Simultaneous Estimation of Gender Male and Atrial Fibrillation as Risk Factors for Adverse Outcomes Following Transcatheter Aortic Valve Implantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Multi-Center TAVI Registry Database
2.2. Participants, Definition of Endpoints and Follow-Up Period
2.3. Statistical Analysis
3. Results
3.1. Baseline Demographics, Medications, Procedural Characteristics and Incidence of Complications of 4 Groups Divided by Gender and AF
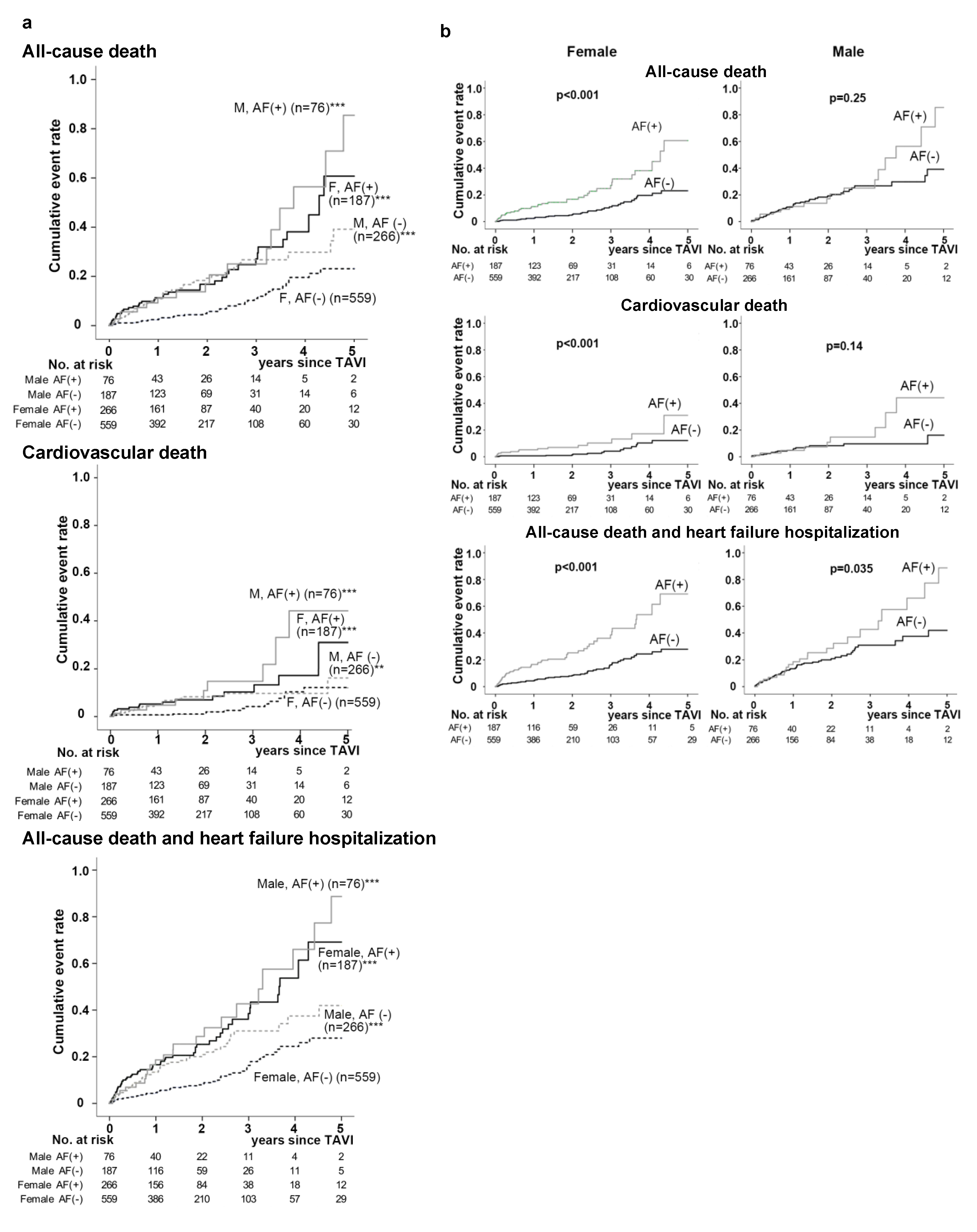
3.2. In-Hospital and Long-Term Outcomes Following TAVI in the 4 Patient Groups Divided According to Gender and AF
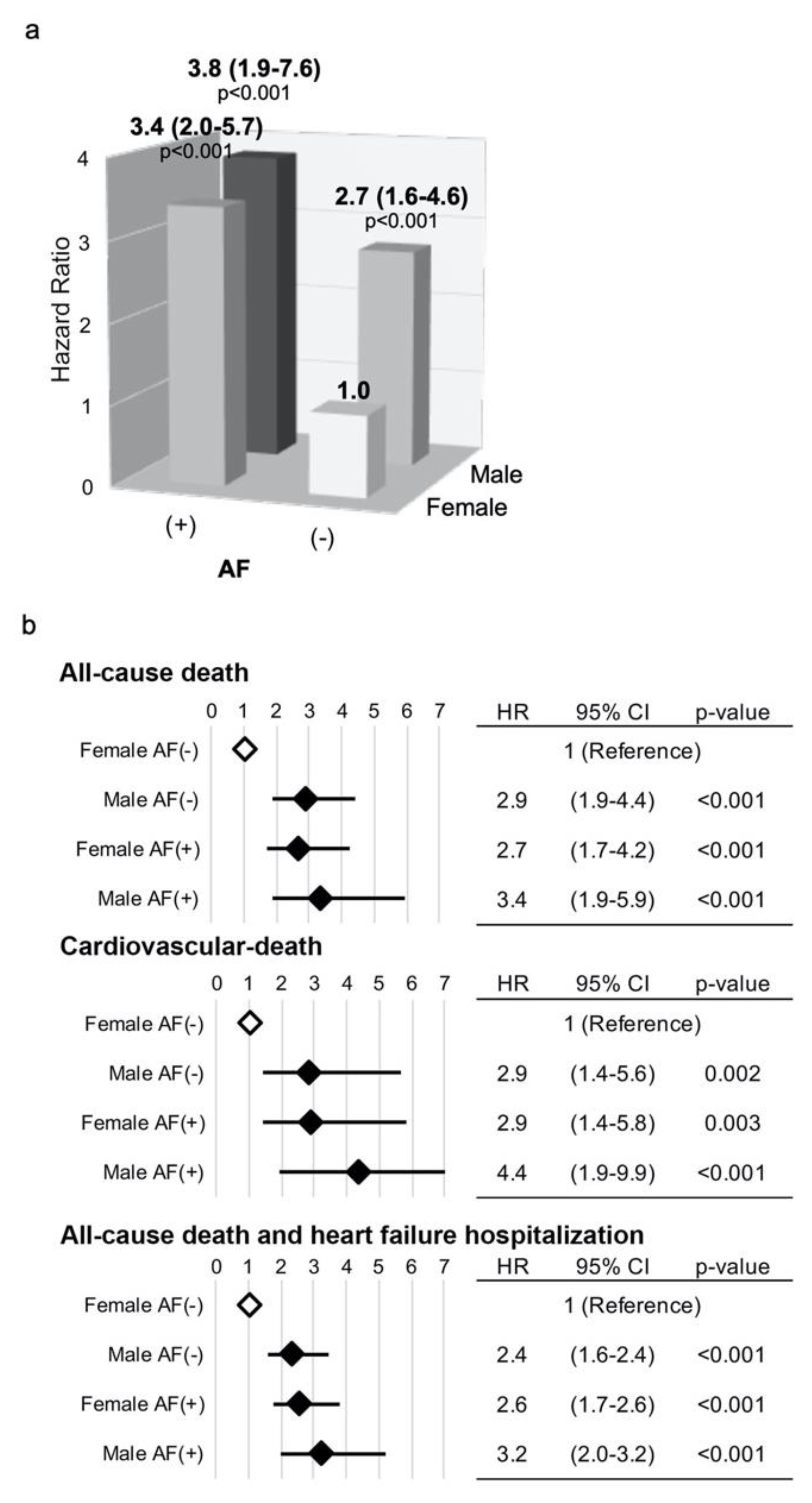
3.3. Simultaneous Risk Assessment of Gender Male and AF Following TAVI for All-Cause and CV Mortalities and the Composite of All-Cause Death with HF Hospitalization
4. Discussions
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Edwards, F.H.; Cohen, D.J.; O’Brien, S.M.; Peterson, E.D.; Mack, M.J.; Shahian, D.M.; Grover, F.L.; Tuzcu, E.M.; Thourani, V.H.; Carroll, J.; et al. Development and validation of a risk prediction model for in-hospital mortality after transcatheter aortic valve replacement. JAMA Cardiol. 2016, 1, 46–52. [Google Scholar] [CrossRef]
- Roques, F.; Michel, P.; Goldstone, A.; Nashef, S. The logistic EuroSCORE. Eur. Hear. J. 2003, 24, 882–883. [Google Scholar] [CrossRef]
- Nashef, S.A.M.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardio-Thorac. Surg. 2012, 41, 734–745. [Google Scholar] [CrossRef]
- O’Brien, S.M.; Shahian, D.M.; Filardo, G.; Ferraris, V.A.; Haan, C.K.; Rich, J.B.; Normand, S.-L.T.; Delong, E.R.; Shewan, C.M.; Dokholyan, R.S.; et al. The society of thoracic surgeons 2008 cardiac surgery risk models: Part 2–isolated valve surgery. Ann. Thorac. Surg. 2009, 88, S23–S42. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Liao, Y.B.; Peng, Y.; Chai, H.; Liu, W.; Li, Q.; Ren, X.; Wang, X.-Q.; Luo, X.-L.; Zhang, C.; et al. Sex-related differences in outcomes after transcatheter aortic valve implantation: A systematic review and meta-analysis. Circ. Cardiovasc. Interv. 2013, 6, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Hart, C.L.; Hole, D.J.; McMurray, J.J. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am. J. Med. 2002, 113, 359–364. [Google Scholar] [CrossRef]
- Tarantini, G.; Mojoli, M.; Urena, M.; Vahanian, A. Atrial fibrillation in patients undergoing transcatheter aortic valve implantation: Epidemiology, timing, predictors, and outcome. Eur. Hear. J. 2017, 38, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, C.; Niiranen, T.J.; Ojeda, F.M.; Gianfagna, F.; Blankenberg, S.; Njølstad, I.; Vartiainen, E.; Sans, S.; Pasterkamp, G.; Hughes, M.; et al. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts. Results From the BiomarCaRE Consortium. Circulation 2017, 136, 1588–1597. [Google Scholar] [CrossRef]
- Nguyen, V.; Michel, M.; Eltchaninoff, H.; Gilard, M.; Dindorf, C.; Iung, B.; Mossialos, E.; Cribier, A.; Vahanian, A.; Chevreul, K.; et al. Implementation of transcatheter aortic valve replacement in France. J. Am. Coll. Cardiol. 2018, 71, 1614–1627. [Google Scholar] [CrossRef]
- Didier, R.; Eltchaninoff, H.; Donzeau-Gouge, P.; Chevreul, K.; Fajadet, J.; Leprince, P.; Leguerrier, A.; Lièvre, M.; Prat, A.; Teiger, E.; et al. Five-Year clinical outcome and valve durability after transcatheter aortic valve replacement in high-risk patients. Circulation 2018, 138, 2597–2607. [Google Scholar] [CrossRef]
- Tamburino, C.; Capodanno, D.; Ramondo, A.; Petronio, A.S.; Ettori, F.; Santoro, G.; Klugmann, S.; Bedogni, F.; Maisano, F.; Marzocchi, A.; et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation 2011, 123, 299–308. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Mack, M.J.; Kaul, S.; Agnihotri, A.; Alexander, K.P.; Bailey, S.R.; Calhoon, J.H.; Carabello, B.A.; Desai, M.Y.; Edwards, F.H.; et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2012, 59, 1200–1254. [Google Scholar] [CrossRef]
- Saji, M.; Tobaru, T.; Higuchi, R.; Mahara, K.; Takamisawa, I.; Iguchi, N.; Doi, S.; Okazaki, S.; Tamura, H.; Takanashi, S.; et al. Usefulness of the transcatheter aortic valve replacement risk score to determine mid-term outcomes. Circ. J. 2019, 83, 1755–1761. [Google Scholar] [CrossRef]
- Humphries, K.H.; Toggweiler, S.; Rodés-Cabau, J.; Nombela-Franco, L.; Dumont, E.; Wood, D.A.; Willson, A.B.; Binder, R.K.; Freeman, M.; Lee, M.K.; et al. Sex differences in mortality after transcatheter aortic valve replacement for severe aortic stenosis. J. Am. Coll. Cardiol. 2012, 60, 882–886. [Google Scholar] [CrossRef]
- Hayashida, K.; Morice, M.-C.; Chevalier, B.; Hovasse, T.; Romano, M.; Garot, P.; Farge, A.; Donzeau-Gouge, P.; Bouvier, E.; Cormier, B.; et al. Sex-Related differences in clinical presentation and outcome of transcatheter aortic valve implantation for severe aortic stenosis. J. Am. Coll. Cardiol. 2012, 59, 566–571. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, F.; Gonella, A.; Moretti, C.; Omedé, P.; Salizzoni, S.; La Torre, M.; Giordana, F.; Barbanti, M.; Ussia, G.P.; Brambilla, N.; et al. Gender differences in patients undergoing TAVI: A multicentre study. EuroIntervention 2013, 9, 367–372. [Google Scholar] [CrossRef]
- Al-Lamee, R.; Broyd, C.; Parker, J.; Davies, J.E.; Mayet, J.; Sutaria, N.; Ariff, B.; Unsworth, B.; Cousins, J.; Bicknell, C.; et al. Influence of gender on clinical outcomes following transcatheter aortic valve implantation from the UK Transcatheter Aortic Valve Implantation Registry and the National Institute for Cardiovascular Outcomes Research. Am. J. Cardiol. 2014, 113, 522–528. [Google Scholar] [CrossRef]
- O’Connor, S.A.; Morice, M.C.; Gilard, M.; Leon, M.B.; Webb, J.G.; Dvir, D.; Rodés-Cabau, J.; Tamburino, C.; Capodanno, D.; D’Ascenzo, F.; et al. Revisiting sex equality with transcatheter aortic valve replacement outcomes: A collaborative; patient-level meta-analysis of 11,310 patients. J. Am. Coll. Cardiol. 2015, 66, 221–228. [Google Scholar]
- Chandrasekhar, J.; Dangas, G.; Yu, J.; Vemulapalli, S.; Suchindran, S.; Vora, A.N.; Baber, U.; Mehran, R.; STS/ACC TVT Registry. Sex-Based differences in outcomes with transcatheter aortic valve therapy: TVT registry from 2011 to 2014. J. Am. Coll. Cardiol. 2016, 68, 2733–2744. [Google Scholar] [CrossRef]
- Greve, A.M.; Gerdts, E.; Boman, K.K.; Gohlke-Baerwolf, C.; Rossebø, A.B.; Nienaber, C.A.; Ray, S.; Egstrup, K.; Pedersen, T.R.; Køber, L.; et al. Prognostic importance of atrial fibrillation in asymptomatic aortic stenosis: The simvastatin and ezetimibe in aortic stenosis study. Int. J. Cardiol. 2013, 166, 72–76. [Google Scholar] [CrossRef]
- Chopard, R.; Teiger, E.; Meneveau, N.; Chocron, S.; Gilard, M.; Laskar, M.; Eltchaninoff, H.; Iung, B.; Leprince, P.; Chevreul, K.; et al. Baseline characteristics and prognostic implications of pre-existing and new-onset atrial fibrillation after transcatheter aortic valve implantation: Results from the FRANCE-2 Registry. JACC Cardiovasc. Interv. 2015, 8, 1346–1355. [Google Scholar] [CrossRef]
- Grover, F.L.; Vemulapalli, S.; Carroll, J.D.; Edwards, F.H.; Mack, M.J.; Thourani, V.H.; Brindis, R.G.; Shahian, D.M.; Ruiz, C.E.; Jacobs, J.P.; et al. 2016 Annual Report of The Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J. Am. Coll. Cardiol. 2017, 69, 1215–1230. [Google Scholar] [CrossRef]
- Auffret, V.; Lefevre, T.; Van Belle, E.; Eltchaninoff, H.; Iung, B.; Koning, R.; Motreff, P.; Leprince, P.; Verhoye, J.P.; Manigold, T.; et al. Temporal trends in transcatheter aortic valve replacement in France: FRANCE 2 to FRANCE TAVI. J. Am. Coll. Cardiol. 2017, 70, 42–55. [Google Scholar] [CrossRef]
- Dahl, J.S.; Videbaek, L.; Poulsen, M.K.; Pellikka, P.A.; Veien, K.; Andersen, L.I.; Haghfelt, T.; Møller, J.E. Noninvasive assessment of filling pressure and left atrial pressure overload in severe aortic valve stenosis: Relation to ventricular remodeling and clinical outcome after aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2011, 142, e77–e83. [Google Scholar] [CrossRef]
- Levy, F.; Tribouilloy, C. Letter by Levy and Tribouilloy Regarding Article, “Atrial fibrillation is associated with increased mortality in patients undergoing transcatheter aortic valve replacement: Insights from the placement of aortic transcatheter valve (PARTNER) trial”. Circ. Cardiovasc. Interv. 2016, 9, e003705. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.G.; Deyell, M.W.; Lee, A.Y.K.; Macle, L. Sex differences in atrial fibrillation. Can. J. Cardiol. 2018, 34, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Ben Zadok, O.I.; Kornowski, R.; Finkelstein, A.; Barbash, I.; Danenberg, H.; Segev, A.; Guetta, V.; Halkin, A.; Vaknin, H.; Planer, D.; et al. Temporal trends in gender-related differences and outcomes in patients who underwent transcatheter aortic valve implantation (from the Israeli Transcatheter Aortic Valve Implantation Multicenter Registry). Am. J. Cardiol. 2019, 123, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Yoshijima, N.; Yanagisawa, R.; Hase, H.; Tanaka, M.; Tsuruta, H.; Shimizu, H.; Fukuda, K.; Naganuma, F.F.M.T.; Mizutani, K.; Araki, M.; et al. Update on the clinical impact of mild aortic regurgitation after transcatheter aortic valve implantation: Insights from the Japanese multicenter OCEAN-TAVI Registry. Catheter. Cardiovasc. Interv. 2020, 95, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.W.; Kim, W.-J.; Ahn, J.-M.; Kook, H.; Kang, S.H.; Han, J.-K.; Ko, Y.-G.; Choi, S.-H.; Koo, B.-K.; Chang, K.; et al. Trends and outcomes of transcatheter aortic valve implantation (TAVI) in Korea: The results of the first cohort of Korean TAVI Registry. Korean Circ. J. 2018, 48, 382–394. [Google Scholar] [CrossRef] [PubMed]

| Overall | FemaleAF (-) | Male AF (−) | FemaleAF (+) | Male AF (+) | p-Value | |
|---|---|---|---|---|---|---|
| n = 1088 | n = 559, 51.4% | n = 266, 24.4% | n = 187, 17.2% | n = 76, 7.0% | ||
| Age, years | 84.0 ± 5.5 | 84.1 ± 5.2 | 83.0 ± 6.4 | 85.2 ± 4.9 | 84.2 ± 5.8 | p < 0.001 |
| BMI, kg/m2 | 22.3 ± 3.7 | 22.4 ± 3.9 | 22.4 ± 3.0 | 22.1 ± 4.3 | 22.4 ± 3.1 | p = 0.78 |
| NYHA class, III or IV, n | 561 (51.7%) | 270 (48.3%) | 133 (50.0%) | 112 (59.9%) | 46 (60.5%) | p = 0.018 |
| Logistic EuroSCORE, % | 12.8 (9.5, 19.0) | 12.8 (9.5, 17.7) | 11.4 (7.6, 19.3) | 16.1 (12.0, 22.4) | 13.6 (8.7, 26.1) | p < 0.001 |
| EuroSCORE II, % | 4.5 (2.8, 7.0) | 4.5 (2.8, 6.3) | 3.5 (2.3, 6.5) | 5.3 (3.5, 8.5) | 5.6 (2.5, 10.0) | p < 0.001 |
| STS-PROM score, % | 5.7 (3.8, 8.3) | 5.6 (3.9, 7.7) | 4.9 (3.3, 7.3) | 7.2 (5.4, 10.3) | 6.0 (3.7, 9.3) | p < 0.001 |
| Comorbidities | ||||||
| History of heart failure, n | 320 (29.4%) | 132 (23.6%) | 65 (24.4%) | 85 (45.5%) | 38 (50.0%) | p < 0.001 |
| Hypertension, n | 841 (77.3%) | 445 (79.6%) | 201 (75.6%) | 140 (74.9%) | 55 (72.4%) | p = 0.28 |
| Diabetes mellitus, n | 262 (24.1%) | 117 (20.9%) | 83 (31.2%) | 41 (21.9%) | 21 (27.6%) | p = 0.0097 |
| Cancer, n | 207 (19.0%) | 96 (17.2%) | 59 (22.2%) | 36 (19.3%) | 16 (21.1%) | p = 0.37 |
| History of stroke, n | 124 (11.4%) | 49 (8.8%) | 35 (13.2%) | 26 (13.9%) | 14 (18.4%) | p = 0.023 |
| COPD, n | 112 (10.4%) | 41 (7.4%) | 41 (15.6%) | 22 (11.9%) | 8 (10.5%) | p = 0.0037 |
| CKD (stage 3 or more), n | 713 (65.5%) | 353 (63.2%) | 160 (60.2%) | 144 (77.0%) | 56 (73.7%) | p < 0.001 |
| PAD, n | 179 (16.5%) | 75 (13.4%) | 53 (19.9%) | 37 (19.8%) | 14 (18.4%) | p = 0.052 |
| OMI, n | 65 (6.0%) | 20 (3.6%) | 31 (11.7%) | 4 (2.1%) | 10 (13.2%) | p < 0.001 |
| History of coronary revascularization *, n | 240 (22.1%) | 94 (16.8%) | 97 (36.5%) | 28 (15.0%) | 21 (27.6%) | p < 0.001 |
| p-PTAV, n | 35 (3.2%) | 20 (3.6%) | 6 (2.3%) | 9 (4.8%) | 0 (0.0%) | p = 0.17 |
| Laboratory data | ||||||
| NT-proBNP, pg/mL | 1154 (479, 3098) | 868 (386, 2510) | 1025 (409, 2563) | 2173 (1111, 5141) | 1784 (951, 5114) | p < 0.001 |
| Creatinine, mg/dL | 0.9 ± 0.4 | 0.8 ± 0.3 | 1.1 ± 0.5 | 0.9 ± 0.4 | 1.1 ± 0.3 | p < 0.001 |
| eGFR, ml/min | 54.2 ± 18.9 | 55.4 ± 19.0 | 56.0 ± 19.4 | 49.3 ± 18.3 | 50.8 ± 15.6 | p < 0.001 |
| Hemoglobin, g/dL | 11.6 ± 1.6 | 11.3 ± 1.4 | 12.1 ± 1.6 | 11.4 ± 1.5 | 12.1 ± 1.8 | p < 0.001 |
| Albumin, g/dL | 3.7 ± 0.4 | 3.8 ± 0.4 | 3.7 ± 0.4 | 3.7 ± 0.4 | 3.7 ± 0.5 | p = 0.018 |
| Echocardiographic findings | ||||||
| LVEF, % | 60.7 ± 10.8 | 62.6 ± 9.7 | 58.8 ± 11.6 | 59.8 ± 10.5 | 55.0 ± 13.1 | p < 0.001 |
| AVA, cm2 | 0.66 ± 0.17 | 0.66 ± 0.17 | 0.70 ± 0.16 | 0.62 ± 0.18 | 0.68 ± 0.20 | p < 0.001 |
| Peak gradient, mmHg | 89.2 ± 31.5 | 92.2 ± 31.3 | 89.0 ± 30.2 | 85.5 ± 33.0 | 77.0 ± 29.8 | p < 0.001 |
| Mean gradient, mmHg | 51.2 ± 19.1 | 53.0 ± 19.6 | 51.0 ± 16.8 | 48.8 ± 20.3 | 43.8 ± 17.9 | p < 0.001 |
| AR ≥ moderate, n | 56 (5.1%) | 18 (3.2%) | 20 (7.5%) | 12 (6.4%) | 6 (7.9%) | p = 0.029 |
| MR ≥ moderate, n | 57 (5.2%) | 21 (3.8%) | 11 (4.1%) | 18 (9.6%) | 7 (9.2%) | p = 0.0045 |
| TR ≥ moderate, n | 57 (5.2%) | 8 (1.4%) | 5 (1.9%) | 33 (17.6%) | 11 (14.5%) | p < 0.001 |
| Medications | ||||||
| Beta-blockers | 382 (35.1%) | 160 (28.6%) | 81 (30.5%) | 99 (52.9%) | 42 (55.3%) | p < 0.001 |
| ACEIs/ARBs | 596 (54.8%) | 320 (57.3%) | 140 (52.6%) | 90 (48.1%) | 46 (60.5%) | p = 0.11 |
| Statins | 571 (52.5%) | 286 (51.2%) | 149 (56.0%) | 94 (50.3%) | 42 (55.3%) | p = 0.48 |
| Diuretics | 516 (47.4%) | 227 (40.6%) | 115 (43.2%) | 117 (62.6%) | 57 (75.0%) | p < 0.001 |
| Oral anticoagulants | 261 (24.0%) | 27 (4.8%) | 15 (5.6%) | 162 (86.6%) | 57 (75.0%) | p < 0.001 |
| Procedural variables | ||||||
| Procedure time, min | 73 (60, 100) | 74 (60, 102) | 73 (60, 97) | 72 (59, 100) | 73 (58, 101) | p = 0.95 |
| Fluoroscopy time, min | 20 (16, 27) | 21 (16, 27) | 20 (16, 27) | 20 (16, 26) | 20 (17, 28) | p = 0.88 |
| Contrast medium volume, ml | 61 (45, 96) | 63 (46, 98) | 60 (45, 94) | 61 (43, 95) | 55 (42, 86) | p = 0.30 |
| Approach plan | ||||||
| Conscious sedation, n | 637 (58.6%) | 336 (60.1%) | 157 (59.0%) | 101 (54.0%) | 43 (56.6%) | p = 0.51 |
| Transfemoral approach, n | 993 (91.3%) | 517 (92.5%) | 242 (91.0%) | 166 (88.8%) | 68 (89.5%) | p = 0.58 |
| Valve size, mm | 24.8 ± 2.3 | 24.1 ± 2.1 | 26.1 ± 2.3 | 24.2 ± 2.1 | 26.2 ± 2.3 | p < 0.001 |
| Valve type | ||||||
| Edwards SAPIEN-XT, n | 171 (15.7%) | 92 (16.5%) | 33 (12.4%) | 32 (17.1%) | 14 (18.4%) | p = 0.38 |
| Edwards SAPIEN3, n | 543 (49.9%) | 275 (49.2%) | 150 (56.4%) | 84 (44.9%) | 34 (44.7%) | p = 0.052 |
| Medtronic CoreValve, n | 29 (2.7%) | 14 (2.5%) | 11 (4.1%) | 3 (1.6%) | 1 (1.3%) | p = 0.30 |
| Medtronic Evolut R, n | 164 (15.1%) | 88 (15.7%) | 27 (10.2%) | 40 (21.4%) | 9 (11.8%) | p = 0.0072 |
| Medtronic Evolut PRO, n | 128 (11.8%) | 66 (11.8%) | 30 (11.3%) | 17 (9.1%) | 15 (19.7%) | p = 0.13 |
| Boston Scientific LOTUS, n | 11 (1.0%) | 5 (0.9%) | 3 (1.1%) | 2 (1.1%) | 1 (1.3%) | p = 0.98 |
| Balloon expandable, n | 714 (65.6%) | 367 (65.7%) | 183 (68.8%) | 116 (62.0%) | 48 (63.2%) | p = 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chikata, Y.; Iwata, H.; Doi, S.; Funamizu, T.; Okazaki, S.; Dohi, S.; Higuchi, R.; Saji, M.; Takamisawa, I.; Tamura, H.; et al. Simultaneous Estimation of Gender Male and Atrial Fibrillation as Risk Factors for Adverse Outcomes Following Transcatheter Aortic Valve Implantation. J. Clin. Med. 2020, 9, 3963. https://doi.org/10.3390/jcm9123963
Chikata Y, Iwata H, Doi S, Funamizu T, Okazaki S, Dohi S, Higuchi R, Saji M, Takamisawa I, Tamura H, et al. Simultaneous Estimation of Gender Male and Atrial Fibrillation as Risk Factors for Adverse Outcomes Following Transcatheter Aortic Valve Implantation. Journal of Clinical Medicine. 2020; 9(12):3963. https://doi.org/10.3390/jcm9123963
Chicago/Turabian StyleChikata, Yuichi, Hiroshi Iwata, Shinichiro Doi, Takehiro Funamizu, Shinya Okazaki, Shizuyuki Dohi, Ryosuke Higuchi, Mike Saji, Itaru Takamisawa, Harutoshi Tamura, and et al. 2020. "Simultaneous Estimation of Gender Male and Atrial Fibrillation as Risk Factors for Adverse Outcomes Following Transcatheter Aortic Valve Implantation" Journal of Clinical Medicine 9, no. 12: 3963. https://doi.org/10.3390/jcm9123963
APA StyleChikata, Y., Iwata, H., Doi, S., Funamizu, T., Okazaki, S., Dohi, S., Higuchi, R., Saji, M., Takamisawa, I., Tamura, H., Amano, A., Daida, H., & Minamino, T. (2020). Simultaneous Estimation of Gender Male and Atrial Fibrillation as Risk Factors for Adverse Outcomes Following Transcatheter Aortic Valve Implantation. Journal of Clinical Medicine, 9(12), 3963. https://doi.org/10.3390/jcm9123963





