New Strategies for Rehabilitation and Pharmacological Treatment of Fatigue Syndrome in Multiple Sclerosis
Abstract
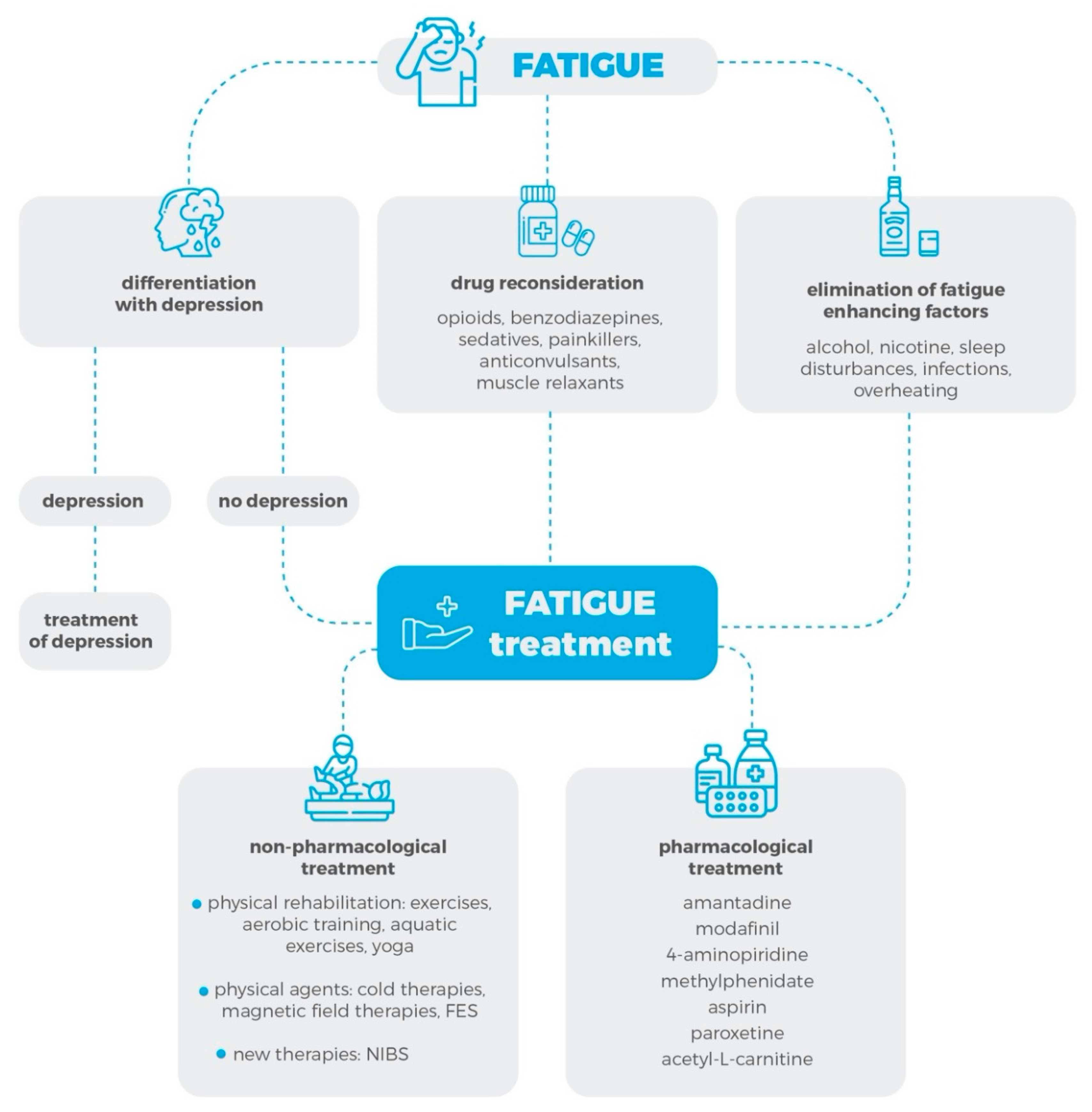
1. Introduction
1.1. Fatigue in Multiple Sclerosis
1.2. Fatigue Syndrome Diagnosis
2. Methods
3. Pharmacological Treatment of the Fatigue Syndrome
4. Non-Pharmacological Treatment of Fatigue Syndrome
4.1. Physical Rehabilitation
4.1.1. Physical Activity and Exercise Therapy
4.1.2. Physical Agents
4.2. New Therapies: Non-Invasive Brain Stimulation
5. Future Research and Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miller, E. Multiple Sclerosis. Adv. Exp. Med. Biol. 2012, 724, 222–238. [Google Scholar] [PubMed]
- Pugliatti, M.; Rosati, G.; Carton, H.; Riise, T.; Drulovic, J.; Vecsei, L.; Milanov, I. The epidemiology of multiple sclerosis in Europe. Eur. J. Neurol. 2006, 13, 700–722. [Google Scholar] [CrossRef]
- Chalah, M.A.; Riachi, N.; Ahdab, R.; Créange, A.; Lefaucheur, J.-P.; Ayache, S.S. Fatigue in Multiple Sclerosis: Neural Correlates and the Role of Non-Invasive Brain Stimulation. Front. Cell. Neurosci. 2015, 9, 460. [Google Scholar] [CrossRef]
- Comi, L.; Leocani, L.; Rossi, P.; Colombo, B. Physiopathology and treatment of fatigue in multiple sclerosis. J. Neurol. 2001, 248, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Flensner, G.; Ek, A.-C.; Söderhamn, O.; Landtblom, A.-M. Sensitivity to heat in MS patients: A factor strongly influencing symptomology—An explorative survey. BMC Neurol. 2011, 11, 27. [Google Scholar] [CrossRef]
- Krupp, L.B.; Christodoulou, C. Fatigue in multiple sclerosis. Curr. Neurol. Neurosci. Rep. 2001, 1, 294–298. [Google Scholar] [CrossRef]
- Mollaoğlu, M.; Üstün, E. Fatigue in multiple sclerosis patients. J. Clin. Nurs. 2009, 18, 1231–1238. [Google Scholar] [CrossRef]
- Weiland, T.J.; Jelinek, G.A.; Marck, C.H.; Hadgkiss, E.J.; Van Der Meer, D.M.; Pereira, N.G.; Taylor, K.L. Clinically Significant Fatigue: Prevalence and Associated Factors in an International Sample of Adults with Multiple Sclerosis Recruited via the Internet. PLoS ONE 2015, 10, e0115541. [Google Scholar] [CrossRef] [PubMed]
- Coates, K.D.; Aboodarda, S.J.; Krüger, R.L.; Martin, T.; Metz, L.M.; Jarvis, S.E.; Millet, G.Y. Multiple sclerosis-related fatigue: The role of impaired corticospinal responses and heightened exercise fatigability. J. Neurophysiol. 2020, 124, 1131–1143. [Google Scholar] [CrossRef]
- Kratz, A.L.; Murphy, S.L.; Braley, T.J. Ecological Momentary Assessment of Pain, Fatigue, Depressive, and Cognitive Symptoms Reveals Significant Daily Variability in Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2017, 98, 2142–2150. [Google Scholar] [CrossRef]
- Giovannoni, G. Multiple sclerosis related fatigue. J. Neurol. Neurosurg. Psychiatry 2006, 77, 2–3. [Google Scholar] [CrossRef]
- Hadjimichael, O.; Vollmer, T.; Oleen-Burkey, M. Fatigue characteristics in multiple sclerosis: The North American Research Committee on Multiple Sclerosis (NARCOMS) survey. Heal. Qual. Life Outcomes 2008, 6, 100. [Google Scholar] [CrossRef]
- Khan, F.; Amatya, B.; Galea, M. Management of Fatigue in Persons with Multiple Sclerosis. Front. Neurol. 2014, 5, 177. [Google Scholar] [CrossRef]
- Chen, M.H.; Wylie, G.R.; Sandroff, B.M.; Dacosta-Aguayo, R.; DeLuca, J.; Genova, H.M. Neural mechanisms underlying state mental fatigue in multiple sclerosis: A pilot study. J. Neurol. 2020, 267, 2372–2382. [Google Scholar] [CrossRef]
- Braley, T.J.; Chervin, R.D. Fatigue in Multiple Sclerosis: Mechanisms, Evaluation, and Treatment. Sleep 2010, 33, 1061–1067. [Google Scholar] [CrossRef]
- Gumus, H. Fatigue Can Be Objectively Measured in Multiple Sclerosis. Arch. Neuropsychiatry 2018, 55, S76–S79. [Google Scholar] [CrossRef] [PubMed]
- Téllez, N.; Río, J.; Tintoré, M.; Nos, C.; Galán, I.; Montalban, X. Does the Modified Fatigue Impact Scale offer a more comprehensive assessment of fatigue in MS? Mult. Scler. J. 2005, 11, 198–202. [Google Scholar] [CrossRef]
- Gobbi, C.; A Rocca, M.; Riccitelli, G.; Pagani, E.; Messina, R.; Preziosa, P.; Colombo, B.; Rodegher, M.; Falini, A.; Comi, G.; et al. Influence of the topography of brain damage on depression and fatigue in patients with multiple sclerosis. Mult. Scler. J. 2013, 20, 192–201. [Google Scholar] [CrossRef]
- Pardini, M.; Bonzano, L.; Bergamino, M.; Bommarito, G.; Feraco, P.; Murugavel, A.; Bove, M.; Brichetto, G.; Uccelli, A.; Mancardi, G.; et al. Cingulum bundle alterations underlie subjective fatigue in multiple sclerosis. Mult. Scler. J. 2014, 21, 442–447. [Google Scholar] [CrossRef]
- Pellicano, C.; Gallo, A.; Li, X.; Ikonomidou, V.N.; Evangelou, I.E.; Ohayon, J.M.; Stern, S.K.; Ehrmantraut, M.; Cantor, F.; McFarland, H.F.; et al. Relationship of Cortical Atrophy to Fatigue in Patients with Multiple Sclerosis. Arch. Neurol. 2010, 67, 447–453. [Google Scholar] [CrossRef]
- Kraft, G.H.; Bowen, J.; Rammohan, K.W.; Lynn, D.J.; Stankoff, B.; Waubant, E.; Lubetzki, C.; Clanet, M.; French Modafinil Study Group. Modafinil for fatigue in MS: A randomized placebo-controlled double-blind study. Neurology 2005, 65, 1995–1997. [Google Scholar] [CrossRef]
- Jensen, H.B.; Ravnborg, M.; Dalgas, U.; Stenager, E. 4-Aminopyridine for symptomatic treatment of multiple sclerosis: A systematic review. Ther. Adv. Neurol. Disord. 2013, 7, 97–113. [Google Scholar] [CrossRef]
- Rossini, P.M.; Pasqualetti, P.; Pozzilli, C.; Grasso, M.G.; Millefiorini, E.; Graceffa, A.; A Carlesimo, G.; Zibellini, G.; Caltagirone, C. Fatigue in progressive multiple sclerosis: Results of a randomized, double-blind, placebo-controlled, crossover trial of oral 4-aminopyridine. Mult. Scler. J. 2001, 7, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Generali, J.A.; Cada, D.J. Amantadine: Multiple Sclerosis–Related Fatigue. Hosp. Pharm. 2014, 49, 710–712. [Google Scholar] [CrossRef]
- Ledinek, A.H.; Sajko, M.C.; Rot, U. Evaluating the effects of amantadin, modafinil and acetyl-l-carnitine on fatigue in multiple sclerosis—Result of a pilot randomized, blind study. Clin. Neurol. Neurosurg. 2013, 115, S86–S89. [Google Scholar] [CrossRef] [PubMed]
- Möller, F.; Poettgen, J.; Broemel, F.; Neuhaus, A.; Daumer, M.; Heesen, C. HAGIL (Hamburg Vigil Study): A randomized placebo-controlled double-blind study with modafinil for treatment of fatigue in patients with multiple sclerosis. Mult. Scler. J. 2011, 17, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Stankoff, B.; Waubant, E.; Confavreux, C.; Edan, G.; Debouverie, M.; Rumbach, L.; Moreau, T.; Pelletier, J.; Lubetzki, C.; Clanet, M.G.; et al. Modafinil for fatigue in MS: A randomized placebo-controlled double-blind study. Neurology 2005, 64, 1139–1143. [Google Scholar] [CrossRef]
- Lange, M.R.; Volkmer, M.; Heesen, C.; Liepert, J. Modafinil effects in multiple sclerosis patients with fatigue. J. Neurol. 2009, 256, 645–650. [Google Scholar] [CrossRef]
- Tomassini, V.; Pozzilli, C.; Onesti, E.; Pasqualetti, P.; Marinelli, F.; Pisani, A.; Fieschi, C. Comparison of the effects of acetyl l-carnitine and amantadine for the treatment of fatigue in multiple sclerosis: Results of a pilot, randomised, double-blind, crossover trial. J. Neurol. Sci. 2004, 218, 103–108. [Google Scholar] [CrossRef]
- Ehde, D.M.; Kraft, G.H.; Chwastiak, L.; Sullivan, M.D.; Gibbons, L.E.; Bombardier, C.H.; Wadhwani, R. Efficacy of paroxetine in treating major depressive disorder in persons with multiple sclerosis. Gen. Hosp. Psychiatry 2008, 30, 40–48. [Google Scholar] [CrossRef]
- Tsou, A.; Treadwell, J.; Erinoff, E.; Schoelles, K. Which treatments improve fatigue and quality of life in Multiple Sclerosis? Evidence appraisal and development of visual interactive evidence maps (P5.2-088). Neurology 2019, 92 (Suppl. 15), P5.2-088. [Google Scholar]
- Yang, T.-T.; Wang, L.; Deng, X.-Y.; Yu, G. Pharmacological treatments for fatigue in patients with multiple sclerosis: A systematic review and meta-analysis. J. Neurol. Sci. 2017, 380, 256–261. [Google Scholar] [CrossRef]
- Nourbakhsh, B.; Revirajan, N.; Waubant, E. Treatment of fatigue with methylphenidate, modafinil and amantadine in multiple sclerosis (TRIUMPHANT-MS): Study design for a pragmatic, randomized, double-blind, crossover clinical trial. Contemp. Clin. Trials 2018, 64, 67–76. [Google Scholar] [CrossRef]
- Triche, E.W.; Ruiz, J.A.; Olson, K.M.; Lo, A.C. Changes in Cognitive Processing Speed, Mood, and Fatigue in an Observational Study of Persons with Multiple Sclerosis Treated with Dalfampridine-ER. Clin. Neuropharmacol. 2016, 39, 73–80. [Google Scholar] [CrossRef]
- Yadav, V.; Bever, C.; Bowen, J.; Bowling, A.; Weinstock-Guttman, B.; Cameron, M.; Bourdette, D.; Gronseth, G.S.; Narayanaswami, P. Summary of evidence-based guideline: Complementary and alternative medicine in multiple sclerosis: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014, 82, 1083–1092. [Google Scholar] [CrossRef]
- Niwald, M.; Redlicka, J.; Miller, E. The effects of aerobic training on the functional status, quality of life, the level of fatigue and disability in patients with multiple sclerosis—A preliminary report. Aktualności. Neurol. 2017, 17, 15–22. [Google Scholar] [CrossRef]
- Asano, M.; Finlayson, M. Meta-Analysis of Three Different Types of Fatigue Management Interventions for People with Multiple Sclerosis: Exercise, Education, and Medication. Mult. Scler. Int. 2014, 2014, 798285. [Google Scholar] [CrossRef] [PubMed]
- Stroud, N.M.; Minahan, C. The impact of regular physical activity on fatigue, depression and quality of life in persons with multiple sclerosis. Health Qual. Life Outcomes 2009, 7, 68. [Google Scholar] [CrossRef]
- Heine, M.; Van De Port, I.; Rietberg, M.B.; Wegen, E.E.H.V.; Kwakkel, G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst. Rev. 2015, CD009956. [Google Scholar] [CrossRef] [PubMed]
- Langeskov-Christensen, M.; Bisson, E.J.; Finlayson, M.; Dalgas, U. Potential pathophysiological pathways that can explain the positive effects of exercise on fatigue in multiple sclerosis: A scoping review. J. Neurol. Sci. 2017, 373, 307–320. [Google Scholar] [CrossRef]
- Kinnett-Hopkins, D.; Adamson, B.; Rougeau, K.; Motl, R.W. People with MS are less physically active than healthy controls but as active as those with other chronic diseases: An updated meta-analysis. Mult. Scler. Relat. Disord. 2017, 13, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Kalb, R.; Brown, T.R.; Coote, S.; Costello, K.; Dalgas, U.; Garmon, E.; Giesser, B.; Halper, J.; Karpatkin, H.; Keller, J.; et al. Exercise and lifestyle physical activity recommendations for people with multiple sclerosis throughout the disease course. Mult. Scler. J. 2020, 26, 1459–1469. [Google Scholar] [CrossRef]
- Kierkegaard, M.; E Lundberg, I.; Olsson, T.; Johansson, S.; Ygberg, S.; Opava, C.H.; Holmqvist, L.W.; Piehl, F. High-intensity resistance training in multiple sclerosis—An exploratory study of effects on immune markers in blood and cerebrospinal fluid, and on mood, fatigue, health-related quality of life, muscle strength, walking and cognition. J. Neurol. Sci. 2016, 362, 251–257. [Google Scholar] [CrossRef]
- Campbell, E.; Coulter, E.H.; Paul, L. High intensity interval training for people with multiple sclerosis: A systematic review. Mult. Scler. Relat. Disord. 2018, 24, 55–63. [Google Scholar] [CrossRef]
- Dehkordi, A.H. Influence of yoga and aerobics exercise on fatigue, pain and psychosocial status in patients with multiple sclerosis: A Randomized Trial. J. Sports Med. Phys. Fit. 2015, 56, 1417–1422. [Google Scholar]
- Mokhtarzade, M.; Ranjbar, R.; Majdinasab, N.; Patel, D.; Malanouri, S.M. Effect of aerobic interval training on serum IL-10, TNFα, and adipokines levels in women with multiple sclerosis: Possible relations with fatigue and quality of life. Endocrine 2017, 57, 262–271. [Google Scholar] [CrossRef]
- Mostert, S.; Kesselring, J. Effects of a short-term exercise training program on aerobic fitness, fatigue, health perception and activity level of subjects with multiple sclerosis. Mult. Scler. J. 2002, 8, 161–168. [Google Scholar] [CrossRef]
- Devasahayam, A.J.; Chaves, A.R.; Lasisi, W.O.; Curtis, M.E.; Wadden, K.P.; Kelly, L.P.; Pretty, R.; Chen, A.; Wallack, E.M.; Newell, C.J.; et al. Vigorous cool room treadmill training to improve walking ability in people with multiple sclerosis who use ambulatory assistive devices: A feasibility study. BMC Neurol. 2020, 20, 1–18. [Google Scholar] [CrossRef]
- Kargarfard, M.; Shariat, A.; Ingle, L.; Cleland, J.A.; Kargarfard, M. Randomized Controlled Trial to Examine the Impact of Aquatic Exercise Training on Functional Capacity, Balance, and Perceptions of Fatigue in Female Patients with Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2018, 99, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Kooshiar, H.; Moshtagh, M.; A Sardar, M.; Foroughipour, M.; Shakeri, M.T.; Vahdatinia, B. Fatigue and quality of life of women with multiple sclerosis: A randomized controlled clinical trial. J. Sports Med. Phys. Fit. 2014, 55, 668–674. [Google Scholar]
- Razazian, N.; Yavari, Z.; Farnia, V.; Azizi, A.; Kordavani, L.; Bahmani, D.S.; Holsboer-Trachsler, E.; Brand, S. Exercising Impacts on Fatigue, Depression, and Paresthesia in Female Patients with Multiple Sclerosis. Med. Sci. Sports Exerc. 2016, 48, 796–803. [Google Scholar] [CrossRef]
- Garrett, M.; Hogan, N.; Larkin, A.; Saunders, J.; Amigo-Benavent, M.; Coote, S. Exercise in the community for people with minimal gait impairment due to MS: An assessor-blind randomized controlled trial. Mult. Scler. J. 2012, 19, 782–789. [Google Scholar] [CrossRef]
- Van Geel, F.; Van Asch, P.; Veldkamp, R.; Feys, P. Effects of a 10-week multimodal dance and art intervention program leading to a public performance in persons with multiple sclerosis - A controlled pilot-trial. Mult. Scler. Relat. Disord. 2020, 44, 102256. [Google Scholar] [CrossRef]
- Garg, H.; Bush, S.; Gappmaier, E. Associations Between Fatigue and Disability, Functional Mobility, Depression, and Quality of Life in People with Multiple Sclerosis. Int. J. MS Care 2016, 18, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Armutlu, K.; Karabudak, R.; Nurlu, G. Physiotherapy approaches in the treatment of ataxic multiple sclerosis: A pilot study. Neurorehabil. Neural Repair 2001, 15, 203–211. [Google Scholar] [CrossRef]
- Tarakci, E.; Yeldan, I.; E Huseyinsinoglu, B.; Zenginler, Y.; Eraksoy, M. Group exercise training for balance, functional status, spasticity, fatigue and quality of life in multiple sclerosis: A randomized controlled trial. Clin. Rehabil. 2013, 27, 813–822. [Google Scholar] [CrossRef]
- Sangelaji, B.; Nabavi, S.M.; Estebsari, F.; Baneshi, M.M.; Rashidian, H.; Jamshidi, E.; Dastoorpoor, M. Effect of Combination Exercise Therapy on Walking Distance, Postural Balance, Fatigue and Quality of Life in Multiple Sclerosis Patients: A Clinical Trial Study. Iran. Red Crescent Med. J. 2014, 16, e17173. [Google Scholar] [CrossRef] [PubMed]
- Mccullagh, R.; Fitzgerald, A.P.; Murphy, R.P.; Cooke, G. Long-term benefits of exercising on quality of life and fatigue in multiple sclerosis patients with mild disability: A pilot study. Clin. Rehabil. 2007, 22, 206–214. [Google Scholar] [CrossRef]
- Feys, P.; Helsen, W.; Liu, X.; Mooren, D.; Albrecht, H.; Nuttin, B.; Ketelaer, P. Effects of peripheral cooling on intention tremor in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2005, 76, 373–379. [Google Scholar] [CrossRef]
- Miller, E.; Mrowicka, M.; Malinowska, K.; Mrowicki, J.; Saluk-Juszczak, J.; Kȩdziora, J. Effects of whole-body cryotherapy on a total antioxidative status and activities of antioxidative enzymes in blood of depressive multiple sclerosis patients. World J. Biol. Psychiatry 2010, 12, 223–227. [Google Scholar] [CrossRef]
- Miller, E.; Kostka, J.; Włodarczyk, T.; Dugué, B. Whole-body cryostimulation (cryotherapy) provides benefits for fatigue and functional status in multiple sclerosis patients. A case-control study. Acta Neurol. Scand. 2016, 134, 420–426. [Google Scholar] [CrossRef]
- Gonzales, B.; Chopard, G.; Charry, B.; Berger, E.; Tripard, J.; Magnin, E.; Groslambert, A. Effects of a Training Program Involving Body Cooling on Physical and Cognitive Capacities and Quality of Life in Multiple Sclerosis Patients: A Pilot Study. Eur. Neurol. 2017, 78, 71–77. [Google Scholar] [CrossRef]
- Tuncay, F. Özkan; Mollaoğlu, M. Effect of the cooling suit method applied to individuals with multiple sclerosis on fatigue and activities of daily living. J. Clin. Nurs. 2017, 26, 4527–4536. [Google Scholar] [CrossRef]
- Nilsagård, Y.; Denison, E.; Gunnarsson, L.-G. Evaluation of a single session with cooling garment for persons with multiple sclerosis—A randomized trial. Disabil. Rehabil. Assist. Technol. 2006, 1, 225–233. [Google Scholar] [CrossRef]
- Lappin, M.S.; Lawrie, F.W.; Richards, T.L.; Kramer, E.D. Effects of a pulsed electromagnetic therapy on multiple sclerosis fatigue and quality of life: A double-blind, placebo controlled trial. Altern. Ther. Health Med. 2003, 9, 38–48. [Google Scholar]
- Piatkowski, J.; Kern, S.; Ziemssen, T. Effect of BEMER Magnetic Field Therapy on the Level of Fatigue in Patients with Multiple Sclerosis: A Randomized, Double-Blind Controlled Trial. J. Altern. Complement. Med. 2009, 15, 507–511. [Google Scholar] [CrossRef]
- De Carvalho, M.L.L.; Motta, R.; Konrad, G.; Battaglia, M.A.; Brichetto, G. A randomized placebo-controlled cross-over study using a low frequency magnetic field in the treatment of fatigue in multiple sclerosis. Mult. Scler. J. 2011, 18, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Mostert, S.; Kesselring, J. Effect of pulsed magnetic field therapy on the level of fatigue in patients with multiple sclerosis—A randomized controlled trial. Mult. Scler. J. 2005, 11, 302–305. [Google Scholar] [CrossRef]
- Barr, C.J.; Patritti, B.L.; Bowes, R.; Crotty, M.; McLoughlin, J.V. Orthotic and therapeutic effect of functional electrical stimulation on fatigue induced gait patterns in people with multiple sclerosis. Disabil. Rehabil. Assist. Technol. 2016, 12, 1–13. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Hsu, M.-J.; Chen, S.-M.; Lin, C.-H.; Wong, A.M.K. Decreased central fatigue in multiple sclerosis patients after 8 weeks of surface functional electrical stimulation. J. Rehabil. Res. Dev. 2011, 48, 555–564. [Google Scholar] [CrossRef]
- Pilutti, L.A.; Edwards, T.A.; Motl, R.W.; Sebastião, E. Functional Electrical Stimulation Cycling Exercise in People with Multiple Sclerosis: Secondary Effects on Cognition, Symptoms, and Quality of Life. Int. J. MS Care 2019, 21, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Fan, S.; Xu, Y.; Cui, L. Non-invasive brain stimulation for fatigue in multiple sclerosis patients: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2019, 36, 101375. [Google Scholar] [CrossRef]
- Chalah, M.A.; Grigorescu, C.; Padberg, F.; Kümpfel, T.; Palm, U.; Ayache, S.S. Bifrontal transcranial direct current stimulation modulates fatigue in multiple sclerosis: A randomized sham-controlled study. J. Neural Transm. 2020, 127, 953–961. [Google Scholar] [CrossRef]
- Cancelli, A.; Cottone, C.; Giordani, A.; Migliore, S.; Lupoi, D.; Porcaro, C.; Mirabella, M.; Rossini, P.M.; Filippi, M.M.; Tecchio, F. Personalized, bilateral whole-body somatosensory cortex stimulation to relieve fatigue in multiple sclerosis. Mult. Scler. J. 2017, 24, 1366–1374. [Google Scholar] [CrossRef]
- Tecchio, F.; Cancelli, A.; Cottone, C.; Zito, G.; Pasqualetti, P.; Ghazaryan, A.; Rossini, P.M.; Filippi, M.M. Multiple sclerosis fatigue relief by bilateral somatosensory cortex neuromodulation. J. Neurol. 2014, 261, 1552–1558. [Google Scholar] [CrossRef]
- Saiote, C.; Goldschmidt, T.; Timäus, C.; Steenwijk, M.D.; Opitz, A.; Antal, A.; Paulus, W.; Nitsche, M.A. Impact of transcranial direct current stimulation on fatigue in multiple sclerosis. Restor. Neurol. Neurosci. 2014, 32, 423–436. [Google Scholar] [CrossRef]
- Gaede, G.; Tiede, M.; Lorenz, I.; Brandt, A.U.; Pfueller, C.; Dörr, J.; Bellmann-Strobl, J.; Piper, S.K.; Roth, Y.; Zangen, A.; et al. Safety and preliminary efficacy of deep transcranial magnetic stimulation in MS-related fatigue. Neurol. Neuroimmunol. Neuroinflamm. 2017, 5, e423. [Google Scholar] [CrossRef]
- Noakes, T.D.; Gibson, A.S.C.; Lambert, E.V. From catastrophe to complexity: A novel model of integrative central neural regulation of effort and fatigue during exercise in humans. Br. J. Sports Med. 2004, 38, 511–514. [Google Scholar] [CrossRef]
- Rudroff, T.; Kindred, J.H.; Ketelhut, N.B. Fatigue in Multiple Sclerosis: Misconceptions and Future Research Directions. Front. Neurol. 2016, 7, 122. [Google Scholar] [CrossRef] [PubMed]
| Study, Year, Reference | Study Design | Specific Treatment | Control Group | Fatigue Outcome Measures | Main Findings |
|---|---|---|---|---|---|
| Rossini et al., 2001 [23] | Randomized, controlled trial, n = 54, 6 weeks | 4-AP | Placebo | FSS |
|
| Ledinek et al., 2013 [25] | Randomized, controlled trial, n = 60, 1 month | Amantadine, modafinil, and ALCAR | Placebo | MFIS |
|
| Möller et al., 2011 [26] | Randomized, controlled trial, n = 121, 8 weeks | Modafinil | Placebo | FSS, MFIS |
|
| Stankoff et al., 2005 [27] | Randomized, controlled, double-blind study n = 115, 35 days | Modafinil | Placebo | MFIS |
|
| Lange R. et al. 2009 [28] | Double-blind, placebo-controlled study, n = 21, 8 weeks | Modafinil | Placebo | FSS |
|
| Tomassini et al., 2004 [29] | Randomized, double-blind, crossover trial, n = 36, 3 months | ALCAR | Amantadine | FSS FIS |
|
| Ehde et al., 2008 [30] | Randomized, controlled trial, n = 42, 12 weeks | Paroxetine | Placebo | MFIS |
|
| Tsou A et al., 2019 [31] | Meta-analysis of RCTs, n = 45 trials | 4-AP, amantadine, modafinil, aspirin, paroxetine | Placebo |
| |
| Yang et al., 2017 [32] | Meta-analysis of RTCs, n = 11 trials (723 patients) | - | MFIS, FSS |
| |
| Nourbaksh et al., 2017–2019 [33] | Randomized, controlled trial, multicenter study, n = 132 | Methyl-phenidate, modafinil, and amantadine | Placebo | MFIS |
|
| Triche et al., 2016 [34] | Observation-al pre–post study, n = 39, 14 weeks | Dalfampridine | No control group | PS |
|
| Study, Year, PEDro Score, Reference | Study Design | Type of Intervention | Outcome Measures | Main Findings |
|---|---|---|---|---|
| Hasanpour et al., 2016 PEDro: 5/10 [45] | Randomized, controlled trial; n = 90 | Yoga, aerobics exercises: 12 weeks, 3 sessions per week, 40 min per session | Rotten fatigue test, SF-36 |
|
| Mokhtarzade et al., 2017 PEDro: 5/10 [46] | Randomized, controlled trial; n = 40 | Aerobic exercise: 8 weeks, 3 days per week, upper and lower limb aerobic interval training program | FSS, MSQOL-54 |
|
| Mostert S, et al., 2002 PEDro: 3/10 [47] | Clinical trial; n = 26 | Aerobics exercise: 4 weeks, 5 sessions a week, 30 min per session, bicycle exercise with individualized intensity | FSS, SF-36 |
|
| Devasahayam et al., 2020 PEDro: none [48] | Clinical trial; n = 10 | Aerobic walking training in a room cooled to 16 °C using bodyweight-supported treadmill | FSS, MFIS, SF-36 |
|
| Kargarfard et al., 2017 PEDro: 7/10 [49] | Randomized, controlled trial; n = 34 | Aquatic exercise: 8 weeks, 3 sessions per week, sessions 45–60 min, water temperature: 28–30 °C | MFIS |
|
| Kooshiar et al., 2015 PEDro: 4/10 [50] | Randomized, controlled trial; n = 37 | Aquatic therapy: 8 weeks, 3 sessions per week and 45 min per session, water temperature: 28–29.5 °C | FSS, MFIS, MQLIM |
|
| Razazian, et al., 2016 PEDro: 6/10 [51] | Randomized, controlled trial; n = 54 | Aquatic exercise: 8 weeks, 3 sessions per week and 1h per session, water temperature: 28–30 °C Yoga: 8 weeks, three times per week, about 60 min | FSS, Beck Depression Inventory, 10-point visual analogue scale for paresthesia |
|
| Garrett et al., 2013 PEDro: 6/10 [52] | Randomized, controlled trial | Physiotherapist (PT)-led exercise (n = 80), yoga (n = 77), fitness instructor (FI)-led exercise (n = 86) | MFIS, MSIS |
|
| Tarakci et al., 2013 PEDro: 8/10 [56] | Randomized, controlled trial; n = 99 | Group exercise led by physical therapist | FSS |
|
| Sangelaji et al., 2014 PEDro: 3/10 [57] | Randomized, controlled trial; n = 59 | Combination exercises: 10 weeks, 3 sessions a week, 20–40 min per session, stretching and aerobic exercises, strengthening exercises with and balancing exercises. | FSS, 6-min Walk Test, EDSS quality of life tests |
|
| McCullagh et al., 2008 PEDro: 4/10 [58] | Randomized, controlled trial; n = 30 | Exercise: 3 months, 2 sessions per week, participants also exercised independently once a week. | MFIS, MSIS-29, FAMS |
|
| Study, Year, PEDro Score, Reference | Study Design | Potential Intervention | Outcome Measures | Main Findings |
|---|---|---|---|---|
| Miller et al., 2016 PEDro: none [61] | Case–control study; n = 24 | 10 × 3 min WBC sessions (one exposure per day at −110 °C or lower) | FSS, RMA, MSIS-29, EDSS |
|
| Gonzales et al., 2017 PEDro: 4/10 [62] | Randomized, controlled trial; n = 18 | 7-week physical training program with a cooling vest during each training session | SEP-59 |
|
| Özkan et al., 2017 PEDro: none [63] | Case–control study; n = 75 | Colling suit (vest) applied once a day for 40 min, 4 weeks | FIS, FSS, and Modified Barthel Index. |
|
| Nilsagård et al., 2006 PEDro: 7/10 [64] | Randomized, controlled crossover study; n = 43 | Single session with Rehband cooling garment | A study-specific questionnaire to evaluate subjective experiences. 10TW, 30TW, TUG, oral temperature, spasticity, standing balance |
|
| Study, Year, PEDro Score, Reference | Study Design | Potential Intervention | Outcome Measures | Main Findings |
|---|---|---|---|---|
| Lappin et al., 2003 PEDro: 7/10 [65] | Multi-site, double-blind, placebo-controlled, crossover trial; n = 117 | Daily exposure to a small, portable PMFT generator | MSQLI |
|
| Piatkowski et al., 2009 PEDro: 7/10 [66] | Randomized, double-blind, controlled trial; n = 37 | BEMER magnetic field treatment for 8 min twice daily in comparison to placebo for 12 weeks | MFIS, FSS |
|
| De Carvalho et al., 2012 PEDro: 6/10 [67] | Randomized, double-blind, crossover trial; n = 50 | Systemic pulsed low-frequency magnetic field with an intensity of 37.5 mT and with a sequence of pulses at 4–7 Hz. Total of 24 sessions, three times a week for 8 weeks, 24 min per session | FSS, MFIS, |
|
| Mostert et al., 2005 PEDro: 6/10 [68] | Randomized, controlled trial; n = 25 | PMFT, single treatment lasted 16 min twice daily over 3–4 weeks | FSS, VAS |
|
| Study, Year, References | Study Design | Potential Intervention | Outcome Measures | Main Findings |
|---|---|---|---|---|
| Chang et al., 2011 [70] | n = 9 | 8 weeks of quadriceps muscle surface FES training | Maximal voluntary contraction, voluntary activation level, twitch force, FI, CFI, Peripheral Fatigue Index, and MFIS |
|
| Pilutti et al., 2019 [71] | Randomized, controlled trial, n = 11 | FES cycling exercise (n = 6) or passive leg cycling (n = 5) for 24 weeks | FSS, MFIS, SF-PMQ, MSIS-29 |
|
| Study, Year | Study Design | Type of Intervention | Outcome Measures | Main Findings |
|---|---|---|---|---|
| Chalah et al., 2020 [73] | randomized, sham-controlled study, n = 11 | bilateral tDCS | FSS, MFIS |
|
| Cancelli et al., 2018 [74] | randomized, double-blind, sham-controlled, crossover study, n = 10 | tDCS | MFIS |
|
| Tecchio et al., 2014 [75] | randomized, double-blind, sham-controlled, crossover study, n = 10 | anodal bilateral primary somatosensory areas tDCS | MFIS |
|
| Saiote et al., 2014 [76] | sham-controlled, double-blind intervention study | excitability-enhancing anodal tDCS | FSS, MSFSS, MFIS |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska-Nowak, E.; Włodarczyk, L.; Kostka, J.; Miller, E. New Strategies for Rehabilitation and Pharmacological Treatment of Fatigue Syndrome in Multiple Sclerosis. J. Clin. Med. 2020, 9, 3592. https://doi.org/10.3390/jcm9113592
Zielińska-Nowak E, Włodarczyk L, Kostka J, Miller E. New Strategies for Rehabilitation and Pharmacological Treatment of Fatigue Syndrome in Multiple Sclerosis. Journal of Clinical Medicine. 2020; 9(11):3592. https://doi.org/10.3390/jcm9113592
Chicago/Turabian StyleZielińska-Nowak, Ewa, Lidia Włodarczyk, Joanna Kostka, and Elżbieta Miller. 2020. "New Strategies for Rehabilitation and Pharmacological Treatment of Fatigue Syndrome in Multiple Sclerosis" Journal of Clinical Medicine 9, no. 11: 3592. https://doi.org/10.3390/jcm9113592
APA StyleZielińska-Nowak, E., Włodarczyk, L., Kostka, J., & Miller, E. (2020). New Strategies for Rehabilitation and Pharmacological Treatment of Fatigue Syndrome in Multiple Sclerosis. Journal of Clinical Medicine, 9(11), 3592. https://doi.org/10.3390/jcm9113592






