Osteoarthritis in Pseudoxanthoma Elasticum Patients: An Explorative Imaging Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ethical Approval
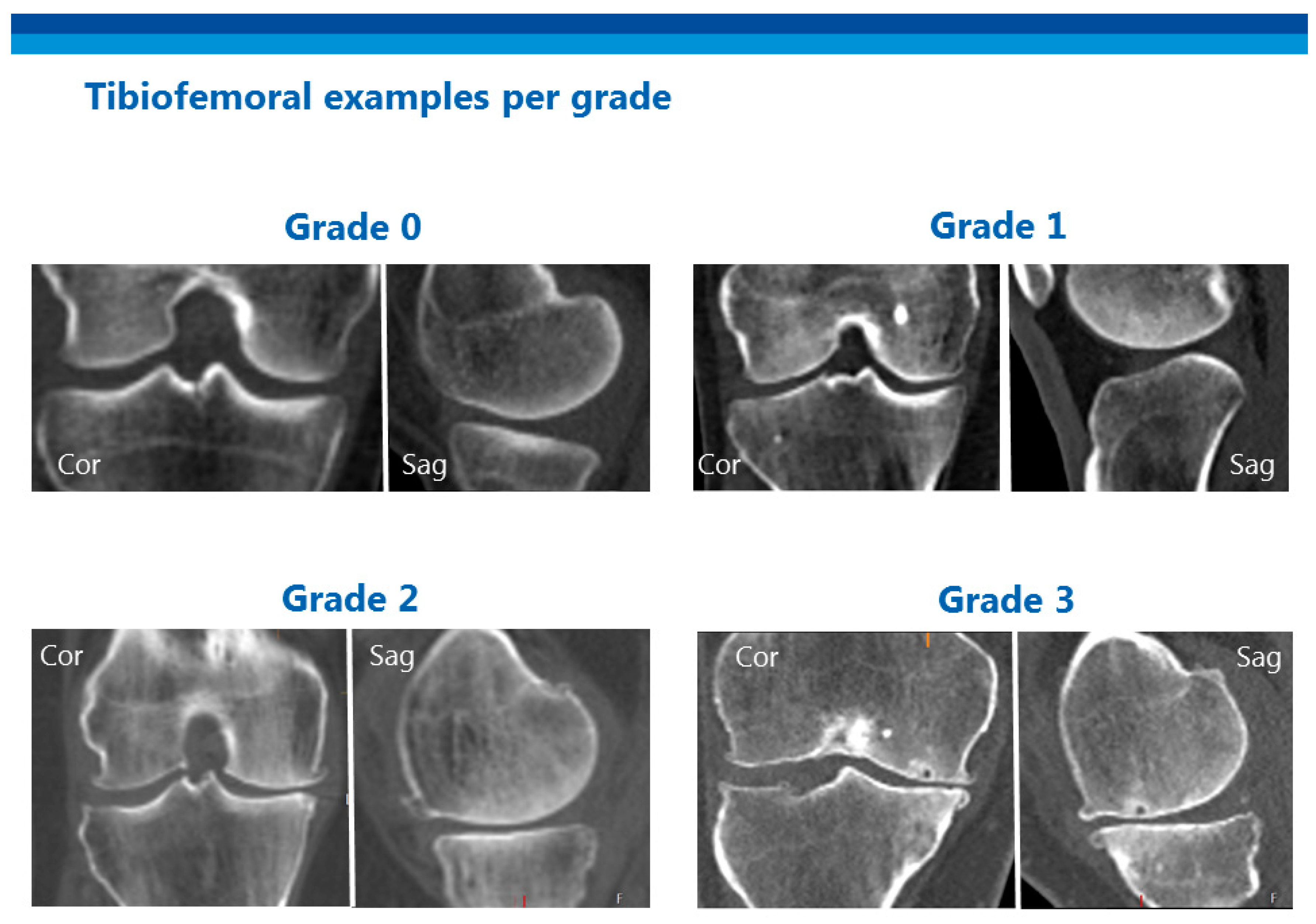
2.3. Scoring
2.4. Statistical Analysis
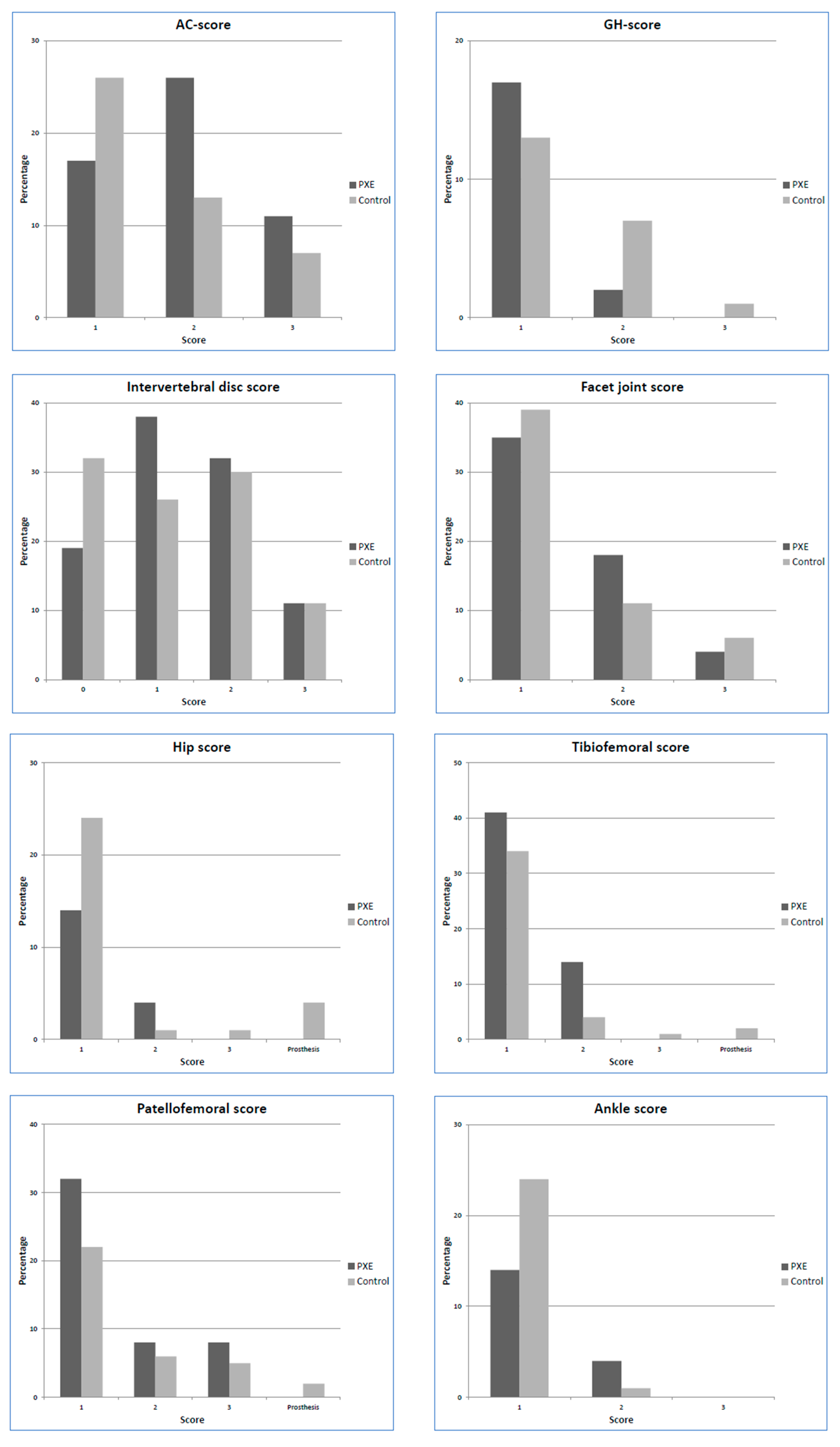
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jansen, R.S.; Duijst, S.; Mahakena, S.; Sommer, D.; Szeri, F.; Váradi, A.; Plomp, A.; Bergen, A.A.; Oude Elferink, R.P.J.; Borst, P.; et al. ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-Brief report. Arter. Thromb. Vasc. Biol. 2014, 34, 1985–1989. [Google Scholar] [CrossRef]
- Mebarek, S.; Hamade, E.; Thouverey, C.; Bandorowicz-Pikula, J.; Pikula, S.; Magne, D.; Buchet, R. Ankylosing spondylitis, late osteoarthritis, vascular calcification, chondrocalcinosis and pseudo gout: Toward a possible drug therapy. Curr. Med. Chem. 2011, 18, 2196–2203. [Google Scholar] [CrossRef]
- Perkins, H.R.; Walker, P.G. The occurence of pyrophosphate in bone. J. Bone Jt. Surg. Br. 1958, 40, 333–339. [Google Scholar] [CrossRef]
- Nitschke, Y.; Baujat, G.; Botschen, U.; Wittkampf, T.; Du Moulin, M.; Stella, J.; Le Merrer, M.; Guest, G.; Lambot, K.; Tazarourte-Pinturier, M.F.; et al. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am. J. Hum. Genet. 2012, 90, 25–39. [Google Scholar] [CrossRef]
- Ichikawa, N.; Taniguchi, A.; Kaneko, H.; Kawamoto, M.; Sekita, C.; Nakajima, A.; Yamanaka, H. Arterial calcification due to deficiency of CD73 (ACDC) as one of rheumatic diseases associated with periarticular calcification. J. Clin. Rheumatol. 2015, 21, 216–220. [Google Scholar] [CrossRef]
- Gutierrez, L.B.; Link, T.; Chaganti, K.; Motamedi, D. Arterial calcification due to CD73 deficiency (ACDC): Imaging manifestations of ectopic mineralization. Skelet. Radiol. 2016, 45, 1583–1587. [Google Scholar] [CrossRef]
- Hilaire, C.S.; Ziegler, S.G.; Markello, T.C.; Brusco, A.; Groden, C.; Gill, F.; Carlson-Donohoe, H.; Lederman, R.J.; Chen, M.Y.; Yang, D.; et al. NT5E mutations and arterial calcifications. N. Engl. J. Med. 2011, 364, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Hoppé, E.; Etienne, M.; Legrand, E.; Rutsch, F.; Leftheriotis, G.; Martin, L. Prevalence of shoulder calcification in pseudoxanthoma elasticum patients. Jt. Bone Spine 2018, 85, 777–778. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.C. Still’s Disease with Pseudoxanthoma Elasticum. Proc. R. Soc. Med. 1957, 50, 473. [Google Scholar] [CrossRef] [PubMed]
- Sairanen, E.; Itkonen, A.; Kangas, S. Pseudoxanthoma elasticum (pxe) and joint manifestations. Acta Rheumatol. Scand. 1970, 16, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Biot, J.; Desbordes, P. A A propos d’un nouveau cas d’elastorrhexie systématisée. Association avec une cervicarthrose et un syndrome de Barré Liéou [Apropos of a new case of pseudoxanthoma elasticum. Association with cervical arthritis and Barre Lieou syndrome]. Med. Trop. 1968, 28, 75–84. [Google Scholar]
- Van Saase, J.L.C.M.; Van Romunde, L.K.J.; Cats, A.; Vandenbroucke, J.P.; Valkenburg, H.A. Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann. Rheum. Dis. 1989, 48, 271–280. [Google Scholar] [CrossRef]
- Bierma-Zeinstra, S.M.A.; Waarsing, J.H. The role of atherosclerosis in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2018. [Google Scholar] [CrossRef]
- Srikanth, V.K.; Fryer, J.L.; Zhai, G.; Winzenberg, T.M.; Hosmer, D.; Jones, G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr. Cartil. 2005, 13, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Corti, M.; Rigon, C. Epidemiology of Osteoarthritis: Prevalence, Risk Factors and Functional Impact. Aging Clin. Exp. Res. 2003, 15. [Google Scholar] [CrossRef] [PubMed]
- Sandell, L.J. Etiology of osteoarthritis: Genetics and synovial joint development. Nat. Rev. Rheumatol. 2012, 8, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Laslett, L.L.; Dore, D.A.; Quinn, S.J.; Boon, P.; Ryan, E.; Winzenberg, T.M.; Jones, G. Zoledronic acid reduces knee pain and bone marrow lesions over 1 year: A randomised controlled trial. Ann. Rheum. Dis. 2012, 71, 1322–1328. [Google Scholar] [CrossRef]
- Pelletier, J.-P.; Roubille, C.; Raynauld, J.-P.; Abram, F.; Dorais, M.; Delorme, P.; Martel-Pelletier, J. Disease-modifying effect of strontium ranelate in a subset of patients from the Phase III knee osteoarthritis study SEKOIA using quantitative MRI: Reduction in bone marrow lesions protects against cartilage loss. Ann. Rheum. Dis. 2015, 74, 422–429. [Google Scholar] [CrossRef]
- Plomp, A.S.; Toonstra, J.; Bergen, A.A.B.; Van Dijk, M.R.; De Jong, P.T.V.M. Proposal for updating the pseudoxanthoma elasticum classification system and a review of the clinical findings. Am. J. Med. Genet. Part A 2010, 152, 1049–1058. [Google Scholar] [CrossRef]
- MRC Holland: Confidence in Copy Number Determination. Available online: https://www.mrcholland.com/ (accessed on 24 November 2020).
- Kranenburg, G.; de Jong, P.A.; Mali, W.P.; Attrach, M.; Visseren, F.L.J.; Spiering, W. Prevalence and severity of arterial calcifications in pseudoxanthoma elasticum (PXE) compared to hospital controls. Novel insights into the vascular phenotype of PXE. Atherosclerosis 2017, 256, 7–14. [Google Scholar] [CrossRef]
- Gielis, W.P.; Weinans, H.; Nap, F.J.; Roewer, F.W.; Foppen, W. Scoring Osteoarthritis Reliably in Large Joints and the Spine Using Whole-Body CT: OsteoArthritis Computed Tomography-Score (OACT-Score). J. Pers. Med. 2021, 11, 5. [Google Scholar] [CrossRef]
- Burns, R.A.; Butterworth, P.; Kiely, K.M.; Bielak, A.A.M.; Luszcz, M.A.; Mitchell, P.; Christensen, H.; Von Sanden, C.; Anstey, K.J. Multiple imputation was an efficient method for harmonizing the Mini-Mental State Examination with missing item-level data. J. Clin. Epidemiol. 2011, 64, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.; Pattrick, M.; Doherty, M. Inorganic pyrophosphate, nucleoside triphosphate pyrophosphatase, and cartilage fragment in normul human synovial fluid. Rheumatology 1991, 30, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Goding, J.; Van Etten, D.; Sali, A.; Hu, S.I.; Farley, D.; Krug, H.; Hessle, L.; Millán, J.L.; Terkeltaub, R. Linked Deficiencies in Extracellular PPi and Osteopontin Mediate Pathologic Calcification Associated With Defective PC-1 and ANK Expression. J. Bone Miner. Res. 2003, 18, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Terkeltaub, R. Inorganic pyrophosphate (PPI) in pathologic calcification of articular cartilage. Front. Biosci. 2005, 10, 988–997. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Brown, M.A.; Peach, C.; Russell, G.; Wordsworth, B.P. Investigation of the role of ENPP1 and TNAP genes in chondrocalcinosis. Rheumatology 2007, 46, 586–589. [Google Scholar] [CrossRef]
- Kranenburg, G.; de Jong, P.A.; Bartstra, J.W.; Lagerweij, S.J.; Lam, M.G.; Norel, J.O.-V.; Risseeuw, S.; van Leeuwen, R.; Imhof, S.M.; Verhaar, H.J.; et al. Etidronate for Prevention of Ectopic Mineralization in Patients With Pseudoxanthoma Elasticum. J. Am. Coll. Cardiol. 2018, 71, 1117–1126. [Google Scholar] [CrossRef]
- Xing, R.L.; Zhao, L.R.; Wang, P.M. Bisphosphonates therapy for osteoarthritis: A meta-analysis of randomized controlled trials. Springerplus 2016, 5, 1–8. [Google Scholar] [CrossRef][Green Version]
- Gielis, W.P.; Welsing, P.M.J.; van Spil, W.E.; Runhaar, J.; Weinans, H.; de Jong, P.A. A sex-specific association between incident radiographic osteoarthritis of hip or knee and incident peripheral arterial calcifications: 8-year prospective data from Cohort Hip and Cohort Knee (CHECK). Osteoarthr. Cartil. 2017. [Google Scholar] [CrossRef]
- Meknas, K.; Johansen, O.; Steigen, S.E.; Olsen, R.; Jørgensen, L.; Kartus, J. Could tendinosis be involved in osteoarthritis? Scand. J. Med. Sci. Sports 2012, 22, 627–634. [Google Scholar] [CrossRef]
- Vanakker, O.M.; Martin, L.; Schurgers, L.J.; Quaglino, D.; Costrop, L.; Vermeer, C.; Pasquali-Ronchetti, I.; Coucke, P.J.; De Paepe, A. Low serum vitamin K in PXE results in defective carboxylation of mineralization inhibitors similar to the GGCX mutations in the PXE-like syndrome. Lab. Investig. 2010, 90, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T.; Booth, S.L.; Zhang, Y.Q.; Jacques, P.F.; Terkeltaub, R.; Aliabadi, P.; Felson, D.T. Low vitamin K status is associated with osteoarthritis in the hand and knee. Arthritis Rheum. 2006, 54, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Neupane, B.; Walter, S.D.; Krueger, P.; Loeb, M. Community controls were preferred to hospital controls in a case-control study where the cases are derived from the hospital. J. Clin. Epidemiol. 2010, 63, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Suri, P.; Morgenroth, D.C.; Hunter, D.J. Epidemiology of Osteoarthritis and Associated Comorbidities. PM R 2012, 4, S10–S19. [Google Scholar] [CrossRef]
- Kranenburg, G.; Baas, A.F.; de Jong, P.A.; Asselbergs, F.W.; Visseren, F.L.J.; Spiering, W. The prevalence of pseudoxanthoma elasticum: Revised estimations based on geotyping in a high vascular risk cohort. Eur. J. Med. Genet. 2019, 62, 90–92. [Google Scholar] [CrossRef]
| Control Group | PXE Group | |
|---|---|---|
| n = 87 | n = 106 | |
| Age (years) | 55 (43–67) | 56 (48–64) |
| Gender (male) | 40 (46%) | 44 (42%) |
| BMI (kg/m2) * | 26.0 (22.5–29.2) | 25.3 (22.7–28.2) |
| Current smoking ** | 16 (20%) | 17 (16%) |
| Joint prosthesis hip | 4 (5%) | 0 (0%) |
| Joint prosthesis knee | 2 (2%) | 0 (0%) |
| Crude OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
|---|---|---|---|---|
| AC score | 1.69 (0.99–2.90) | 0.054 | 2.00 (1.12–3.61) | 0.020 |
| GH score | 0.83 (0.41–1.70) | 0.606 | 1.19 (0.49–2.54) | 0.822 |
| Intervertebral disc score | 1.34 (0.80–2.26) | 0.265 | 1.61 (0.91–2.87) | 0.102 |
| Facet joint score | 1.08 (0.65–1.83) | 0.782 | 1.31 (0.73–2.38) | 0.369 |
| Hip score | 0.51 (0.26–1.00) | 0.050 | 0.56 (0.27–1.15) | 0.117 |
| Tibiofemoral score | 1.83 (1.06–3.22) | 0.033 | 2.63 (1.40–5.07) | 0.003 |
| Patellofemoral score | 1.70 (0.97–3.02) | 0.066 | 2.22 (1.18–4.24) | 0.014 |
| Ankle score | 0.68 (0.34–1.35) | 0.267 | 0.78 (0.37–1.66) | 0.522 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gielis, W.P.; de Jong, P.A.; Bartstra, J.W.; Foppen, W.; Spiering, W.; den Harder, A.M. Osteoarthritis in Pseudoxanthoma Elasticum Patients: An Explorative Imaging Study. J. Clin. Med. 2020, 9, 3898. https://doi.org/10.3390/jcm9123898
Gielis WP, de Jong PA, Bartstra JW, Foppen W, Spiering W, den Harder AM. Osteoarthritis in Pseudoxanthoma Elasticum Patients: An Explorative Imaging Study. Journal of Clinical Medicine. 2020; 9(12):3898. https://doi.org/10.3390/jcm9123898
Chicago/Turabian StyleGielis, Willem Paul, Pim A. de Jong, Jonas W. Bartstra, Wouter Foppen, Wilko Spiering, and Annemarie M. den Harder. 2020. "Osteoarthritis in Pseudoxanthoma Elasticum Patients: An Explorative Imaging Study" Journal of Clinical Medicine 9, no. 12: 3898. https://doi.org/10.3390/jcm9123898
APA StyleGielis, W. P., de Jong, P. A., Bartstra, J. W., Foppen, W., Spiering, W., & den Harder, A. M. (2020). Osteoarthritis in Pseudoxanthoma Elasticum Patients: An Explorative Imaging Study. Journal of Clinical Medicine, 9(12), 3898. https://doi.org/10.3390/jcm9123898







