Effects of Netarsudil on Actin-Driven Cellular Functions in Normal and Glaucomatous Trabecular Meshwork Cells: A Live Imaging Study
Abstract
:1. Introduction
2. Experimental Section
2.1. TM Cell Culture and Actin Staining
2.2. Live-Cell Imaging
2.3. Confocal Microscopy of Fixed TM Cells
2.4. EV Counts and Statistical Analyses
3. Results
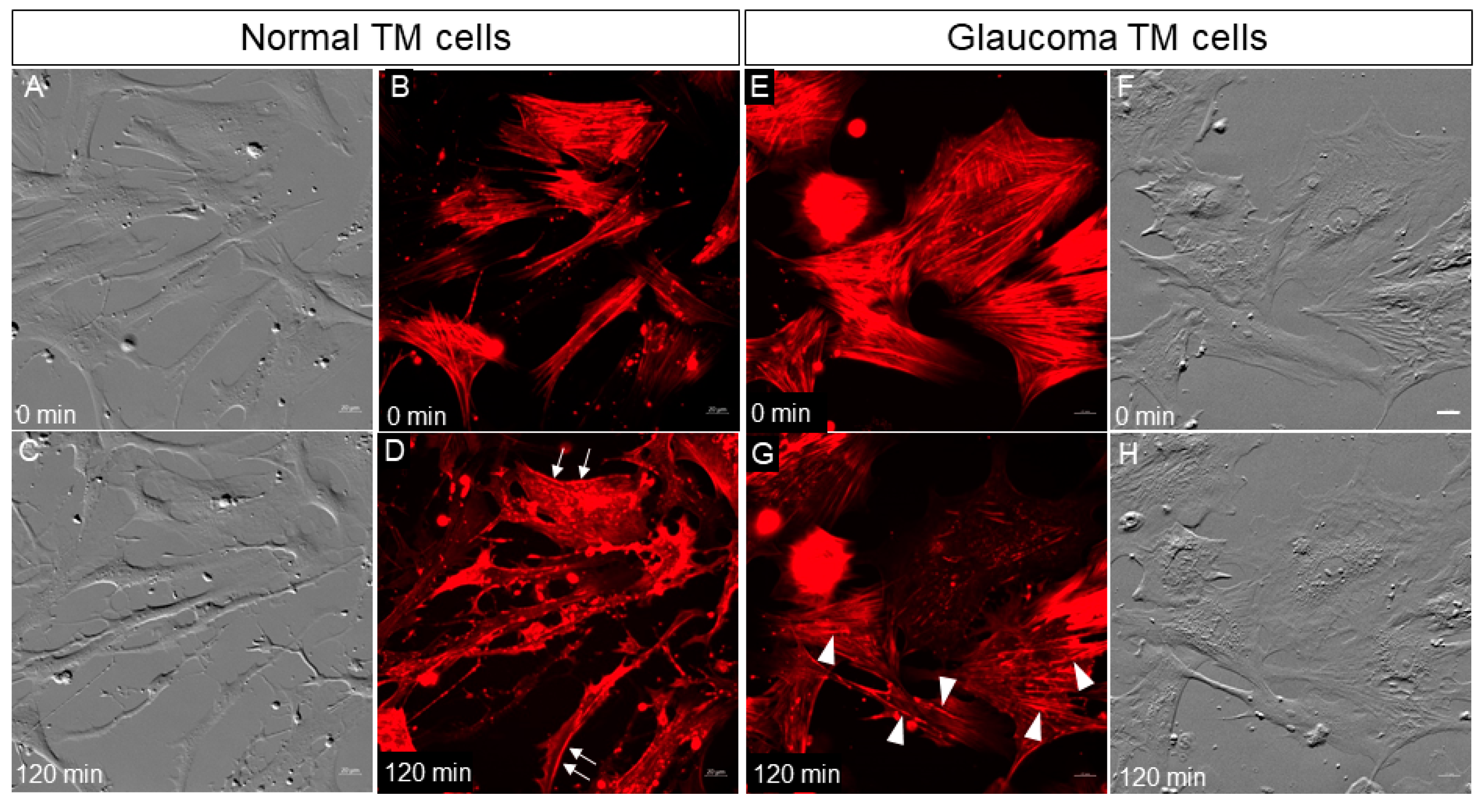
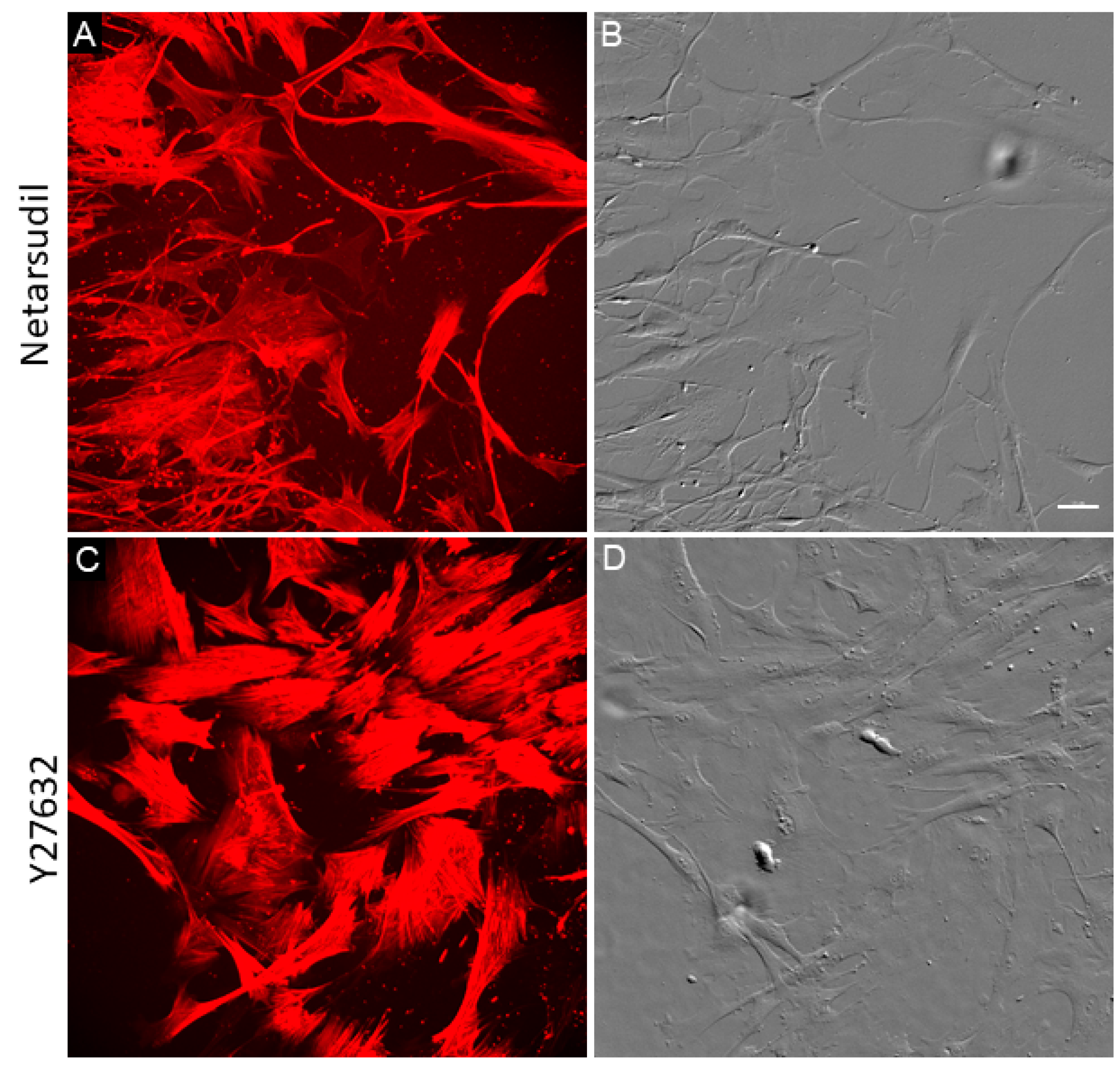
3.1. Normal and Glaucomatous TM Cells Treated with Netarsudil
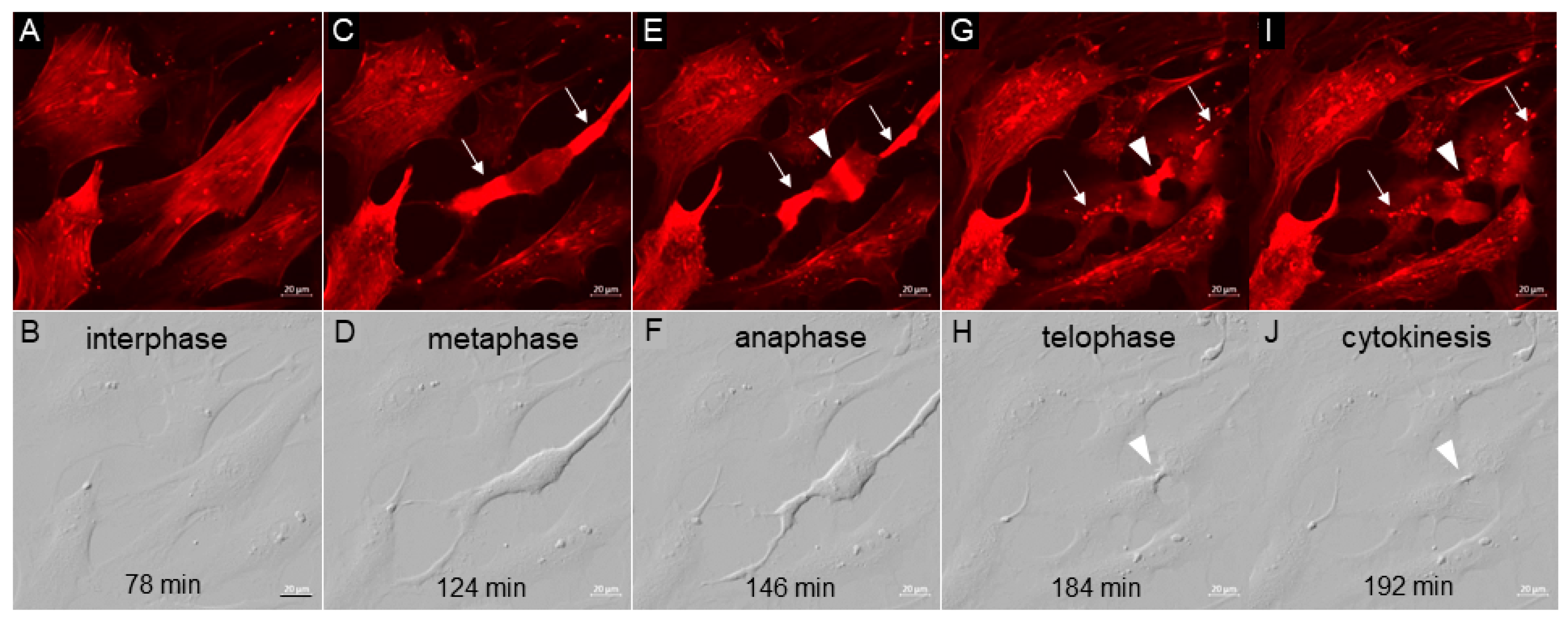
3.2. TM Cell Mitosis
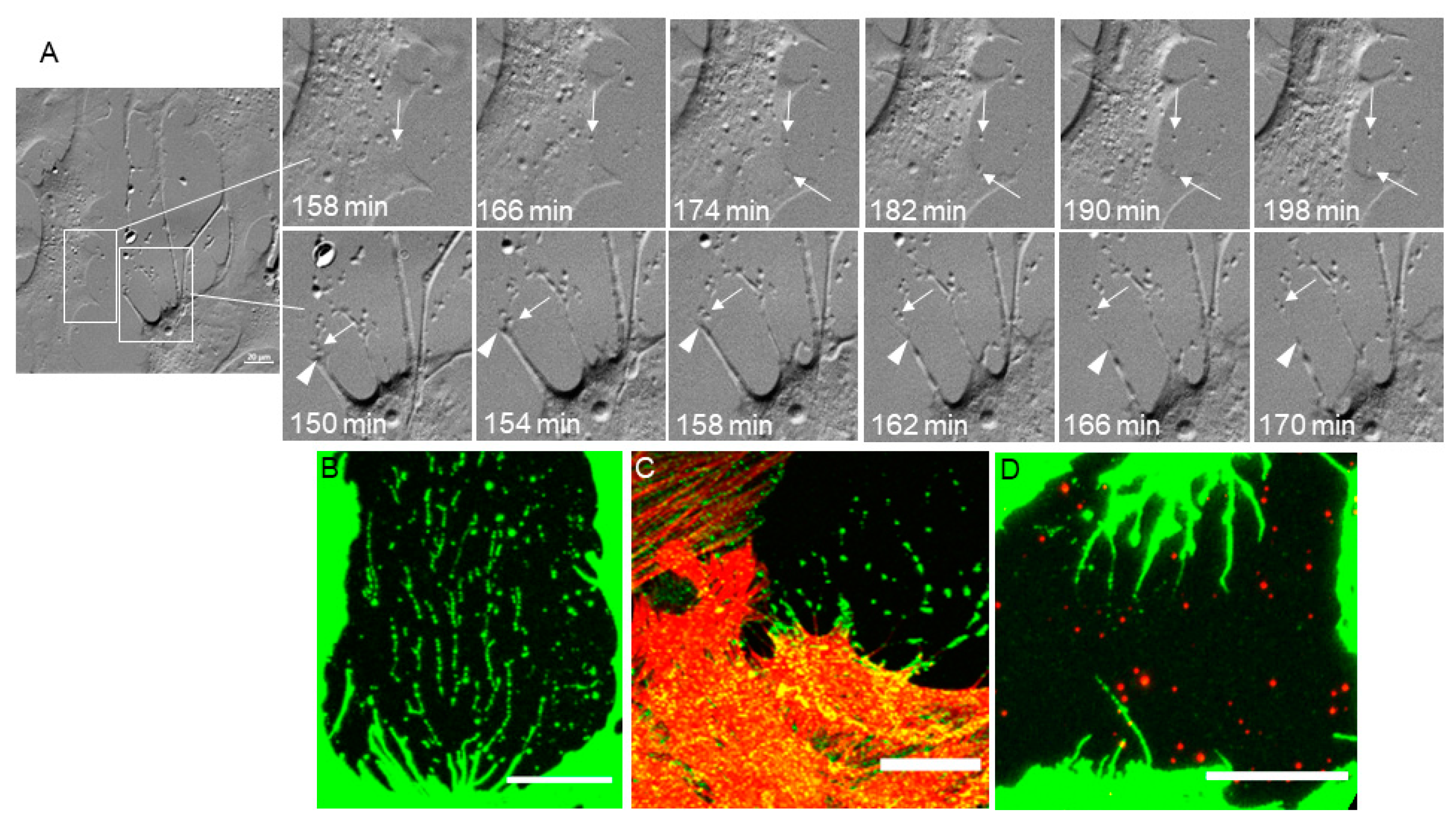
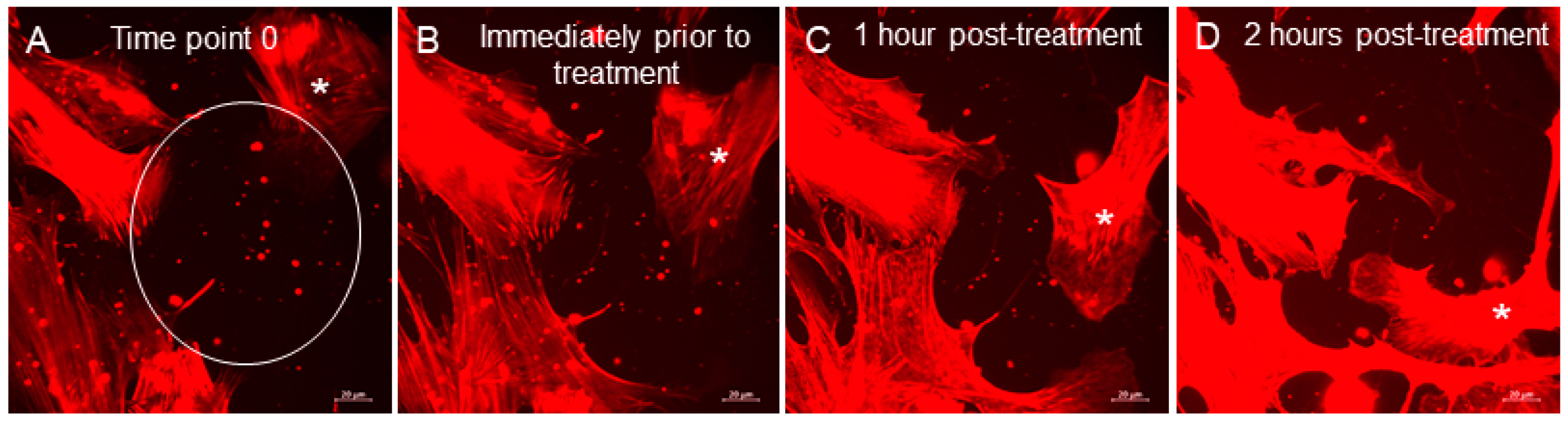
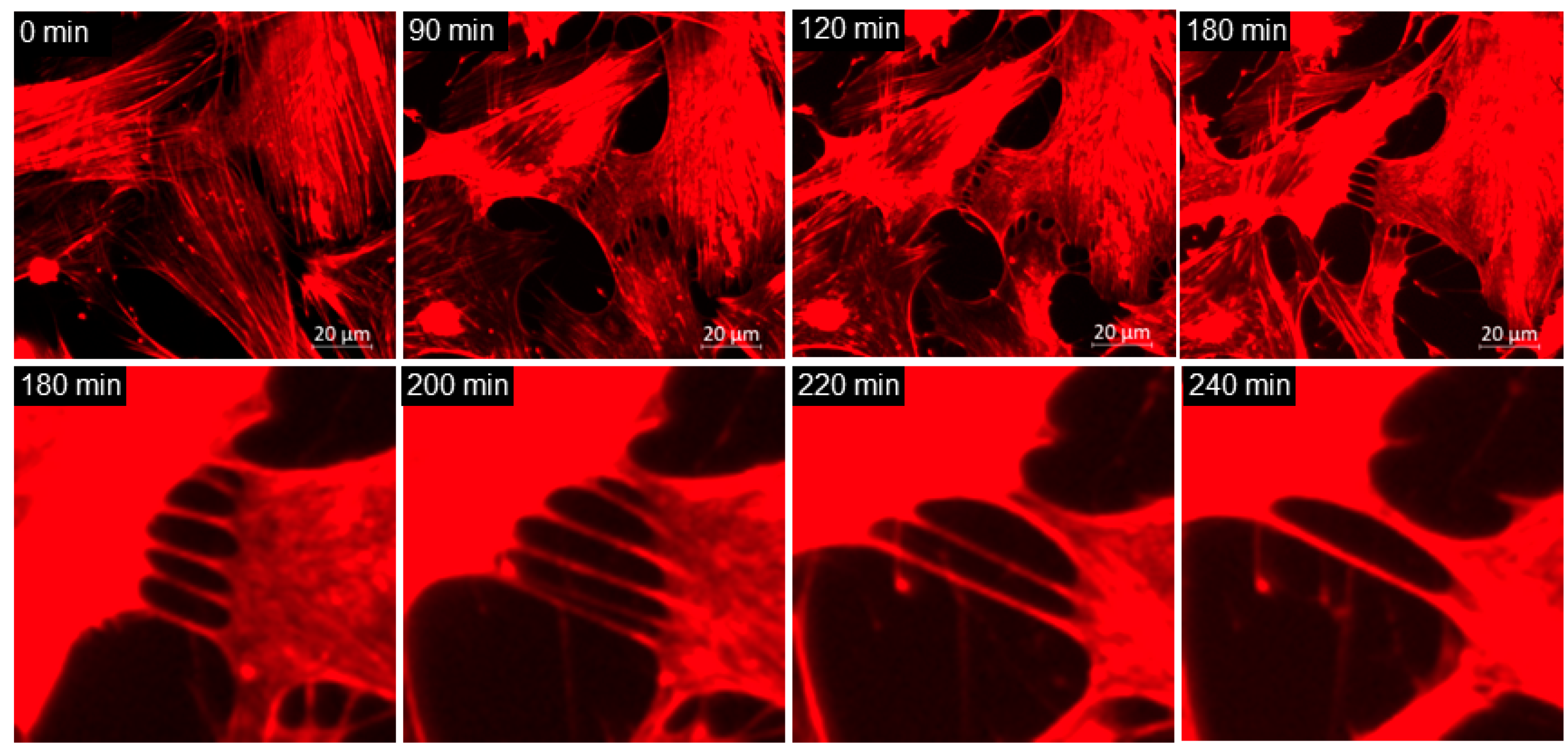
3.3. Extracellular Vesicles (EVs)
3.4. Phagocytosis
3.5. TNT Lateral Fusion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stamer, W.D.; Clark, A.F. The many faces of the trabecular meshwork cell. Exp. Eye Res. 2017, 158, 112–123. [Google Scholar] [CrossRef] [Green Version]
- Vranka, J.A.; Kelley, M.J.; Acott, T.S.; Keller, K.E. Extracellular matrix in the trabecular meshwork: Intraocular pressure regulation and dysregulation in glaucoma. Exp. Eye Res. 2015, 133, 112–125. [Google Scholar] [CrossRef] [Green Version]
- Pollard, T.D.; Cooper, J.A. Actin, a central player in cell shape and movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, R.P.; Veltman, D.; Machesky, L.M. Actin-bundling proteins in cancer progression at a glance. J. Cell Sci. 2012, 125, 1073–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobes, C.D.; Hall, A. Rho, rac and cdc42 GTPases: Regulators of actin structures, cell adhesion and motility. Biochem. Soc. Trans. 1995, 23, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.A.; Tian, B.; Geiger, B.; Kaufman, P.L. Effect of Latrunculin-B on Outflow Facility in Monkeys. Exp. Eye Res. 2000, 70, 307–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, P.V.; Deng, P.F.; Kumar, J.; Epstein, D.L. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1029–1037. [Google Scholar]
- Rao, P.V.; Pattabiraman, P.P.; Kopczynski, C. Role of the Rho GTPase/Rho kinase signaling pathway in pathogenesis and treatment of glaucoma: Bench to bedside research. Exp. Eye Res. 2016, 158, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Tian, B.; Kaufman, P.L. Comparisons of actin filament disruptors and Rho kinase inhibitors as potential antiglaucoma medications. Expert Rev. Ophthalmol. 2012, 7, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Inoue, T.; Tanihara, H. Rho-associated kinase inhibitors: A novel glaucoma therapy. Prog. Retin. Eye Res. 2013, 37, 1–12. [Google Scholar] [CrossRef]
- Ren, R.; Li, G.; Le, T.D.; Kopczynski, C.; Stamer, W.D.; Gong, H. Netarsudil Increases Outflow Facility in Human Eyes Through Multiple Mechanisms. Investig. Opthalmol. Vis. Sci. 2016, 57, 6197–6209. [Google Scholar] [CrossRef]
- Li, G.; Mukherjee, D.; Navarro, I.; Ashpole, N.E.; Sherwood, J.M.; Chang, J.; Overby, D.R.; Yuan, F.; Gonzalez, P.; Kopczynski, C.C.; et al. Visualization of conventional outflow tissue responses to netarsudil in living mouse eyes. Eur. J. Pharmacol. 2016, 787, 20–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-W.; Sherman, B.; Moore, L.A.; Laethem, C.L.; Lu, D.-W.; Pattabiraman, P.P.; Rao, P.V.; Delong, M.A.; Kopczynski, C.C. Discovery and Preclinical Development of Netarsudil, a Novel Ocular Hypotensive Agent for the Treatment of Glaucoma. J. Ocul. Pharmacol. Ther. 2018, 34, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-F.; Williamson, J.E.; Kopczynski, C.; Serle, J.B. Effect of 0.04% AR-13324, a ROCK, and Norepinephrine Transporter Inhibitor, on Aqueous Humor Dynamics in Normotensive Monkey Eyes. J. Glaucoma 2015, 24, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; McLaren, J.W.; Kopczynski, C.C.; Heah, T.G.; Novack, G.D.; Sit, A.J. The Effects of Netarsudil Ophthalmic Solution on Aqueous Humor Dynamics in a Randomized Study in Humans. J. Ocul. Pharmacol. Ther. 2018, 34, 380–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, A.F.; Miggans, S.T.; Wilson, K.; Browder, S.; McCartney, M.D. Cytoskeletal Changes in Cultured Human Glaucoma Trabecular Meshwork Cells. J. Glaucoma 1995, 4, 183–188. [Google Scholar] [CrossRef]
- Grierson, I.; Rahi, A.H. Microfilaments in the cells of the human trabecular meshwork. Br. J. Ophthalmol. 1979, 63, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.E. Tunneling nanotubes and actin cytoskeleton dynamics in glaucoma. Neural Regen. Res. 2020, 15, 2031–2032. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Bradley, J.M.; Keller, K.E. Phenotypic and Functional Alterations in Tunneling Nanotubes Formed by Glaucomatous Trabecular Meshwork Cells. Investig. Opthalmol. Vis. Sci. 2019, 60, 4583–4595. [Google Scholar] [CrossRef] [Green Version]
- Keller, K.E.; Bradley, J.M.; Sun, Y.Y.; Yang, Y.-F.; Acott, T.S. Tunneling Nanotubes are Novel Cellular Structures That Communicate Signals Between Trabecular Meshwork Cells. Investig. Opthalmol. Vis. Sci. 2017, 58, 5298–5307. [Google Scholar] [CrossRef] [Green Version]
- Chhabra, E.S.; Higgs, H.N. The many faces of actin: Matching assembly factors with cellular structures. Nat. Cell Biol. 2007, 9, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
- Chugh, P.; Paluch, E.K. The actin cortex at a glance. J. Cell Sci. 2018, 131, 186254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Bademosi, A.T.; Luo, J.; Meunier, F.A. Actin Remodeling in Regulated Exocytosis: Toward a Mesoscopic View. Trends Cell Biol. 2018, 28, 685–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunda, P.; Baum, B. The actin cytoskeleton in spindle assembly and positioning. Trends Cell Biol. 2009, 19, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Niedergang, F.; Chavrier, P. Signaling and membrane dynamics during phagocytosis: Many roads lead to the phagos(R)ome. Curr. Opin. Cell Biol. 2004, 16, 422–428. [Google Scholar] [CrossRef]
- Mattila, P.K.; Lappalainen, P. Filopodia: Molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 446–454. [Google Scholar] [CrossRef]
- Melak, M.; Plessner, M.; Grosse, R. Actin visualization at a glance. J. Cell Sci. 2017, 130, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Flores, L.R.; Keeling, M.C.; Zhang, X.; Sliogeryte, K.; Gavara, N. Lifeact-TagGFP2 alters F-actin organization, cellular morphology and biophysical behaviour. Sci. Rep. 2019, 9, 3241. [Google Scholar] [CrossRef]
- Fujimoto, T.; Inoue, T.; Inoue-Mochita, M.; Tanihara, H. Live cell imaging of actin dynamics in dexamethasone-treated porcine trabecular meshwork cells. Exp. Eye Res. 2016, 145, 393–400. [Google Scholar] [CrossRef]
- Lukinavičius, G.; Reymond, L.; D’Este, E.; Masharina, A.; Göttfert, F.; Ta, H.; Güther, A.; Fournier, M.; Rizzo, S.; Waldmann, H.; et al. Fluorogenic probes for live-cell imaging of the cytoskeleton. Nat. Methods 2014, 11, 731–733. [Google Scholar] [CrossRef]
- Davis, D.M.; Sowinski, S. Membrane nanotubes: Dynamic long-distance connections between animal cells. Nat. Rev. Mol. Cell Biol. 2008, 9, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.-H. Nanotubular Highways for Intercellular Organelle Transport. Sciense 2004, 303, 1007–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinnery, H.R.; Keller, K.E. Tunneling Nanotubes and the Eye: Intercellular Communication and Implications for Ocular Health and Disease. BioMed Res. Int. 2020, 2020, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherer, N.M. Long-distance relationships: Do membrane nanotubes regulate cell–cell communication and disease progression? Mol. Biol. Cell 2013, 24, 1095–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, K.E.; Bhattacharya, S.K.; Borrás, T.; Brunner, T.M.; Chansangpetch, S.; Clark, A.F.; Dismuke, W.M.; Du, Y.; Elliott, M.H.; Ethier, C.R.; et al. Consensus recommendations for trabecular meshwork cell isolation, characterization and culture. Exp. Eye Res. 2018, 171, 164–173. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Yang, Y.-F.; Keller, K.E. Myosin-X Silencing in the Trabecular Meshwork Suggests a Role for Tunneling Nanotubes in Outflow Regulation. Investig. Opthalmol. Vis. Sci. 2019, 60, 843–851. [Google Scholar] [CrossRef] [Green Version]
- Sturdivant, J.M.; Royalty, S.M.; Lin, C.-W.; Moore, L.A.; Yingling, J.D.; Laethem, C.L.; Sherman, B.; Heintzelman, G.R.; Kopczynski, C.C.; Delong, M.A. Discovery of the ROCK inhibitor netarsudil for the treatment of open-angle glaucoma. Bioorg. Med. Chem. Lett. 2016, 26, 2475–2480. [Google Scholar] [CrossRef]
- Davies, S.P.; Reddy, H.; Caivano, M.; Cohen, P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000, 351, 95–105. [Google Scholar] [CrossRef]
- Keller, K.E.; Bradley, J.M.; Acott, T.S. Differential Effects of ADAMTS-1, -4, and -5 in the Trabecular Meshwork. Investig. Opthalmol. Vis. Sci. 2009, 50, 5769–5777. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Rosenblatt, J.; Cramer, L.P.; Baum, B.; McGee, K.M. Myosin II-Dependent Cortical Movement Is Required for Centrosome Separation and Positioning during Mitotic Spindle Assembly. Cell 2004, 117, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Klingeborn, M.; Dismuke, W.M.; Rickman, C.B.; Stamer, W.D. Roles of exosomes in the normal and diseased eye. Prog. Retin. Eye Res. 2017, 59, 158–177. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Hardy, K.M.; Hoffman, E.A.; Gonzalez, P.; McKay, B.S.; Stamer, W.D. Extracellular Trafficking of Myocilin in Human Trabecular Meshwork Cells. J. Biol. Chem. 2005, 280, 28917–28926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, E.A.; Perkumas, K.M.; Highstrom, L.M.; Stamer, W. Regulation of myocilin-associated exosome release from human trabecular meshwork cells. Investig. Opthalmol. Vis. Sci. 2008, 50, 1313–1318. [Google Scholar] [CrossRef] [Green Version]
- Stamer, W.; Hoffman, E.; Luther, J.M.; Hachey, D.; Schey, K. Protein profile of exosomes from trabecular meshwork cells. J. Proteom. 2011, 74, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Filla, M.S.; Faralli, J.A.; Peotter, J.L.; Peters, D.M. The role of integrins in glaucoma. Exp. Eye Res. 2016, 158, 124–136. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, Y.; Johnson, D.H. Trabecular Meshwork Phagocytosis in Glaucomatous Eyes. Ophthalmologica 1997, 211, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Buller, C.; Johnson, D.H.; Tschumper, R.C. Human trabecular meshwork phagocytosis. Observations in an organ culture system. Investig. Ophthalmol. Vis. Sci. 1990, 31, 2156–2163. [Google Scholar]
- Zhang, X.; Ognibene, C.M.; Clark, A.F.; Yorio, T. Dexamethasone inhibition of trabecular meshwork cell phagocytosis and its modulation by glucocorticoid receptor beta. Exp. Eye Res. 2007, 84, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, J.M.; Ko, M.K.; Pouw, A.; Tan, J.C.H. Tissue-based multiphoton analysis of actomyosin and structural responses in human trabecular meshwork. Sci. Rep. 2016, 6, 21315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bovellan, M.; Romeo, Y.; Biro, M.; Boden, A.; Chugh, P.; Yonis, A.; Vaghela, M.; Fritzsche, M.; Moulding, D.; Thorogate, R.; et al. Cellular Control of Cortical Actin Nucleation. Curr. Biol. 2014, 24, 1628–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, C.; Charniga, C.; Temple, S.; Finnemann, S.C. Quantified F-Actin Morphology Is Predictive of Phagocytic Capacity of Stem Cell-Derived Retinal Pigment Epithelium. Stem Cell Rep. 2018, 10, 1075–1087. [Google Scholar] [CrossRef] [Green Version]
- Dang, Y.; Wang, C.; Shah, P.; Waxman, S.; Loewen, R.T.; Loewen, N. RKI-1447, a Rho kinase inhibitor, causes ocular hypotension, actin stress fiber disruption, and increased phagocytosis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 257, 101–109. [Google Scholar] [CrossRef]
- Wang, C.; Dang, Y.; Waxman, S.; Hong, Y.; Shah, P.; Loewen, R.T.; Xia, X.; Loewen, N.A. Ripasudil in a Model of Pigmentary Glaucoma. Transl. Vis. Sci. Technol. 2020, 9, 27. [Google Scholar] [CrossRef]
- Fujimoto, T.; Sato-Ohira, S.; Tanihara, H.; Inoue, T. RhoA activation decreases phagocytosis of trabecular meshwork cells. Curr. Eye Res. 2020. [Google Scholar] [CrossRef]
- Fu, P.C.; Tang, R.H.; Wan, Y.; Xie, M.J.; Wang, W.; Luo, X.; Yu, Z.Y. Rock inhibition with fasudil promotes early functional recovery of spinal cord injury in rats by enhancing microglia phagocytosis. Acta Acad. Med. Wuhan 2016, 36, 31–36. [Google Scholar] [CrossRef]
- Jung, K.-T.; Kim, H.-R.; Lee, B.-H.; Kim, S.-H.; So, K.Y.; An, T.-H.; Lee, H.-Y.; Oh, S.-H. Correction: Differential effects of p38 and JNK activation by GSK3 on cadmium-induced autophagy and apoptosis. Toxicol. Res. 2015, 4, 1426. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.L.; Bement, W.M. Regulation of cytokinesis by Rho GTPase flux. Nat. Cell Biol. 2009, 11, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Longo, F.M.; Zhou, H.; Massa, S.M.; Chen, Y.-H. Signaling Through Rho GTPase Pathway as Viable Drug Target. Curr. Med. Chem. 2009, 16, 1355–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dismuke, W.M.; Challa, P.; Navarro, I.; Stamer, W.D.; Liu, Y. Human aqueous humor exosomes. Exp. Eye Res. 2015, 132, 73–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayaram, H.; Phillips, J.I.; Lozano, D.C.; Choe, T.E.; Cepurna, W.O.; Johnson, E.C.; Morrison, J.C.; Gattey, D.M.; Saugstad, J.A.; Keller, K.E. Comparison of MicroRNA Expression in Aqueous Humor of Normal and Primary Open-Angle Glaucoma Patients Using PCR Arrays: A Pilot Study. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2884–2890. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, M.; Shah, N.; Zanetti, B.R.; Maugeri, M.; Silvestre, R.N.; Fatima, F.; Neder, L.; Valadi, H. Extracellular Vesicles and Matrix Remodeling Enzymes: The Emerging Roles in Extracellular Matrix Remodeling, Progression of Diseases and Tissue Repair. Cells 2018, 7, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimoda, M.; Khokha, R. Proteolytic factors in exosomes. Proteomics 2013, 13, 1624–1636. [Google Scholar] [CrossRef]
- Raghunathan, V.; Benoit, J.; Kasetti, R.; Zode, G.; Salemi, M.; Phinney, B.S.; Keller, K.E.; Staverosky, J.A.; Murphy, C.J.; Acott, T.; et al. Glaucomatous cell derived matrices differentially modulate non-glaucomatous trabecular meshwork cellular behavior. Acta Biomater. 2018, 71, 444–459. [Google Scholar] [CrossRef]
- Patel, G.; Fury, W.; Yang, H.; Gomez-Caraballo, M.; Bai, Y.; Yang, T.; Adler, C.; Wei, Y.; Ni, M.; Schmitt, H.; et al. Molecular taxonomy of human ocular outflow tissues defined by single-cell transcriptomics. Proc. Natl. Acad. Sci. USA 2020, 117, 12856–12867. [Google Scholar] [CrossRef]
- Van Zyl, T.; Yan, W.; McAdams, A.; Peng, Y.-R.; Shekhar, K.; Regev, A.; Juric, D.; Sanes, J.R. Cell atlas of aqueous humor outflow pathways in eyes of humans and four model species provides insight into glaucoma pathogenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 10339–10349. [Google Scholar] [CrossRef]
- Stamer, W.D.; Roberts, B.C.; Howell, D.N.; Epstein, D.L. Isolation, culture, and characterization of endothelial cells from Schlemm’s canal. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1804–1812. [Google Scholar]
- Tian, B.; Kiland, J.A.; Kaufman, P.L. Effects of the marine macrolides swinholide A and jasplakinolide on outflow facility in monkeys. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3187–3192. [Google Scholar]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Netarsudil (accessed on 29 October 2020).
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/448042 (accessed on 29 October 2020).
- Last, J.A.; Pan, T.; Ding, Y.; Reilly, C.M.; Keller, K.E.; Acott, T.S.; Fautsch, M.P.; Murphy, C.J.; Russell, P. Elastic Modulus Determination of Normal and Glaucomatous Human Trabecular Meshwork. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2147–2152. [Google Scholar] [CrossRef] [PubMed]
| Cell Strain | Sex | Age | Cause of Death | Glaucoma? |
|---|---|---|---|---|
| NTM 2018-0070 | M | 54 | Cardiac arrest | No |
| NTM 2018-1341 | M | 55 | Myocardial infarction | No |
| NTM 2018-1233 | M | 53 | Lung cancer | No |
| NTM 2012-1457 | M | 47 | Ventricular fibrillation arrest | No |
| NTM 2007-0125 | M | 4 | Respiratory failure | No |
| NTM 2011-1808 | M | 19 | Multiple trauma | No |
| GTM 2018-1672 | M | 57 | Respiratory failure | Yes |
| GTM 2018-0374 | M | 79 | Ischemic cerebrovascular accident | Yes |
| GTM 2017-1729 | F | 64 | Anoxic brain injury, cardiopulmonary arrest | Yes |
| NTM | GTM | |
|---|---|---|
| # of movies analyzed | 8 | 5 |
| # of EVs prior to Netarsudil treatment | 18.25 ± 4.4 | 9.4 ± 3.1 |
| # of EVs 1 h after Netarsudil treatment | 9.25 ± 2.8 | 7 ± 2.7 |
| # of EVs 2 h after Netarsudil treatment | 6.13 ± 2.1 | 3 ± 0.9 |
| % of EVs engulfed (prior vs. 1 h) | 49.3% | 25.5% |
| % of EVs engulfed (1 h vs. 2 h) | 33.7% | 57.1% |
| Total EVs engulfed (prior vs. 2 h) | 66% * | 68% ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, K.E.; Kopczynski, C. Effects of Netarsudil on Actin-Driven Cellular Functions in Normal and Glaucomatous Trabecular Meshwork Cells: A Live Imaging Study. J. Clin. Med. 2020, 9, 3524. https://doi.org/10.3390/jcm9113524
Keller KE, Kopczynski C. Effects of Netarsudil on Actin-Driven Cellular Functions in Normal and Glaucomatous Trabecular Meshwork Cells: A Live Imaging Study. Journal of Clinical Medicine. 2020; 9(11):3524. https://doi.org/10.3390/jcm9113524
Chicago/Turabian StyleKeller, Kate E., and Casey Kopczynski. 2020. "Effects of Netarsudil on Actin-Driven Cellular Functions in Normal and Glaucomatous Trabecular Meshwork Cells: A Live Imaging Study" Journal of Clinical Medicine 9, no. 11: 3524. https://doi.org/10.3390/jcm9113524




