Cytokines and Pathogenesis of Central Retinal Vein Occlusion
Abstract
:1. Introduction
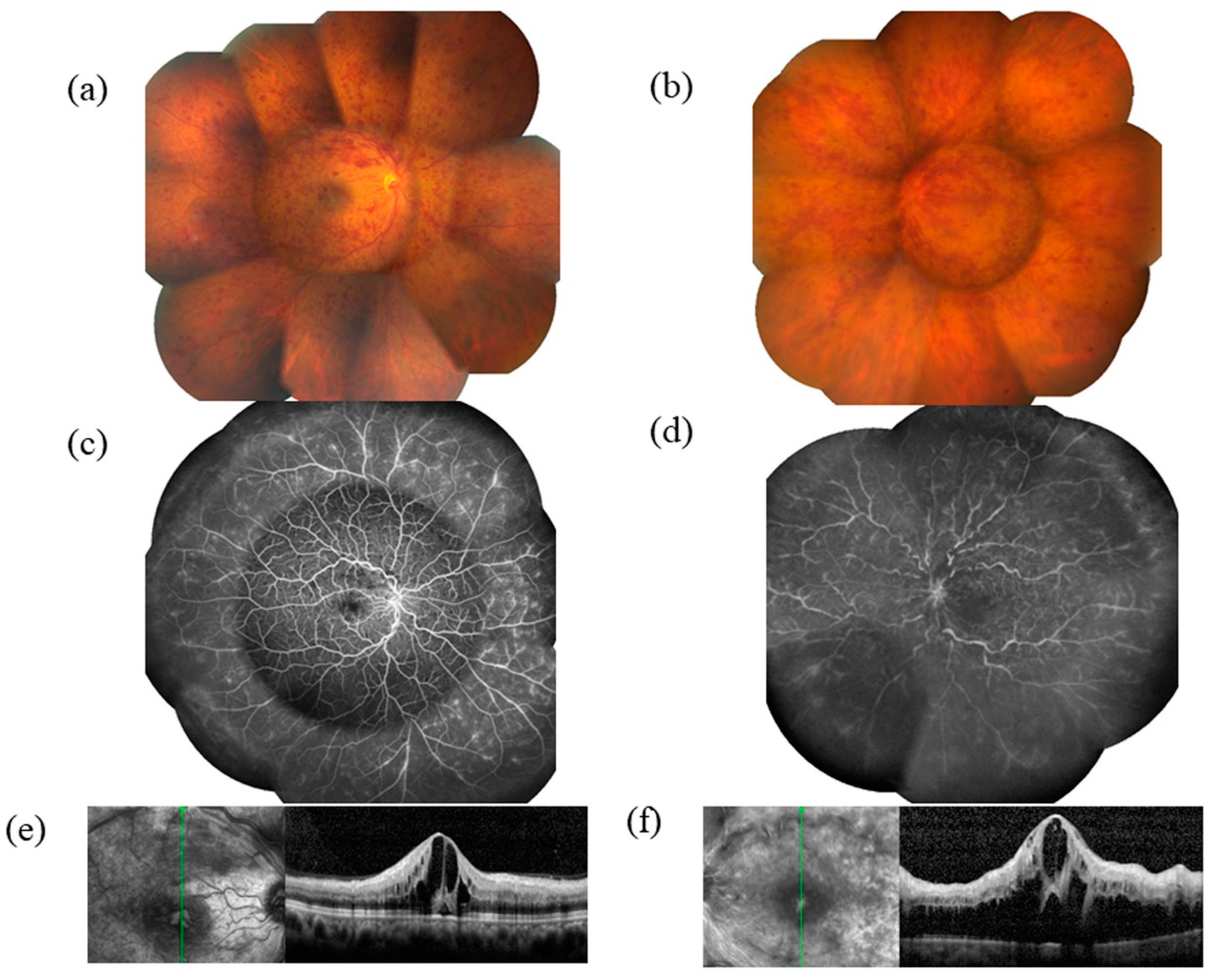
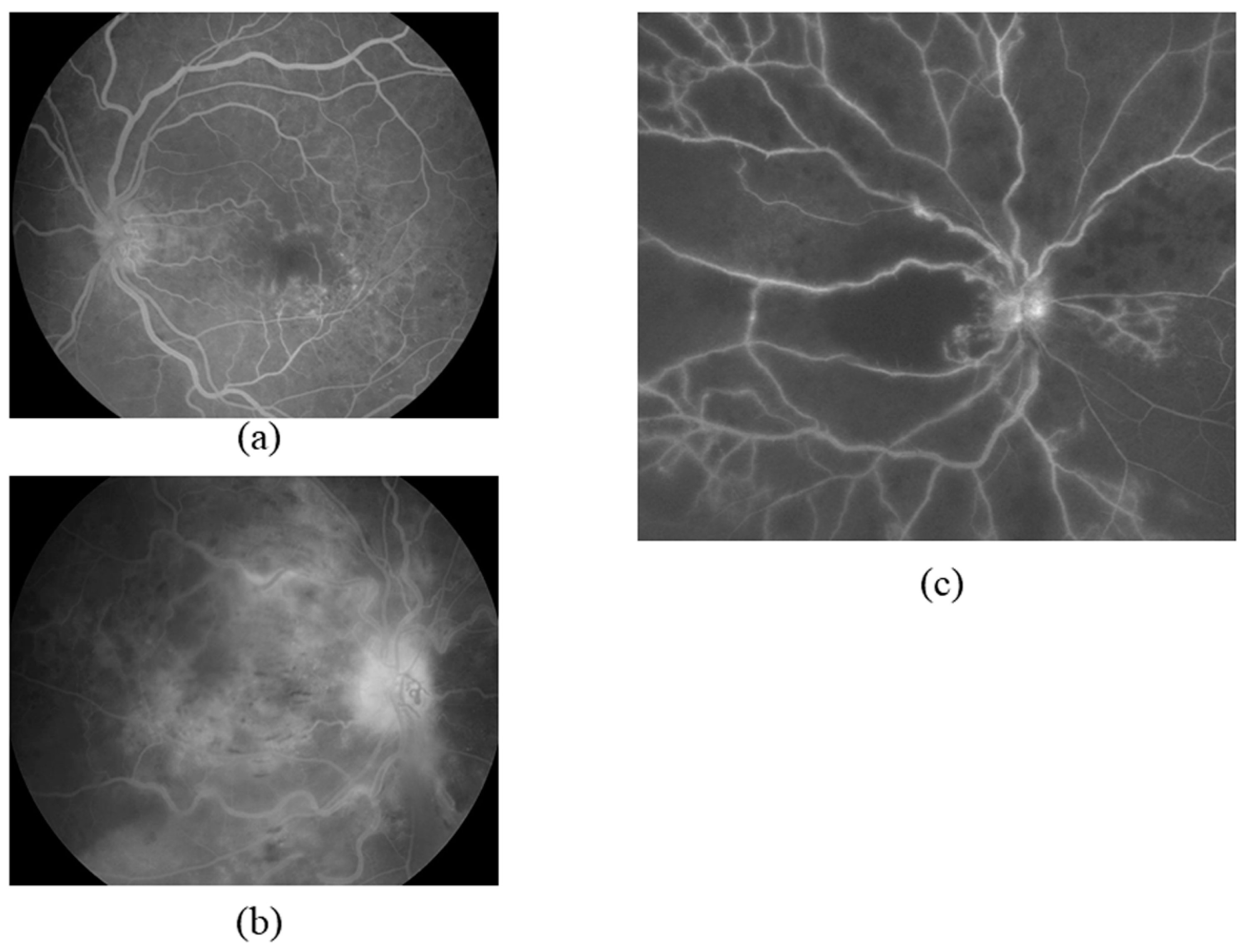
2. Pathogenesis of CRVO
3. VEGF and Macular Edema
4. Inflammation and Other Putative Mechanisms of Macular Edema in CRVO
4.1. Cytokines
4.1.1. Interleukin 6
4.1.2. Interleukin 8
4.2. Growth Factors
4.2.1. Placental Growth Factor
4.2.2. Platelet-Derived Growth Factor
4.3. Monocyte Chemoattractant Protein
4.4. Intercellular Adhesion Molecule 1
4.5. Interferon-Inducible 10-kDa Protein
4.6. Pentraxin 3
4.7. Erythropoietin
4.8. Other Putative Mechanisms
5. VEGF Receptors and Macular Edema
6. Inflammation and Retinal Blood Flow Velocity
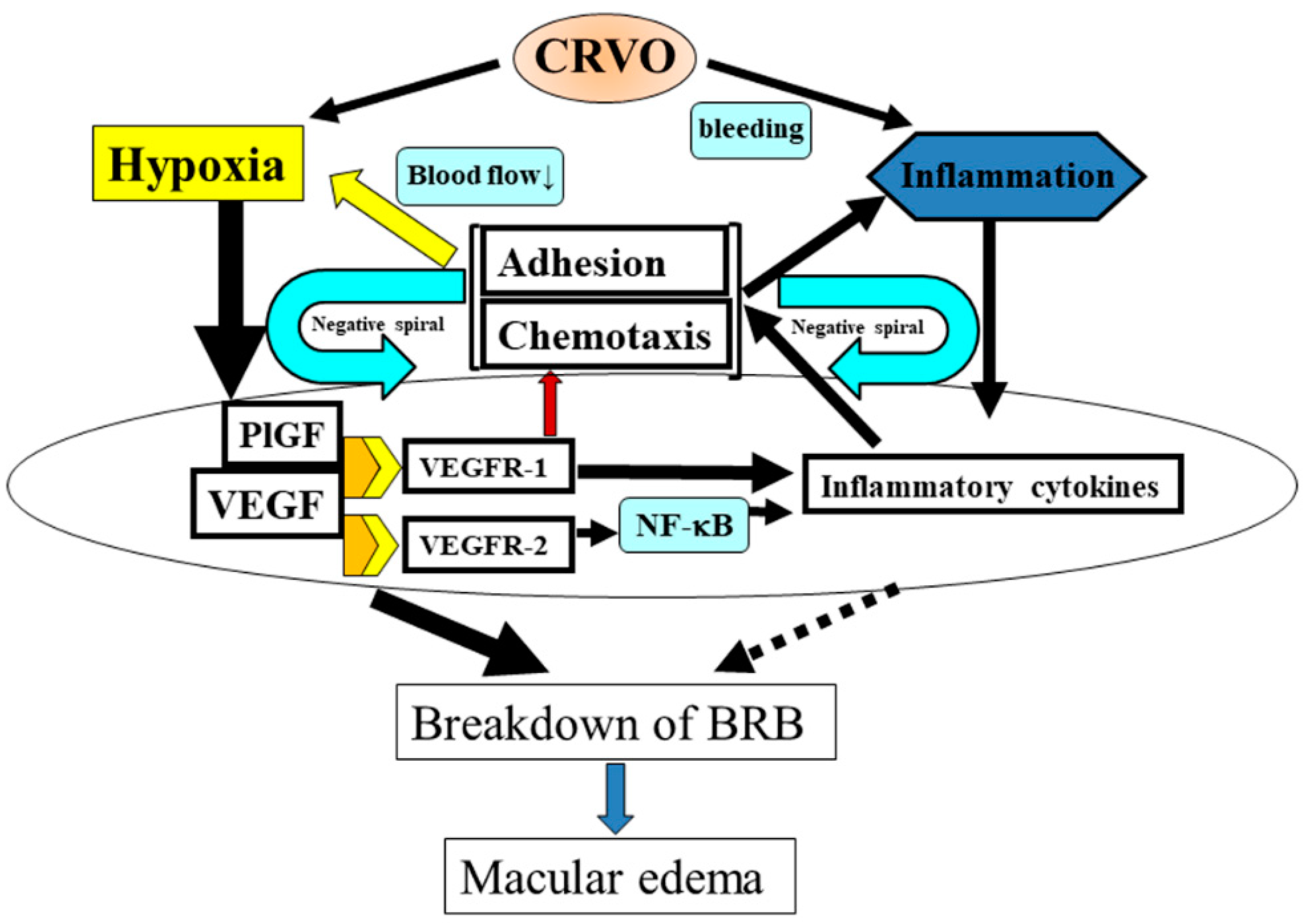
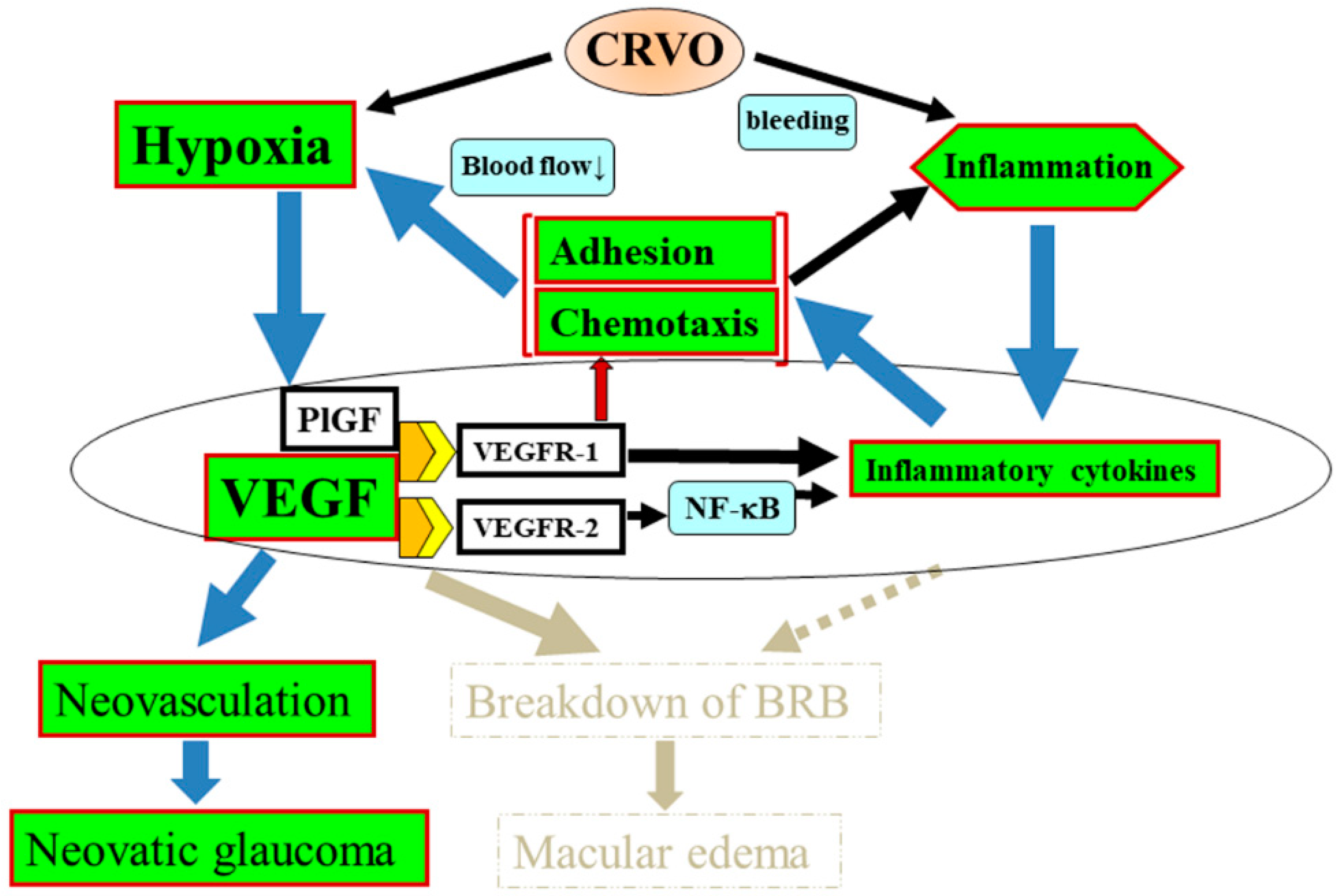
7. Proposed Mechanism of CRVO Pathogenesis
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mohamed, Q.; McIntosh, R.L.; Saw, S.M.; Wong, T.Y. Interventions for central retinal vein occlusion: An evidence-based systematic review. Ophthalmology 2007, 114, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Campochiaro, P.A.; Singh, R.P.; Li, Z.; Gray, S.; Saroj, N.; Rundle, A.C.; Rubio, R.G.; Murahashi, W.Y. Ranibizumab for macular edema following central retinal vein occlusion: Six-month primary end point results of a phase III study. Ophthalmology 2010, 117, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Yasuda, K.; Shimura, M. Cytokines and the Pathogenesis of Macular Edema in Branch Retinal Vein Occlusion. J. Ophthalmol. 2019, 2, 5185128. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S.; Zimmerman, M.B.; Podhajsky, P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am. J. Ophthalmol. 1994, 117, 429–441. [Google Scholar] [CrossRef]
- Hayreh, S.S. So-called “central retinal vein occlusion”. I. Pathogenesis, terminology, clinical features. Int. J. Ophthalmol. 1976, 172, 1. [Google Scholar]
- Walters, R.F.; Spalton, D.J. Central retinal vein occlusion in people aged 40 years or less: A review of 17 patients. Br. J. Ophthalmol. 1990, 74, 30–35. [Google Scholar] [CrossRef]
- Fong, A.C.; Schatz, H. Central retinal vein occlusion in young adults. Surv. Ophthalmol. 1993, 37, 393–417. [Google Scholar] [CrossRef]
- Moisseiev, E.; Sagiv, O.; Lazar, M. Intense exercise causing central retinal vein occlusion in a young patient: Case report and review of the literature. Case Rep. Ophthalmol. 2014, 5, 116–120. [Google Scholar] [CrossRef]
- Tsujikawa, A.; Sakamoto, A.; Ota, M.; Kotera, Y.; Oh, H.; Miyamoto, K.; Kita, M.; Yoshimura, N. Serous retinal detachment associated with retinal vein occlusion. Am. J. Ophthalmol. 2010, 149, 291–301. [Google Scholar] [CrossRef]
- Cunha-Vaz, J. Mechanisms of Retinal Fluid Accumulation and Blood-Retinal Barrier Breakdown. Dev. Ophthalmol. 2017, 58, 11–20. [Google Scholar]
- Silva, R.M.; de Abreu, J.F.; Cunha-Vaz, J.G. Blood-retina barrier in acute retinal branch vein occlusion. Graefes Arch. Clin. Exp. Ophthalmol. 1995, 233, 721–726. [Google Scholar] [CrossRef]
- Saika, S.; Tanaka, T.; Miyamoto, T.; Ohnishi, Y. Surgical posterior vitreous detachment combined with gas/air tamponade for treating macular edema associated with branch retinal vein occlusion: Retinal tomography and visual outcome. Graefes Arch. Clin. Exp. Ophthalmol. 2001, 239, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Kida, T. Mystery of Retinal Vein Occlusion: Vasoactivity of the Vein and Possible Involvement of Endothelin-1. BioMed Res. Int. 2017, 2017, 4816527. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Northrup, J.M.; Keyt, B.A.; Takagi, H.; Iwamoto, M.A. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch. Ophthalmol. 1995, 113, 1538–1544. [Google Scholar] [CrossRef]
- Dvorak, H.F.; Brown, L.F.; Detmar, M.; Dvorak, A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995, 146, 1029–1039. [Google Scholar]
- Antonetti, D.A.; Barber, A.J.; Hollinger, L.A.; Wolpert, E.B.; Gardner, T.W. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J. Biol. Chem. 1999, 274, 23463–23467. [Google Scholar] [CrossRef] [Green Version]
- Noma, H.; Funatsu, H.; Mimura, T.; Harino, S.; Hori, S. Vitreous levels of interleukin-6 and vascular endothelial growth factor in macular edema with central retinal vein occlusion. Ophthalmology 2009, 116, 87–93. [Google Scholar] [CrossRef]
- Pe’er, J.; Folberg, R.; Itin, A.; Gnessin, H.; Hemo, I.; Keshet, E. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology 1998, 105, 412–416. [Google Scholar] [CrossRef]
- Kriechbaum, K.; Michels, S.; Prager, F.; Georgopoulos, M.; Funk, M.; Geitzenauer, W.; Schmidt-Erfurth, U. Intravitreal Avastin for macular oedema secondary to retinal vein occlusion: A prospective study. Br. J. Ophthalmol. 2008, 92, 518–522. [Google Scholar] [CrossRef] [Green Version]
- Ach, T.; Hoeh, A.E.; Schaal, K.B.; Scheuerle, A.F.; Dithmar, S. Predictive factors for changes in macular edema in intravitreal bevacizumab therapy of retinal vein occlusion. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Ip, M.S.; Scott, I.U.; VanVeldhuisen, P.C.; Oden, N.L.; Blodi, B.A.; Fisher, M.; Singerman, L.J.; Tolentino, M.; Chan, C.K.; Gonzalez, V.H. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch. Ophthalmol. 2009, 127, 1101–1114. [Google Scholar] [PubMed] [Green Version]
- Funk, M.; Kriechbaum, K.; Prager, F.; Benesch, T.; Georgopoulos, M.; Zlabinger, G.J.; Schmidt-Erfurth, U. Intraocular concentrations of growth factors and cytokines in retinal vein occlusion and the effect of therapy with bevacizumab. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1025–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, T.; Sonoda, K.H.; Sugahara, M.; Mochizuki, Y.; Enaida, H.; Oshima, Y.; Ueno, A.; Hata, Y.; Yoshida, H.; Ishibashi, T. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS ONE 2009, 4, e8158. [Google Scholar] [CrossRef]
- Noma, H.; Funatsu, H.; Harino, S.; Mimura, T.; Eguchi, S.; Hori, S. Vitreous inflammatory factors in macular edema with central retinal vein occlusion. Jpn. J. Ophthalmol. 2011, 55, 248–255. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nakazawa, M.; Suzuki, K.; Yamazaki, H.; Miyagawa, Y. Expression profiles of cytokines and chemokines in vitreous fluid in diabetic retinopathy and central retinal vein occlusion. Jpn. J. Ophthalmol. 2011, 55, 256–263. [Google Scholar] [CrossRef]
- Noma, H.; Mimura, T.; Masahara, H.; Shimada, K. Pentraxin 3 and other inflammatory factors in central retinal vein occlusion and macular edema. Retina 2014, 34, 352–359. [Google Scholar] [CrossRef]
- Maruo, N.; Morita, I.; Shirao, M.; Murota, S. IL-6 increases endothelial permeability in vitro. Endocrinology 1992, 131, 710–714. [Google Scholar]
- Echevarria, F.D.; Rickman, A.E.; Sappington, R.M. Interleukin-6: A Constitutive Modulator of Glycoprotein 130, Neuroinflammatory and Cell Survival Signaling in Retina. J. Clin. Cell Immunol. 2016, 7, 439. [Google Scholar] [CrossRef] [Green Version]
- Echevarria, F.D.; Formichella, C.R.; Sappington, R.M. Interleukin-6 Deficiency Attenuates Retinal Ganglion Cell Axonopathy and Glaucoma-Related Vision Loss. Front. Neurosci. 2017, 11, 318. [Google Scholar] [CrossRef] [Green Version]
- Rojas, M.; Zhang, W.; Lee, D.L.; Romero, M.J.; Nguyen, D.T.; Al-Shabrawey, M.; Tsai, N.T.; Liou, G.I.; Brands, M.W.; Caldwell, R.W.; et al. Role of IL-6 in angiotensin II-induced retinal vascular inflammation. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.F.; Tritto, I.; Pinsky, D.; Liao, H.; Huang, J.; Fuller, G.; Brett, J.; May, L.; Stern, D. Induction of interleukin 6 (IL-6) by hypoxia in vascular cells. Central role of the binding site for nuclear factor-IL-6. J. Biol. Chem. 1995, 270, 11463–11471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.H.; Schlidt, S.A.; Chandel, N.S.; Hynes, K.L.; Schumacker, P.T.; Gewertz, B.L. Endothelial permeability and IL-6 production during hypoxia: Role of ROS in signal transduction. Am. J. Physiol. 1999, 277, L1057–L1065. [Google Scholar] [CrossRef]
- Pearlstein, D.P.; Ali, M.H.; Mungai, P.T.; Hynes, K.L.; Gewertz, B.L.; Schumacker, P.T. Role of mitochondrial oxidant generation in endothelial cell responses to hypoxia. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 566–573. [Google Scholar] [CrossRef]
- Karakurum, M.; Shreeniwas, R.; Chen, J.; Pinsky, D.; Yan, S.D.; Anderson, M.; Sunouchi, K.; Major, J.; Hamilton, T.; Kuwabara, K.; et al. Hypoxic induction of interleukin-8 gene expression in human endothelial cells. J. Clin. Investig. 1994, 93, 1564–1570. [Google Scholar] [CrossRef] [Green Version]
- Shono, T.; Ono, M.; Izumi, H.; Jimi, S.I.; Matsushima, K.; Okamoto, T.; Kohno, K.; Kuwano, M. Involvement of the transcription factor NF-kappaB in tubular morphogenesis of human microvascular endothelial cells by oxidative stress. Mol. Cell. Biol. 1996, 16, 4231–4239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taub, D.D.; Anver, M.; Oppenheim, J.J.; Longo, D.L.; Murphy, W.J. T lymphocyte recruitment by interleukin-8 (IL-8). IL-8-induced degranulation of neutrophils releases potent chemoattractants for human T lymphocytes both in vitro and in vivo. J. Clin. Investig. 1996, 97, 1931–1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuchtey, J.; Rezaei, K.A.; Jaru-Ampornpan, P.; Sternberg, P., Jr.; Kuchtey, R.W. Multiplex cytokine analysis reveals elevated concentration of interleukin-8 in glaucomatous aqueous humor. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6441–6447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, S.; Adachi, K.; Suzuki, Y.; Maeno, A.; Nakazawa, M. Profiles of Inflammatory Cytokines in the Vitreous Fluid from Patients with Rhegmatogenous Retinal Detachment and Their Correlations with Clinical Features. BioMed Res. Int. 2016, 2016, 4256183. [Google Scholar] [CrossRef]
- Moyer, K.E.; Saggers, G.C.; Allison, G.M.; Mackay, D.R.; Ehrlich, H.P. Effects of interleukin-8 on granulation tissue maturation. J. Cell. Physiol. 2002, 193, 173–179. [Google Scholar] [CrossRef]
- Yu, H.; Huang, X.; Ma, Y.; Gao, M.; Wang, O.; Gao, T.; Shen, Y.; Liu, X. Interleukin-8 regulates endothelial permeability by down-regulation of tight junction but not dependent on integrins induced focal adhesions. Int. J. Biol. Sci. 2013, 9, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Detmers, P.A.; Lo, S.K.; Olsen-Egbert, E.; Walz, A.; Baggiolini, M.; Cohn, Z.A. Neutrophil-activating protein 1/interleukin 8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. J. Exp. Med. 1990, 171, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Paccaud, J.P.; Schifferli, J.A.; Baggiolini, M. NAP-1/IL-8 induces up-regulation of CR1 receptors in human neutrophil leukocytes. Biochem. Biophys. Res. Commun. 1990, 166, 187–192. [Google Scholar] [CrossRef]
- Maglione, D.; Guerriero, V.; Viglietto, G.; Delli-Bovi, P.; Persico, M.G. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc. Natl. Acad. Sci. USA 1991, 88, 9267–9271. [Google Scholar] [CrossRef] [Green Version]
- De Falco, S.; Gigante, B.; Persico, M.G. Structure and function of placental growth factor. Trends. Cardiovasc. Med. 2002, 12, 241–246. [Google Scholar] [CrossRef]
- Noma, H.; Yasuda, K.; Mimura, T.; Ofusa, A.; Shimura, M. Relationship between retinal blood flow and cytokines in central retinal vein occlusion. BMC Ophthalmol. 2020, 20, 215. [Google Scholar] [CrossRef]
- DiSalvo, J.; Bayne, M.L.; Conn, G.; Kwok, P.W.; Trivedi, P.G.; Soderman, D.D.; Palisi, T.M.; Sullivan, K.A.; Thomas, K.A. Purification and characterization of a naturally occurring vascular endothelial growth factor.placenta growth factor heterodimer. J. Biol. Chem. 1995, 270, 7717–7723. [Google Scholar] [CrossRef] [Green Version]
- Park, J.E.; Chen, H.H.; Winer, J.; Houck, K.A.; Ferrara, N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J. Biol. Chem. 1994, 269, 25646–25654. [Google Scholar]
- Olofsson, B.; Korpelainen, E.; Pepper, M.S.; Mandriota, S.J.; Aase, K.; Kumar, V.; Gunji, Y.; Jeltsch, M.M.; Shibuya, M.; Alitalo, K.; et al. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc. Natl. Acad. Sci. USA 1998, 95, 11709–11714. [Google Scholar] [CrossRef] [Green Version]
- Clauss, M.; Weich, H.; Breier, G.; Knies, U.; Rockl, W.; Waltenberger, J.; Risau, W. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J. Biol. Chem. 1996, 271, 17629–17634. [Google Scholar] [CrossRef] [Green Version]
- Noma, H.; Mimura, T.; Yasuda, K.; Shimura, M. Role of soluble vascular endothelial growth factor receptor signaling and other factors or cytokines in central retinal vein occlusion with macular edema. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1122–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [Green Version]
- Edqvist, P.H.; Niklasson, M.; Vidal-Sanz, M.; Hallböök, F.; Forsberg-Nilsson, K. Platelet-derived growth factor over-expression in retinal progenitors results in abnormal retinal vessel formation. PLoS ONE 2012, 7, e42488. [Google Scholar] [CrossRef] [PubMed]
- Kraiss, L.W.; Geary, R.L.; Mattsson, E.J.; Vergel, S.; Au, Y.P.; Clowes, A.W. Acute reductions in blood flow and shear stress induce platelet-derived growth factor-A expression in baboon prosthetic grafts. Circ. Res. 1996, 79, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Ao, P.; Boynton, A.L. Rapid disruption of gap junctional communication and phosphorylation of connexin43 by platelet-derived growth factor in T51B rat liver epithelial cells expressing platelet-derived growth factor receptor. J. Cell. Physiol. 1998, 174, 66–77. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Keep, R.F.; Kunkel, S.L.; Andjelkovic, A.V. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: Signaling via Rho and Rho kinase. J. Cell Sci. 2003, 116, 4615–4628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.R.; Liu, M.T.; Lei, H.Y.; Liu, C.C.; Wu, J.M.; Tung, Y.C.; Lin, Y.S.; Yeh, T.M.; Chen, S.H.; Liu, H.S. MCP-1, a highly expressed chemokine in dengue haemorrhagic fever/dengue shock syndrome patients, may cause permeability change, possibly through reduced tight junctions of vascular endothelium cells. J. Gen. Virol. 2006, 87, 3623–3630. [Google Scholar] [CrossRef]
- Koss, M.; Pfister, M.; Rothweiler, F.; Rejdak, R.; Ribeiro, R.; Cinatl, J.; Schubert, R.; Kohnen, T.; Koch, F. Correlation from undiluted vitreous cytokines of untreated central retinal vein occlusion with spectral domain optical coherence tomography. Open Ophthalmol. J. 2013, 7, 11–17. [Google Scholar] [CrossRef]
- Chen, Y.L.; Chang, Y.J.; Jiang, M.J. Monocyte chemotactic protein-1 gene and protein expression in atherogenesis of hypercholesterolemic rabbits. Atherosclerosis 1999, 143, 115–123. [Google Scholar] [CrossRef]
- Chen, P.; Shibata, M.; Zidovetzki, R.; Fisher, M.; Zlokovic, B.V.; Hofman, F.M. Endothelin-1 and monocyte chemoattractant protein-1 modulation in ischemia and human brain-derived endothelial cell cultures. J. Neuroimmunol. 2001, 116, 62–73. [Google Scholar] [CrossRef]
- Lee, P.C.; Ho, I.C.; Lee, T.C. Oxidative stress mediates sodium arsenite-induced expression of heme oxygenase-1, monocyte chemoattractant protein-1, and interleukin-6 in vascular smooth muscle cells. Toxicol. Sci. 2005, 85, 541–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elner, S.G.; Elner, V.M.; Pavilack, M.A.; Todd, R.F., 3rd; Mayo-Bond, L.; Franklin, W.A.; Strieter, R.M.; Kunkel, S.L.; Huber, A.R. Modulation and function of intercellular adhesion molecule-1 (CD54) on human retinal pigment epithelial cells. Lab. Investig. 1992, 66, 200–211. [Google Scholar] [PubMed]
- Nishiwaki, A.; Ueda, T.; Ugawa, S.; Shimada, S.; Ogura, Y. Upregulation of P-selectin and intercellular adhesion molecule-1 after retinal ischemia-reperfusion injury. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4931–4935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirose, F.; Kiryu, J.; Miyamoto, K.; Nishijima, K.; Miyahara, S.; Katsuta, H.; Tamura, H.; Honda, Y. In vivo evaluation of retinal injury after transient ischemia in hypertensive rats. Hypertension 2004, 43, 1098–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, K.; Khosrof, S.; Bursell, S.E.; Rohan, R.; Murata, T.; Clermont, A.C.; Aiello, L.P.; Ogura, Y.; Adamis, A.P. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc. Natl. Acad. Sci. USA 1999, 96, 10836–10841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsujikawa, A.; Ogura, Y.; Hiroshiba, N.; Miyamoto, K.; Kiryu, J.; Honda, Y. In vivo evaluation of leukocyte dynamics in retinal ischemia reperfusion injury. Investig. Ophthalmol. Vis. Sci. 1998, 39, 793–800. [Google Scholar]
- Feldman, E.D.; Weinreich, D.M.; Carroll, N.M.; Burness, M.L.; Feldman, A.L.; Turner, E.; Xu, H.; Alexander, H.R., Jr. Interferon gamma-inducible protein 10 selectively inhibits proliferation and induces apoptosis in endothelial cells. Ann. Surg. Oncol. 2006, 13, 125–133. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Yates, C.C.; Wells, A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ. Res. 2006, 98, 617–625. [Google Scholar] [CrossRef]
- Woo, J.M.; Kwon, M.Y.; Shin, D.Y.; Kang, Y.H.; Hwang, N.; Chung, S.W. Human retinal pigment epithelial cells express the long pentraxin PTX3. Mol. Vis. 2013, 19, 303–310. [Google Scholar]
- Park, K.S.; Kim, J.W.; An, J.H.; Woo, J.M. Elevated plasma pentraxin 3 and its association with retinal vein occlusion. Korean J. Ophthalmol. 2014, 28, 460–465. [Google Scholar] [CrossRef] [Green Version]
- Dodson, P.M.; Shine, B. Retinal vein occlusion: C-reactive protein and arterial hypertension. Acta Ophthalmol. 1984, 62, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Breviario, F.; d’Aniello, E.M.; Golay, J.; Peri, G.; Bottazzi, B.; Bairoch, A.; Saccone, S.; Marzella, R.; Predazzi, V.; Rocchi, M.; et al. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J. Biol. Chem. 1992, 267, 22190–22197. [Google Scholar] [PubMed]
- Garlanda, C.; Bottazzi, B.; Bastone, A.; Mantovani, A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu. Rev. Immunol. 2005, 23, 337–366. [Google Scholar] [CrossRef]
- Toniatti, C.; Demartis, A.; Monaci, P.; Nicosia, A.; Ciliberto, G. Synergistic trans-activation of the human C-reactive protein promoter by transcription factor HNF-1 binding at two distinct sites. EMBO J. 1990, 9, 4467–4475. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Alles, V.V.; Bottazzi, B.; Peri, G.; Golay, J.; Introna, M.; Mantovani, A. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood 1994, 84, 3483–3493. [Google Scholar] [CrossRef]
- Introna, M.; Alles, V.V.; Castellano, M.; Picardi, G.; De Gioia, L.; Bottazzai, B.; Peri, G.; Breviario, F.; Salmona, M.; De Gregorio, L.; et al. Cloning of mouse ptx3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood 1996, 87, 1862–1872. [Google Scholar] [CrossRef] [Green Version]
- Goodman, A.R.; Levy, D.E.; Reis, L.F.; Vilcek, J. Differential regulation of TSG-14 expression in murine fibroblasts and peritoneal macrophages. J. Leukoc. Biol. 2000, 67, 387–395. [Google Scholar] [CrossRef]
- Doni, A.; Peri, G.; Chieppa, M.; Allavena, P.; Pasqualini, F.; Vago, L.; Romani, L.; Garlanda, C.; Mantovani, A. Production of the soluble pattern recognition receptor PTX3 by myeloid, but not plasmacytoid, dendritic cells. Eur. J. Immunol. 2003, 33, 2886–2893. [Google Scholar] [CrossRef]
- Klouche, M.; Peri, G.; Knabbe, C.; Eckstein, H.H.; Schmid, F.X.; Schmitz, G.; Mantovani, A. Modified atherogenic lipoproteins induce expression of pentraxin-3 by human vascular smooth muscle cells. Atherosclerosis 2004, 175, 221–228. [Google Scholar] [CrossRef]
- Souza, D.G.; Soares, A.C.; Pinho, V.; Torloni, H.; Reis, L.F.; Teixeira, M.M.; Dias, A.A. Increased mortality and inflammation in tumor necrosis factor-stimulated gene-14 transgenic mice after ischemia and reperfusion injury. Am. J. Pathol. 2002, 160, 1755–1765. [Google Scholar] [CrossRef] [Green Version]
- Deban, L.; Russo, R.C.; Sironi, M.; Moalli, F.; Scanziani, M.; Zambelli, V.; Cuccovillo, I.; Bastone, A.; Gobbi, M.; Valentino, S. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat. Immunol. 2010, 11, 328–334. [Google Scholar] [CrossRef]
- Souza, D.G.; Amaral, F.A.; Fagundes, C.T.; Coelho, F.M.; Arantes, R.M.; Sousa, L.P.; Matzuk, M.M.; Garlanda, C.; Mantovani, A.; Dias, A.A.; et al. The long pentraxin PTX3 is crucial for tissue inflammation after intestinal ischemia and reperfusion in mice. Am. J. Pathol. 2009, 174, 1309–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenza, G.L. Regulation of erythropoietin production. New insights into molecular mechanisms of oxygen homeostasis. Hematol. Oncol. Clin. N. Am. 1994, 8, 863–884. [Google Scholar] [CrossRef]
- Junk, A.K.; Mammis, A.; Savitz, S.I.; Singh, M.; Roth, S.; Malhotra, S.; Rosenbaum, P.S.; Cerami, A.; Brines, M.; Rosenbaum, D.M. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 2002, 99, 10659–10664. [Google Scholar] [CrossRef] [Green Version]
- Stahl, A.; Buchwald, A.; Martin, G.; Junker, B.; Chen, J.; Hansen, L.L.; Agostini, H.T.; Smith, L.E.; Feltgen, N. Vitreal levels of erythropoietin are increased in patients with retinal vein occlusion and correlate with vitreal VEGF and the extent of macular edema. Retina 2010, 30, 1524–1529. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.J.; Kim, H.C.; Moon, J.W. Aqueous levels of erythropoietin in acute retinal vein occlusion with macular edema. Int. J. Ophthalmol. 2014, 7, 501–506. [Google Scholar]
- Böcker-Meffert, S.; Rosenstiel, P.; Röhl, C.; Warneke, N.; Held-Feindt, J.; Sievers, J.; Lucius, R. Erythropoietin and VEGF promote neural outgrowth from retinal explants in postnatal rats. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2021–2026. [Google Scholar]
- Noma, H.; Mimura, T.; Tatsugawa, M.; Shimada, K. Aqueous flare and inflammatory factors in macular edema with central retinal vein occlusion: A case series. BMC Ophthalmol. 2013, 13, 78. [Google Scholar] [CrossRef] [Green Version]
- Noma, H.; Mimura, T.; Shimada, K. Role of inflammation in previously untreated macular edema with branch retinal vein occlusion. BMC Ophthalmol. 2014, 14, 67. [Google Scholar] [CrossRef] [Green Version]
- Xia, P.; Aiello, L.P.; Ishii, H.; Jiang, Z.Y.; Park, D.J.; Robinson, G.S.; Takagi, H.; Newsome, W.P.; Jirousek, M.R.; King, G.L. Characterization of vascular endothelial growth factor’s effect on the activation of protein kinase C, its isoforms, and endothelial cell growth. J. Clin. Investig. 1996, 98, 2018–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Venema, V.J.; Gu, X.; Venema, R.C.; Marrero, M.B.; Caldwell, R.B. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J. Biol. Chem. 1999, 274, 25130–25135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.W.; Mayo, L.D.; Dunbar, J.D.; Kessler, K.M.; Baerwald, M.R.; Jaffe, E.A.; Wang, D.; Warren, R.S.; Donner, D.B. Utilization of distinct signaling pathways by receptors for vascular endothelial cell growth factor and other mitogens in the induction of endothelial cell proliferation. J. Biol. Chem. 2000, 275, 5096–5103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiba, A.; Sagara, H.; Hara, T.; Shibuya, M. VEGFR-2-specific ligand VEGF-E induces non-edematous hyper-vascularization in mice. Biochem. Biophys. Res. Commun. 2003, 301, 371–377. [Google Scholar] [CrossRef]
- Murakami, M.; Iwai, S.; Hiratsuka, S.; Yamauchi, M.; Nakamura, K.; Iwakura, Y.; Shibuya, M. Signaling of vascular endothelial growth factor receptor-1 tyrosine kinase promotes rheumatoid arthritis through activation of monocytes/macrophages. Blood 2006, 108, 1849–1856. [Google Scholar] [CrossRef]
- Shibuya, M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J. Biochem. Mol. Biol. 2006, 39, 469–478. [Google Scholar] [CrossRef]
- Deobhakta, A.; Chang, L.K. Inflammation in retinal vein occlusion. Int. J. Inflamm. 2013, 438412. [Google Scholar] [CrossRef] [Green Version]
- Selvaraj, S.K.; Giri, R.K.; Perelman, N.; Johnson, C.; Malik, P.; Kalra, V.K. Mechanism of monocyte activation and expression of proinflammatory cytochemokines by placenta growth factor. Blood 2003, 102, 1515–1524. [Google Scholar] [CrossRef] [Green Version]
- Ledebur, H.C.; Parks, T.P. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NF-kappa B site and p65 homodimers. J. Biol. Chem. 1995, 270, 933–943. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, A.S., Jr. The NF-kappa B and I kappa B proteins: New discoveries and insights. Annu. Rev. Immunol. 1996, 14, 649–683. [Google Scholar] [CrossRef] [Green Version]
- Marumo, T.; Schini-Kerth, V.B.; Fisslthaler, B.; Busse, R. Platelet-derived growth factor-stimulated superoxide anion production modulates activation of transcription factor NF-kappaB and expression of monocyte chemoattractant protein 1 in human aortic smooth muscle cells. Circulation 1997, 96, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Usui, T.; Yamashiro, K.; Kaji, Y.; Ahmed, E.; Carrasquillo, K.G.; Amano, S.; Hida, T.; Oguchi, Y.; Adamis, A.P. VEGF164 is proinflammatory in the diabetic retina. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2155–2162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelson, G.; Harazny, J. Increased vascular resistance for venous outflow in central retinal vein occlusion. Ophthalmology 1997, 104, 659–663. [Google Scholar] [CrossRef]
- Horio, N.; Horiguchi, M. Retinal blood flow and macular edema after radial optic neurotomy for central retinal vein occlusion. Am. J. Ophthalmol. 2006, 141, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Crama, N.; Gualino, V.; Restori, M.; Charteris, D.G. Central retinal vessel blood flow after surgical treatment for central retinal vein occlusion. Retina 2010, 30, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Funatsu, H.; Sakata, K.; Harino, S.; Okuzawa, Y.; Noma, H.; Hori, S. Tracing method in the assessment of retinal capillary blood flow velocity by fluorescein angiography with scanning laser ophthalmoscope. Jpn. J. Ophthalmol. 2006, 50, 25–32. [Google Scholar] [CrossRef]
- Tanaka, T.; Muraoka, K.; Shimizu, K. Fluorescein fundus angiography with scanning laser ophthalmoscope. Visibility of leukocytes and platelets in perifoveal capillaries. Ophthalmology 1991, 98, 1824–1829. [Google Scholar] [CrossRef]
- Noma, H.; Funatsu, H.; Mimura, T.; Shimada, K. Perifoveal microcirculation in macular oedema with retinal vein occlusion. Open Ophthalmol. J. 2012, 6, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Funatsu, H.; Sakata, K.; Mimura, T.; Hori, S. Association between macular microcirculation and soluble intercellular adhesion molecule-1 in patients with macular edema and retinal vein occlusion. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 1515–1518. [Google Scholar] [CrossRef]
- Henry, P.D.; Chen, C.H. Inflammatory mechanisms of atheroma formation. Influence of fluid mechanics and lipid-derived inflammatory mediators. Am. J. Hypertens. 1993, 6, 328S–334S. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Duraiswamy, N.; Frank, A.O.; Moore, J.E., Jr. Blood flow in stented arteries: A parametric comparison of strut design patterns in three dimensions. J. Biomech. Eng. 2005, 127, 637–647. [Google Scholar] [CrossRef]
- Marin, T.; Gongol, B.; Chen, Z.; Woo, B.; Subramaniam, S.; Chien, S.; Shyy, J.Y. Mechanosensitive microRNAs-role in endothelial responses to shear stress and redox state. Free Radic. Biol. Med. 2013, 64, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Dawson, R.; Crane, I.J.; Liversidge, J. Leukocyte diapedesis in vivo induces transient loss of tight junction protein at the blood-retina barrier. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2487–2494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abcouwer, S.F.; Lin, C.M.; Shanmugam, S.; Muthusamy, A.; Barber, A.J.; Antonetti, D.A. Minocycline prevents retinal inflammation and vascular permeability following ischemia-reperfusion injury. J. Neuroinflamm. 2013, 10, 1742–2094. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Wykoff, C.C.; Shapiro, H.; Rubio, R.G.; Ehrlich, J.S. Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema. Ophthalmology 2014, 121, 1783–1789. [Google Scholar] [CrossRef]
- The Central Vein Occlusion Study. Baseline and early natural history report. Arch. Ophthalmol. 1993, 111, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- The Central Vein Occlusion Study Group M report. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. Ophthalmology 1995, 102, 1425–1433. [Google Scholar] [CrossRef]
- The Central Vein Occlusion Study Group. Natural history and clinical management of central retinal vein occlusion. Arch. Ophthalmol. 1997, 115, 486–491. [Google Scholar] [CrossRef]
- Brown, D.M.; Wykoff, C.C.; Wong, T.P.; Mariani, A.F.; Croft, D.E.; Schuetzle, K.L. Ranibizumab in preproliferative (ischemic) central retinal vein occlusion: The rubeosis anti-VEGF (RAVE) trial. Retina 2014, 34, 1728–1735. [Google Scholar] [CrossRef]
- Larsen, M.; Waldstein, S.M.; Boscia, F.; Gerding, H.; Mones, J.; Tadayoni, R.; Priglinger, S.; Wenzel, A.; Barnes, E.; Pilz, S.; et al. Individualized Ranibizumab Regimen Driven by Stabilization Criteria for Central Retinal Vein Occlusion: Twelve-Month Results of the CRYSTAL Study. Ophthalmology 2016, 123, 1101–1111. [Google Scholar] [CrossRef] [Green Version]
- Heier, J.S.; Campochiaro, P.A.; Yau, L.; Li, Z.; Saroj, N.; Rubio, R.G.; Lai, P. Ranibizumab for macular edema due to retinal vein occlusions: Long-term follow-up in the HORIZON trial. Ophthalmology 2012, 119, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Mimura, T.; Yasuda, K.; Shimura, M. Cytokine Kinetics after Monthly Intravitreal Bevacizumab for Retinal Vein Occlusion Associated with Macular Oedema. Ophthalmic Res. 2016, 56, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Bhisitkul, R.B.; Shapiro, H.; Rubio, R.G. Vascular endothelial growth factor promotes progressive retinal nonperfusion in patients with retinal vein occlusion. Ophthalmology 2013, 120, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Mashima, A.; Noma, H.; Yasuda, K.; Goto, H.; Shimura, M. Anti-vascular endothelial growth factor agent reduces inflammation in macular edema with central retinal vein occlusion. J. Inflamm. 2019, 16, 9. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noma, H.; Yasuda, K.; Shimura, M. Cytokines and Pathogenesis of Central Retinal Vein Occlusion. J. Clin. Med. 2020, 9, 3457. https://doi.org/10.3390/jcm9113457
Noma H, Yasuda K, Shimura M. Cytokines and Pathogenesis of Central Retinal Vein Occlusion. Journal of Clinical Medicine. 2020; 9(11):3457. https://doi.org/10.3390/jcm9113457
Chicago/Turabian StyleNoma, Hidetaka, Kanako Yasuda, and Masahiko Shimura. 2020. "Cytokines and Pathogenesis of Central Retinal Vein Occlusion" Journal of Clinical Medicine 9, no. 11: 3457. https://doi.org/10.3390/jcm9113457
APA StyleNoma, H., Yasuda, K., & Shimura, M. (2020). Cytokines and Pathogenesis of Central Retinal Vein Occlusion. Journal of Clinical Medicine, 9(11), 3457. https://doi.org/10.3390/jcm9113457




