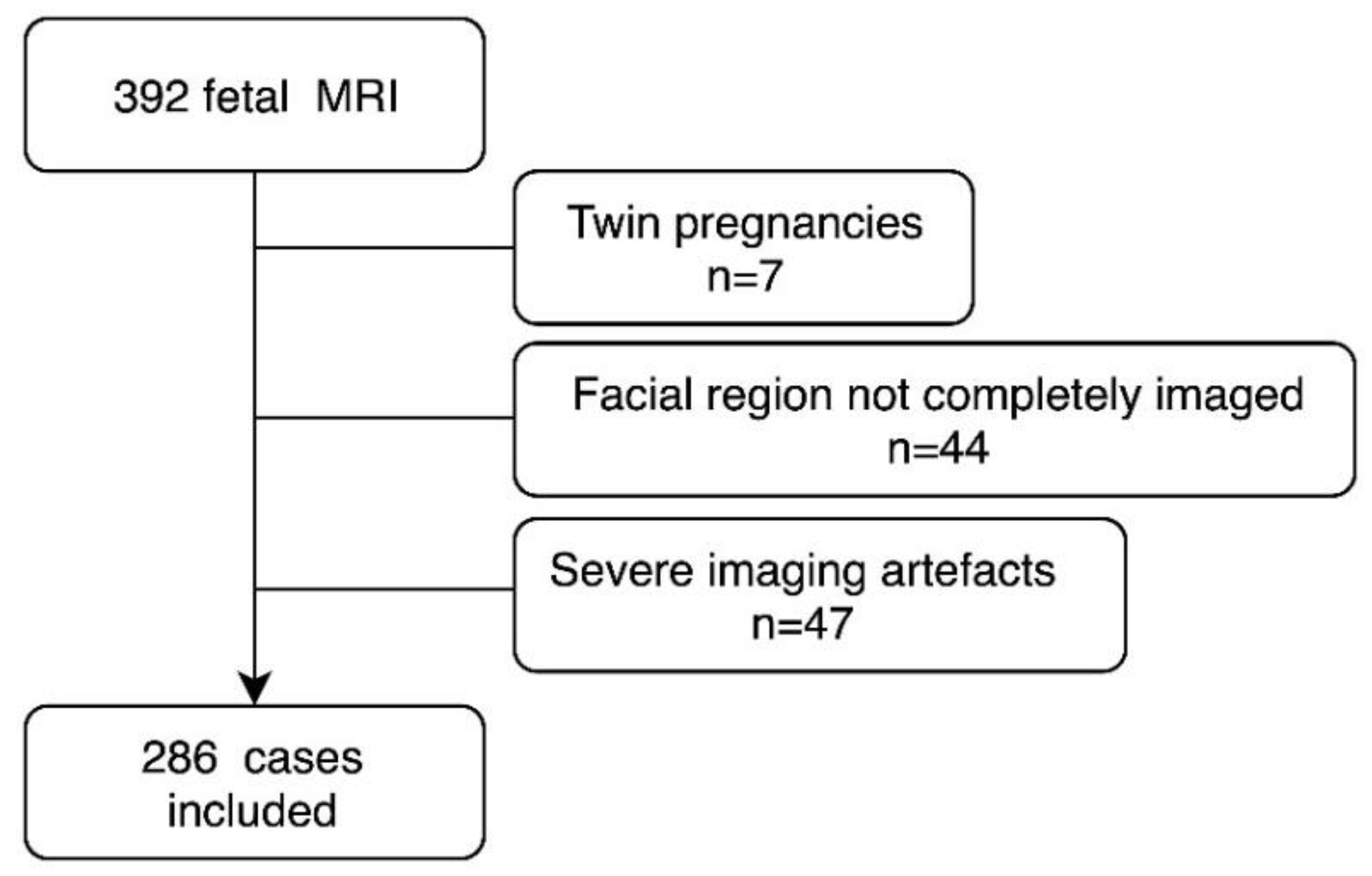
2. Materials and Methods
2.1. Patients and Setting
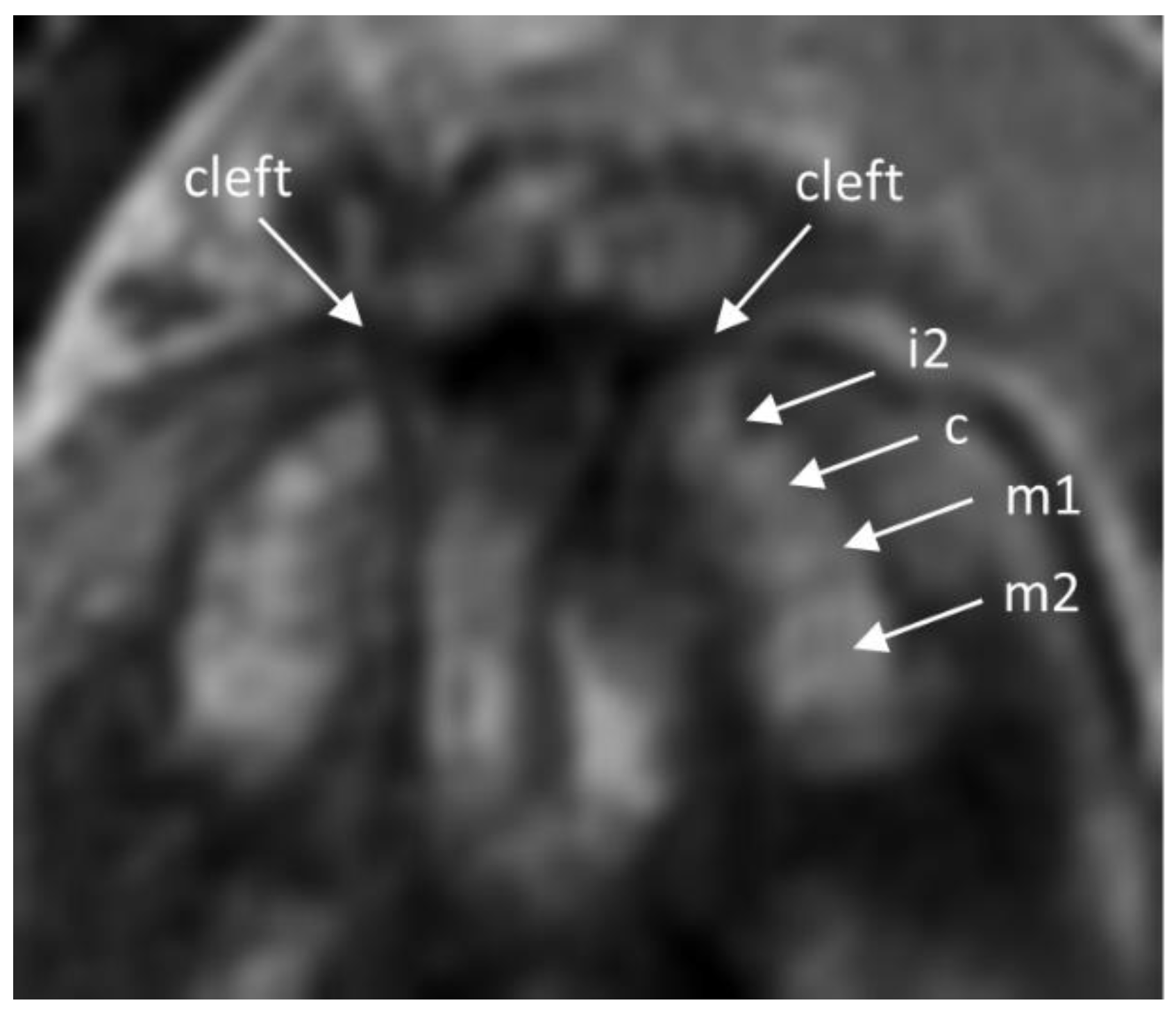
2.2. Imaging Analysis
2.3. Statistic Analysis
3. Results
4. Discussion
4.1. Principal Findings
4.2. Results
4.3. Clinical Implications
4.4. Strength and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Radlanski, R.J. Orale Struktur-und Entwicklungsbiologie: Quintessenz Verlags-Gmgh; Qunitessenz Verlags Gmbh: Berlin, Germany, 2011; 606p. [Google Scholar]
- Mukhopadhyay, S.; Mitra, S. Anomalies in primary dentition: Their distribution and correlation with permanent dentition. J. Nat. Sci. Boil. Med. 2014, 5, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Jarvinen, S.; Lehtinen, L. Supernumerary and congenitally missing primary teeth in Finnish children. An epidemiologic study. Acta Odontol. Scand. 1981, 39, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, K.; Miskelly, J.; Voge, E.; Macfarlane, T.V. Prevalence of hypodontia and associated factors: A systematic review and meta-analysis. J. Orthod. 2014, 41, 299–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulm, M.R.; Kratochwil, A.; Ulm, B.; Solar, P.; Aro, G.; Bernaschek, G. Three-dimensional ultrasound evaluation of fetal tooth germs. Ultrasound Obstet. Gynecol. 1998, 12, 240–243. [Google Scholar] [CrossRef]
- Klein, O.D.; Oberoi, S.; Huysseune, A.; Hovorakova, M.; Peterka, M.; Peterkova, R. Developmental disorders of the dentition: An update. Am. J. Med. Genet. Part C Semin. Med. Genet. 2013, 163, 318–332. [Google Scholar] [CrossRef] [Green Version]
- Haque, S.; Alam, M.K. Common dental anomalies in cleft lip and palate patients. Malays. J. Med. Sci. MJMS 2015, 22, 55–60. [Google Scholar]
- Palaska, P.K.; Antonarakis, G.S. Prevalence and patterns of permanent tooth agenesis in individuals with Down syndrome: A meta-analysis. Eur. J. Oral Sci. 2016, 124, 317–328. [Google Scholar] [CrossRef]
- Prokocimer, T.; Amir, E.; Blumer, S.; Peretz, B. Birth-Weight, Pregnancy Term, Pre-Natal and Natal Complications Related to Child’s Dental Anomalies. J. Clin. Pediatr. Dent. 2015, 39, 371–376. [Google Scholar] [CrossRef]
- Khetarpal, S.; Kempf, E.; Mostow, E. Congenital syphilis: Early- and late-stage findings of rhagades and dental anomalies. Pediatr. Dermatol. 2011, 28, 401–403. [Google Scholar] [CrossRef]
- Needleman, H.L.; Allred, E.; Bellinger, D.; Leviton, A.; Rabinowitz, M.; Iverson, K. Antecedents and correlates of hypoplastic enamel defects of primary incisors. Pediatr. Dent. 1992, 14, 158–166. [Google Scholar]
- Prayer, D.; Brugger, P.C. Investigation of normal organ development with fetal MRI. Eur. Radiol. 2007, 17, 2458–2471. [Google Scholar] [CrossRef]
- Jarvis, D.; Mooney, C.; Cohen, J.; Papaioannou, D.; Bradburn, M.; Sutton, A.; Griffiths, P.D. A systematic review and meta-analysis to determine the contribution of mr imaging to the diagnosis of foetal brain abnormalities in Utero. Eur. Radiol. 2017, 27, 2367–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, N.; Windrim, R.; Chong, K.; Viero, S.; Thompson, M.; Blaser, S. Prenatal diagnosis of solitary median maxillary central incisor syndrome by magnetic resonance imaging. Ultrasound Obstet. Gynecol. 2008, 32, 120–122. [Google Scholar] [CrossRef]
- Wünsche, S.; Jüngert, J.; Faschingbauer, F.; Mommsen, H.; Goecke, T.; Schwanitz, K.; Stepan, H.; Schneider, H. Noninvasive Prenatal Diagnosis of Hypohidrotic Ectodermal Dysplasia by Tooth Germ Sonography. Ultraschall Med. (Stuttg. Ger. 1980) 2015, 36, 381–385. [Google Scholar] [CrossRef]
- Seabra, M.; Felino, A.; Nogueira, R.; Valente, F.; Braga, A.C.; Vaz, P. Prenatal ultrasound and postmortem histologic evaluation of tooth germs: An observational, transversal study. Head Face Med. 2015, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Davidovich, E.; Kooby, E.; Shapira, J.; Ram, D. The traditional practice of canine bud removal in the offspring of Ethiopian immigrants. BMC Oral Health 2013, 13, 34. [Google Scholar] [CrossRef] [Green Version]
- Seabra, M.; Vaz, P.; Valente, F.; Braga, A.; Felino, A. Two-Dimensional Identification of Fetal Tooth Germs. Cleft Palate Craniofac. J. 2017, 54, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Couly, G.; Nicot, R.; Kverneland, B.; Ferri, J.; Levaillant, J.M. Fetal dental panorama on three-dimensional ultrasound imaging. Ultrasound Obstet. Gynecol. 2016, 48, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Brugger, P.C.; Weber, M.; Prayer, D. Magnetic resonance imaging of the fetal efferent lacrimal pathways. Eur. Radiol. 2010, 20, 1965–1973. [Google Scholar] [CrossRef]
- Mailath-Pokorny, M.; Worda, C.; Krampl-Bettelheim, E.; Watzinger, F.; Brugger, P.C.; Prayer, D. What does magnetic resonance imaging add to the prenatal ultrasound diagnosis of facial clefts? Ultrasound Obstet. Gynecol. 2010, 36, 445–451. [Google Scholar] [CrossRef]
- Mailath-Pokorny, M.; Klein, K.; Worda, C.; Weber, M.; Brugger, P.C.; Czerny, C.; Nemec, U.; Prayer, D. Maxillary dental arch biometry: Assessment with fetal MR imaging. Prenat. Diagn. 2012, 32, 530–535. [Google Scholar] [CrossRef]
- Victoria, T.; Johnson, A.M.; Edgar, J.C.; Zarnow, D.M.; Vossough, A.; Jaramillo, D. Comparison Between 1.5-T and 3-T MRI for Fetal Imaging: Is There an Advantage to Imaging with a Higher Field Strength? AJR Am. J. Roentgenol. 2016, 206, 195–201. [Google Scholar] [CrossRef]
- Ranta, R. A review of tooth formation in children with cleft lip/palate. Am. J. Orthod. Dentofac. Orthop. 1986, 90, 11–18. [Google Scholar] [CrossRef]
- Nik-Hussein, N.N.; Majid, Z.A. Dental anomalies in the primary dentition: Distribution and correlation with the permanent dentition. J. Clin. Pediatr. Dent. 1996, 21, 15–19. [Google Scholar]
- Aka, P.S.; Canturk, N.; Dagalp, R.; Yagan, M. Age determination from central incisors of fetuses and infants. Forensic Sci. Int. 2009, 184, 15–20. [Google Scholar] [CrossRef]
- Ozcan, U.A.; Yildiz, M.E.; Ulus, S.; Turk, A.; Erzen, C.; Canter, H.I. Magnetic resonance imaging evaluation of fetal maxillary sinuses. J. Craniofacial Surg. 2014, 25, 363–366. [Google Scholar] [CrossRef]




| GW Group | 18–21 | 22–25 | 26–29 | 30–33 | 34+ | Total (n) | Total (%) |
|---|---|---|---|---|---|---|---|
| 1.5 Tesla | |||||||
| n= | 23 | 51 | 33 | 39 | 24 | 170 | 100% |
| no abnormalities | 1 | 9 | 0 | 11 | 10 | 31 | 18% |
| brain abnormalities | 7 | 16 | 16 | 17 | 7 | 63 | 37% |
| thoracic abnormalities | 7 | 10 | 7 | 6 | 2 | 32 | 19% |
| abdominal abnormalities | 7 | 12 | 8 | 3 | 3 | 33 | 19% |
| extra-fetal and limp abnormalities | 1 | 4 | 2 | 2 | 2 | 11 | 7% |
| 3 Tesla | |||||||
| n= | 13 | 50 | 20 | 23 | 10 | 116 | 100% |
| no abnormalities | 1 | 6 | 1 | 5 | 1 | 14 | 12% |
| brain abnormalities | 7 | 21 | 10 | 12 | 4 | 54 | 46% |
| thoracic abnormalities | 5 | 13 | 3 | 2 | 1 | 24 | 21% |
| abdominal abnormalities | 0 | 7 | 4 | 2 | 2 | 15 | 13% |
| extra-fetal and limb abnormalities | 0 | 3 | 2 | 2 | 2 | 9 | 8% |
| GW | All Tooth Buds Visible n/Total (%) | 1.5T | 3T | Cat. 1 (%) | Cat. 2 (%) | Cat. 3 (%) | Cat. 4 (%) | Cat. 5 (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||||
| GW 18–21 | 2/37 (5.4) | 4.6 | 2.9 | 6.0 | 1.9 | 7.2 | 45.0 | 37.8 | 9.0 | 0.9 |
| GW 22–25 | 37/103 (35.9) | 7.6 | 2.4 | 7.7 | 2.1 | 0.3 | 17.6 | 41.8 | 14.4 | 25.8 |
| GW 26–29 | 40/53 (75.5) | 8.8 | 1.8 | 9.0 | 1.8 | 0.0 | 7.5 | 18.9 | 16.4 | 57.2 |
| GW 30–33 | 47/61 (77) | 9.2 | 1.1 | 9.0 | 1.6 | 0.0 | 1.6 | 25.7 | 14.8 | 57.9 |
| GW 34–38 | 29/32 (90.6) | 9.7 | 0.7 | 9.7 | 0.7 | 0.0 | 0.0 | 8.3 | 11.5 | 80.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunzendorf, B.; Diogo, M.C.; Covini, D.I.; Weber, M.; Gruber, G.M.; Zeilhofer, H.-F.; Berg, B.-I.; Prayer, D. Comparison of the Visibility of Fetal Tooth Buds on 1.5 and 3 Tesla MRI. J. Clin. Med. 2020, 9, 3424. https://doi.org/10.3390/jcm9113424
Kunzendorf B, Diogo MC, Covini DI, Weber M, Gruber GM, Zeilhofer H-F, Berg B-I, Prayer D. Comparison of the Visibility of Fetal Tooth Buds on 1.5 and 3 Tesla MRI. Journal of Clinical Medicine. 2020; 9(11):3424. https://doi.org/10.3390/jcm9113424
Chicago/Turabian StyleKunzendorf, Burkhard, Mariana C. Diogo, Delfina I. Covini, Michael Weber, Gerlinde M. Gruber, Hans-Florian Zeilhofer, Britt-Isabelle Berg, and Daniela Prayer. 2020. "Comparison of the Visibility of Fetal Tooth Buds on 1.5 and 3 Tesla MRI" Journal of Clinical Medicine 9, no. 11: 3424. https://doi.org/10.3390/jcm9113424
APA StyleKunzendorf, B., Diogo, M. C., Covini, D. I., Weber, M., Gruber, G. M., Zeilhofer, H.-F., Berg, B.-I., & Prayer, D. (2020). Comparison of the Visibility of Fetal Tooth Buds on 1.5 and 3 Tesla MRI. Journal of Clinical Medicine, 9(11), 3424. https://doi.org/10.3390/jcm9113424





