When Should We Perform Endoscopic Drainage and Necrosectomy for Walled-Off Necrosis?
Abstract
1. Introduction
2. Evolution of Pancreatic Fluid Collection
3. Treatment of Walled-Off Necrosis
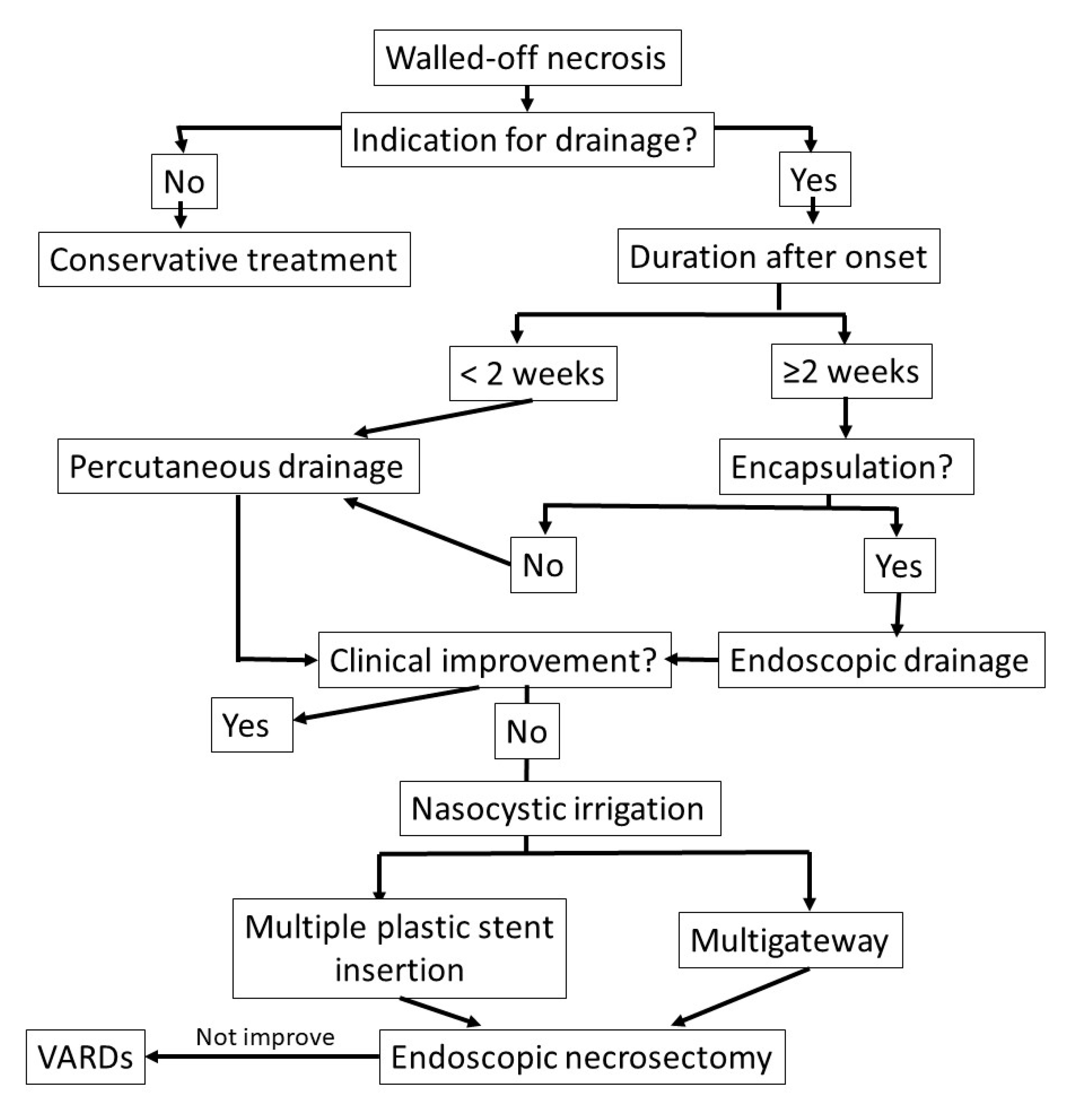
3.1. Indications for Walled-Off Necrosis Drainage
3.2. Timing for Treatment of Walled-Off Necrosis
3.3. Step-Up Approach
3.3.1. Endoscopic Step-Up Approach
3.3.2. Percutaneous and Surgical Drainage with Step-Up Approach
4. Endoscopic Drainage
4.1. SEMS as an Adjunctive Strategy to Improve Endoscopic Drainage
4.2. Endoscopic Necrosectomy
4.2.1. Technical Aspects of Endoscopic Necrosectomy
4.2.2. Timing of Endoscopic Necrosectomy
4.2.3. Adjunctive Techniques for Endoscopic Necrosectomy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S.; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis-2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Bollen, T.L.; Wu, B.U.; Repas, K.; Maurer, R.; Yu, S.; Mortele, K.J.; Conwell, D.L.; Banks, P.A. An assessment of the severity of interstitial pancreatitis. Clin. Gastroenterol. Hepatol. 2011, 9, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Van Brunschot, S.; Bakker, O.J.; Besselink, M.G.; Bollen, T.L.; Fockens, P.; Gooszen, H.G.; van Santvoort, H.C.; Dutch Pancreatitis Study, G. Treatment of necrotizing pancreatitis. Clin. Gastroenterol. Hepatol. 2012, 10, 1190–1201. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, M.; Dumonceau, J.M.; Albert, J.; Badaoui, A.; Bali, M.A.; Barthet, M.; Besselink, M.; Deviere, J.; Oliveira Ferreira, A.; Gyokeres, T.; et al. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy 2018, 50, 524–546. [Google Scholar] [CrossRef]
- Baron, T.H.; DiMaio, C.J.; Wang, A.Y.; Morgan, K.A. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology 2020, 158, 67–75. [Google Scholar] [CrossRef]
- Windsor, J.A. Infected pancreatic necrosis: Drain first, but do it better. HPB 2011, 13, 367–368. [Google Scholar] [CrossRef]
- Muthusamy, V.R.; Chandrasekhara, V.; Acosta, R.D.; Bruining, D.H.; Chathadi, K.V.; Eloubeidi, M.A.; Faulx, A.L.; Fonkalsrud, L.; Gurudu, S.R.; Yang, J.; et al. The role of endoscopy in the diagnosis and treatment of inflammatory pancreatic fluid collections. Gastrointest. Endosc. 2016, 83, 481–488. [Google Scholar] [CrossRef]
- Mier, J.; Leon, E.L.; Castillo, A.; Robledo, F.; Blanco, R. Early versus late necrosectomy in severe necrotizing pancreatitis. Am. J. Surg. 1997, 173, 71–75. [Google Scholar] [CrossRef]
- Van Grinsven, J.; van Santvoort, H.C.; Boermeester, M.A.; Dejong, C.H.; van Eijck, C.H.; Fockens, P.; Besselink, M.G.; Dutch Pancreatitis Study, G. Timing of catheter drainage in infected necrotizing pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 306–312. [Google Scholar] [CrossRef]
- Van Santvoort, H.C.; Bakker, O.J.; Bollen, T.L.; Besselink, M.G.; Ahmed Ali, U.; Schrijver, A.M.; Boermeester, M.A.; van Goor, H.; Dejong, C.H.; van Eijck, C.H.; et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology 2011, 141, 1254–1263. [Google Scholar] [CrossRef]
- Van Santvoort, H.C.; Besselink, M.G.; Bakker, O.J.; Hofker, H.S.; Boermeester, M.A.; Dejong, C.H.; van Goor, H.; Schaapherder, A.F.; van Eijck, C.H.; Bollen, T.L.; et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N. Engl. J. Med. 2010, 362, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Isayama, H.; Nakai, Y.; Rerknimitr, R.; Khor, C.; Lau, J.; Wang, H.P.; Seo, D.W.; Ratanachu-Ek, T.; Lakhtakia, S.; Ang, T.L.; et al. Asian consensus statements on endoscopic management of walled-off necrosis Part 1: Epidemiology, diagnosis, and treatment. J. Gastroenterol. Hepatol. 2016, 31, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Baron, T.H.; Kozarek, R.A. Endotherapy for organized pancreatic necrosis: Perspectives after 20 years. Clin. Gastroenterol. Hepatol. 2012, 10, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.S.; Gupta, R.; Kang, M.; Sharma, V.; Sharma, R.; Gorsi, U.; Bhasin, D.K. Percutaneous catheter drainage followed by endoscopic transluminal drainage/necrosectomy for treatment of infected pancreatic necrosis in early phase of illness. Endosc. Ultrasound 2018, 7, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Varadarajulu, S.; Phadnis, M.A.; Christein, J.D.; Wilcox, C.M. Multiple transluminal gateway technique for EUS-guided drainage of symptomatic walled-off pancreatic necrosis. Gastrointest. Endosc. 2011, 74, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Dabizzi, E.; Anderloni, A.; Cennamo, V.; Fiscaletti, M.; Fugazza, A.; Jovine, E.; Ercolani, G.; Gasbarrini, A.; Fabbri, C. Single-step endoscopic ultrasound-guided multiple gateway drainage of complex walled-off necrosis with lumen apposing metal stents. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1401–1404. [Google Scholar] [CrossRef]
- Mukai, S.; Itoi, T.; Sofuni, A.; Itokawa, F.; Kurihara, T.; Tsuchiya, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; Tanaka, R.; et al. Expanding endoscopic interventions for pancreatic pseudocyst and walled-off necrosis. J. Gastroenterol. 2015, 50, 211–220. [Google Scholar] [CrossRef]
- Sugimoto, M.; Sonntag, D.P.; Flint, G.S.; Boyce, C.J.; Kirkham, J.C.; Harris, T.J.; Carr, S.M.; Nelson, B.D.; Bell, D.A.; Barton, J.G.; et al. Better Outcomes if Percutaneous Drainage Is Used Early and Proactively in the Course of Necrotizing Pancreatitis. J. Vasc. Interv. Radiol. 2016, 27, 418–425. [Google Scholar] [CrossRef]
- Van Baal, M.C.; van Santvoort, H.C.; Bollen, T.L.; Bakker, O.J.; Besselink, M.G.; Gooszen, H.G.; Dutch Pancreatitis Study, G. Systematic review of percutaneous catheter drainage as primary treatment for necrotizing pancreatitis. Br. J. Surg. 2011, 98, 18–27. [Google Scholar] [CrossRef]
- Mallick, B.; Dhaka, N.; Gupta, P.; Gulati, A.; Malik, S.; Sinha, S.K.; Yadav, T.D.; Gupta, V.; Kochhar, R. An audit of percutaneous drainage for acute necrotic collections and walled off necrosis in patients with acute pancreatitis. Pancreatology 2018, 18, 727–733. [Google Scholar] [CrossRef]
- Besselink, M.G.; Verwer, T.J.; Schoenmaeckers, E.J.; Buskens, E.; Ridwan, B.U.; Visser, M.R.; Nieuwenhuijs, V.B.; Gooszen, H.G. Timing of surgical intervention in necrotizing pancreatitis. Arch. Surg. 2007, 142, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.H.; Cicurel, N.J.; Seed, R.W. Transgastric needle aspiration of pancreatic pseudocyst through an endoscope. Gastrointest. Endosc. 1975, 21, 133–134. [Google Scholar] [CrossRef]
- Yip, H.C.; Teoh, A.Y.B. Endoscopic Management of Peri-Pancreatic Fluid Collections. Gut Liver 2017, 11, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Trikudanathan, G.; Tawfik, P.; Amateau, S.K.; Munigala, S.; Arain, M.; Attam, R.; Beilman, G.; Flanagan, S.; Freeman, M.L.; Mallery, S. Early (<4 Weeks) Versus Standard (>/= 4 Weeks) Endoscopically Centered Step-Up Interventions for Necrotizing Pancreatitis. Am. J. Gastroenterol. 2018, 113, 1550–1558. [Google Scholar] [CrossRef]
- Chantarojanasiri, T.; Yamamoto, N.; Nakai, Y.; Saito, T.; Saito, K.; Hakuta, R.; Ishigaki, K.; Takeda, T.; Uchino, R.; Takahara, N.; et al. Comparison of early and delayed EUS-guided drainage of pancreatic fluid collection. Endosc. Int. Open 2018, 6, E1398–E1405. [Google Scholar] [CrossRef]
- Hritz, I.; Fejes, R.; Szekely, A.; Szekely, I.; Horvath, L.; Sarkany, A.; Altorjay, A.; Madacsy, L. Endoscopic transluminal pancreatic necrosectomy using a self-expanding metal stent and high-flow water-jet system. World J. Gastroenterol. 2013, 19, 3685–3692. [Google Scholar] [CrossRef]
- Belle, S.; Collet, P.; Post, S.; Kaehler, G. Temporary cystogastrostomy with self-expanding metallic stents for pancreatic necrosis. Endoscopy 2010, 42, 493–495. [Google Scholar] [CrossRef]
- Attam, R.; Trikudanathan, G.; Arain, M.; Nemoto, Y.; Glessing, B.; Mallery, S.; Freeman, M.L. Endoscopic transluminal drainage and necrosectomy by using a novel, through-the-scope, fully covered, large-bore esophageal metal stent: Preliminary experience in 10 patients. Gastrointest. Endosc. 2014, 80, 312–318. [Google Scholar] [CrossRef]
- Tellez-Avila, F.I.; Villalobos-Garita, A.; Ramirez-Luna, M.A. Use of a novel covered self-expandable metal stent with an anti-migration system for endoscopic ultrasound-guided drainage of a pseudocyst. World J. Gastrointest. Endosc. 2013, 5, 297–299. [Google Scholar] [CrossRef]
- Teoh, A.Y.; Ng, E.K.; Chan, S.M.; Lai, M.; Moran, S.; Binmoeller, K.F.; Moon, J.H.; Ho, K.Y. Ex vivo comparison of the lumen-apposing properties of EUS-specific stents (with video). Gastrointest. Endosc. 2016, 84, 62–68. [Google Scholar] [CrossRef]
- Bank, J.; Adler, D. Lumen apposing metal stents: A review of current uses and outcomes. Gastrointest. Interv. 2017, 6, 9–14. [Google Scholar] [CrossRef]
- Braden, B.; Koutsoumpas, A.; Silva, M.A.; Soonawalla, Z.; Dietrich, C.F. Endoscopic ultrasound-guided drainage of pancreatic walled-off necrosis using self-expanding metal stents without fluoroscopy. World J. Gastrointest. Endosc. 2018, 10, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Weilert, F.; Binmoeller, K.F. Specially designed stents for translumenal drainage. Gastrointest. Interv. 2015, 4, 40–45. [Google Scholar] [CrossRef]
- Anderloni, A.; Fabbri, C.; Nieto, J.; Uwe, W.; Dollhopf, M.; Aparicio, J.R.; Perez-Miranda, M.; Tarantino, I.; Arlt, A.; Vleggaar, F.; et al. The safety and efficacy of a new 20 mm lumen apposing metal stent (lams) for the endoscopic treatment of pancreatic and peripancreatic fluid collections: A large international, multicenter study. Surg. Endosc. 2020. [Google Scholar] [CrossRef]
- Adler, D.G.; Taylor, L.J.; Hasan, R.; Siddiqui, A.A. A retrospective study evaluating endoscopic ultrasound-guided drainage of pancreatic fluid collections using a novel lumen-apposing metal stent on an electrocautery enhanced delivery system. Endosc. Ultrasound 2017, 6, 389–393. [Google Scholar] [CrossRef]
- Weigand, K.; Mehrl, A.; Goessmann, H.; Mueller, M.; Kandulski, A. Endoscopic Necrosectomy of Walled-Off Necrosis following Severe Pancreatitis Using a Hot AxiosTM Stent-A Case Series. Dig. Dis. 2019, 1–4. [Google Scholar] [CrossRef]
- Bang, J.Y.; Hasan, M.; Navaneethan, U.; Hawes, R.; Varadarajulu, S. Lumen-apposing metal stents (LAMS) for pancreatic fluid collection (PFC) drainage: May not be business as usual. Gut 2017, 66, 2054–2056. [Google Scholar] [CrossRef]
- Ge, P.S.; Young, J.Y.; Jirapinyo, P.; Dong, W.; Ryou, M.; Thompson, C.C. Comparative Study Evaluating Lumen Apposing Metal Stents Versus Double Pigtail Plastic Stents for Treatment of Walled-Off Necrosis. Pancreas 2020, 49, 236–241. [Google Scholar] [CrossRef]
- Bang, J.Y.; Navaneethan, U.; Hasan, M.K.; Sutton, B.; Hawes, R.; Varadarajulu, S. Non-superiority of lumen-apposing metal stents over plastic stents for drainage of walled-off necrosis in a randomised trial. Gut 2019, 68, 1200–1209. [Google Scholar] [CrossRef]
- Mohan, B.P.; Jayaraj, M.; Asokkumar, R.; Shakhatreh, M.; Pahal, P.; Ponnada, S.; Navaneethan, U.; Adler, D.G. Lumen apposing metal stents in drainage of pancreatic walled-off necrosis, are they any better than plastic stents? A systematic review and meta-analysis of studies published since the revised Atlanta classification of pancreatic fluid collections. Endosc. Ultrasound 2019, 8, 82–90. [Google Scholar] [CrossRef]
- Chen, Y.I.; Yang, J.; Friedland, S.; Holmes, I.; Law, R.; Hosmer, A.; Stevens, T.; Franco, M.C.; Jang, S.; Pawa, R.; et al. Lumen apposing metal stents are superior to plastic stents in pancreatic walled-off necrosis: A large international multicenter study. Endosc. Int. Open 2019, 7, E347–E354. [Google Scholar] [CrossRef] [PubMed]
- Kayal, A.; Taghizadeh, N.; Ishikawa, T.; Gonzalez-Moreno, E.; Bass, S.; Cole, M.J.; Heitman, S.J.; Mohamed, R.; Turbide, C.; Chen, Y.I.; et al. Endosonography-guided transmural drainage of pancreatic fluid collections: Comparative outcomes by stent type. Surg. Endosc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.D.; Fritz, C.; Bhat, T.; Das, K.K.; Murad, F.M.; Early, D.S.; Edmundowicz, S.A.; Kushnir, V.M.; Mullady, D.K. EUS-guided drainage of peripancreatic fluid collections with lumen-apposing metal stents and plastic double-pigtail stents: Comparison of efficacy and adverse event rates. Gastrointest. Endosc. 2018, 87, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.S.; Sharma, R.; Dhalaria, L.; Gupta, R. Efficacy and safety of plastic versus lumen-apposing metal stents for transmural drainage of walled-off necrosis: A retrospective single-center study. Ann. Gastroenterol. 2020, 33, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Mukai, S.; Itoi, T.; Baron, T.H.; Sofuni, A.; Itokawa, F.; Kurihara, T.; Tsuchiya, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; et al. Endoscopic ultrasound-guided placement of plastic vs. biflanged metal stents for therapy of walled-off necrosis: A retrospective single-center series. Endoscopy 2015, 47, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ang, T.L.; Kongkam, P.; Kwek, A.B.; Orkoonsawat, P.; Rerknimitr, R.; Fock, K.M. A two-center comparative study of plastic and lumen-apposing large diameter self-expandable metallic stents in endoscopic ultrasound-guided drainage of pancreatic fluid collections. Endosc. Ultrasound 2016, 5, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Bapaye, A.; Dubale, N.A.; Sheth, K.A.; Bapaye, J.; Ramesh, J.; Gadhikar, H.; Mahajani, S.; Date, S.; Pujari, R.; Gaadhe, R. Endoscopic ultrasonography-guided transmural drainage of walled-off pancreatic necrosis: Comparison between a specially designed fully covered bi-flanged metal stent and multiple plastic stents. Dig. Endosc. 2017, 29, 104–110. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Kowalski, T.E.; Loren, D.E.; Khalid, A.; Soomro, A.; Mazhar, S.M.; Isby, L.; Kahaleh, M.; Karia, K.; Yoo, J.; et al. Fully covered self-expanding metal stents versus lumen-apposing fully covered self-expanding metal stent versus plastic stents for endoscopic drainage of pancreatic walled-off necrosis: Clinical outcomes and success. Gastrointest. Endosc. 2017, 85, 758–765. [Google Scholar] [CrossRef]
- Abu Dayyeh, B.K.; Mukewar, S.; Majumder, S.; Zaghlol, R.; Vargas Valls, E.J.; Bazerbachi, F.; Levy, M.J.; Baron, T.H.; Gostout, C.J.; Petersen, B.T.; et al. Large-caliber metal stents versus plastic stents for the management of pancreatic walled-off necrosis. Gastrointest. Endosc. 2018, 87, 141–149. [Google Scholar] [CrossRef]
- Law, S.T.; De La Serna Higuera, C.; Simon, P.G.; Castillo, M.P.-M. Comparison of clinical efficacies and safeties of lumen-apposing metal stent and conventional-type metal stent-assisted EUS-guided pancreatic wall-off necrosis drainage: A real-life experience in a tertiary hospital. Surg. Endosc. 2018, 32, 2448–2453. [Google Scholar] [CrossRef]
- Cho, I.R.; Chung, M.J.; Jo, J.H.; Lee, H.S.; Park, J.Y.; Bang, S.; Park, S.W.; Song, S.Y. A novel lumen-apposing metal stent with an anti-reflux valve for endoscopic ultrasound-guided drainage of pseudocysts and walled-off necrosis: A pilot study. PLoS ONE 2019, 14, e0221812. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xie, P.; Wang, Y.; Jin, Z.; Li, Z.; Du, Y. The role of solid debris in endoscopic ultrasound-guided drainage of walled-off necrosis: A large cohort study. J. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Parsa, N.; Nieto, J.M.; Powers, P.; Mitsuhashi, S.; Abdelqader, A.; Hadzinakos, G.; Anderloni, A.A.; Fugazza, A.; James, T.W.; Arlt, A.; et al. Endoscopic ultrasound-guided drainage of pancreatic walled-off necrosis using 20 mm versus 15 mm lumen-apposing metal stents: An international, multicenter, case-matched study. Endoscopy 2020, 52, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.C.; Kumar, N.; Slattery, J.; Clancy, T.E.; Ryan, M.B.; Ryou, M.; Swanson, R.S.; Banks, P.A.; Conwell, D.L. A standardized method for endoscopic necrosectomy improves complication and mortality rates. Pancreatology 2016, 16, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.B.; Chahal, P.; Papachristou, G.I.; Vege, S.S.; Petersen, B.T.; Gostout, C.J.; Topazian, M.D.; Takahashi, N.; Sarr, M.G.; Baron, T.H. A comparison of direct endoscopic necrosectomy with transmural endoscopic drainage for the treatment of walled-off pancreatic necrosis. Gastrointest. Endosc. 2009, 69, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Puri, R.; Choudhary, N.; Bhatia, S.; Patel, N.; Patle, S.; Patil, G.; Agarwal, A.; Prabha, C.; Sud, R. Endoscopic pancreatic necrosectomy: Why scuff when you can flush the muck-Make it an easy row to hoe. Endosc. Int. Open 2017, 5, E847–E853. [Google Scholar] [CrossRef]
- Isayama, H.; Nakai, Y.; Rerknimitr, R.; Khor, C.; Lau, J.; Wang, H.P.; Seo, D.W.; Ratanachu-Ek, T.; Lakhtakia, S.; Ang, T.L.; et al. Asian consensus statements on endoscopic management of walled-off necrosis. Part 2: Endoscopic management. J. Gastroenterol. Hepatol. 2016, 31, 1555–1565. [Google Scholar] [CrossRef]
- Yamamoto, N.; Isayama, H.; Takahara, N.; Sasahira, N.; Miyabayashi, K.; Mizuno, S.; Kawakubo, K.; Mohri, D.; Kogure, H.; Sasaki, T.; et al. Percutaneous direct-endoscopic necrosectomy for walled-off pancreatic necrosis. Endoscopy 2013, 45, E44–E45. [Google Scholar] [CrossRef]
- Ke, L.; Mao, W.; Zhou, J.; Ye, B.; Li, G.; Zhang, J.; Wang, P.; Tong, Z.; Windsor, J.; Li, W. Stent-Assisted Percutaneous Endoscopic Necrosectomy for Infected Pancreatic Necrosis: Technical Report and a Pilot Study. World J. Surg. 2019, 43, 1121–1128. [Google Scholar] [CrossRef]
- Yan, L.; Dargan, A.; Nieto, J.; Shariaha, R.Z.; Binmoeller, K.F.; Adler, D.G.; DeSimone, M.; Berzin, T.; Swahney, M.; Draganov, P.V.; et al. Direct endoscopic necrosectomy at the time of transmural stent placement results in earlier resolution of complex walled-off pancreatic necrosis: Results from a large multicenter United States trial. Endosc. Ultrasound 2019, 8, 172–179. [Google Scholar] [CrossRef]
- Powers, P.C.; Siddiqui, A.; Sharaiha, R.Z.; Yang, G.; Dawod, E.; Novikov, A.A.; Javia, A.; Edirisuriya, C.; Noor, A.; Mumtaz, T.; et al. Discontinuation of proton pump inhibitor use reduces the number of endoscopic procedures required for resolution of walled-off pancreatic necrosis. Endosc. Ultrasound 2019, 8, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Mukai, S.; Itoi, T.; Sofuni, A.; Itokawa, F.; Kurihara, T.; Tsuchiya, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; Tanaka, R.; et al. Novel single transluminal gateway transcystic multiple drainages after EUS-guided drainage for complicated multilocular walled-off necrosis (with videos). Gastrointest. Endosc. 2014, 79, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Fagenholz, P.J.; Thabet, A.; Mueller, P.R.; Forcione, D.G. Combined endoscopic trangastric drainage and video assisted retroperitoneal pancreatic debridement–The best of both worlds for extensive pancreatic necrosis with enteric fistulae. Pancreatology 2016, 16, 788–790. [Google Scholar] [CrossRef] [PubMed]
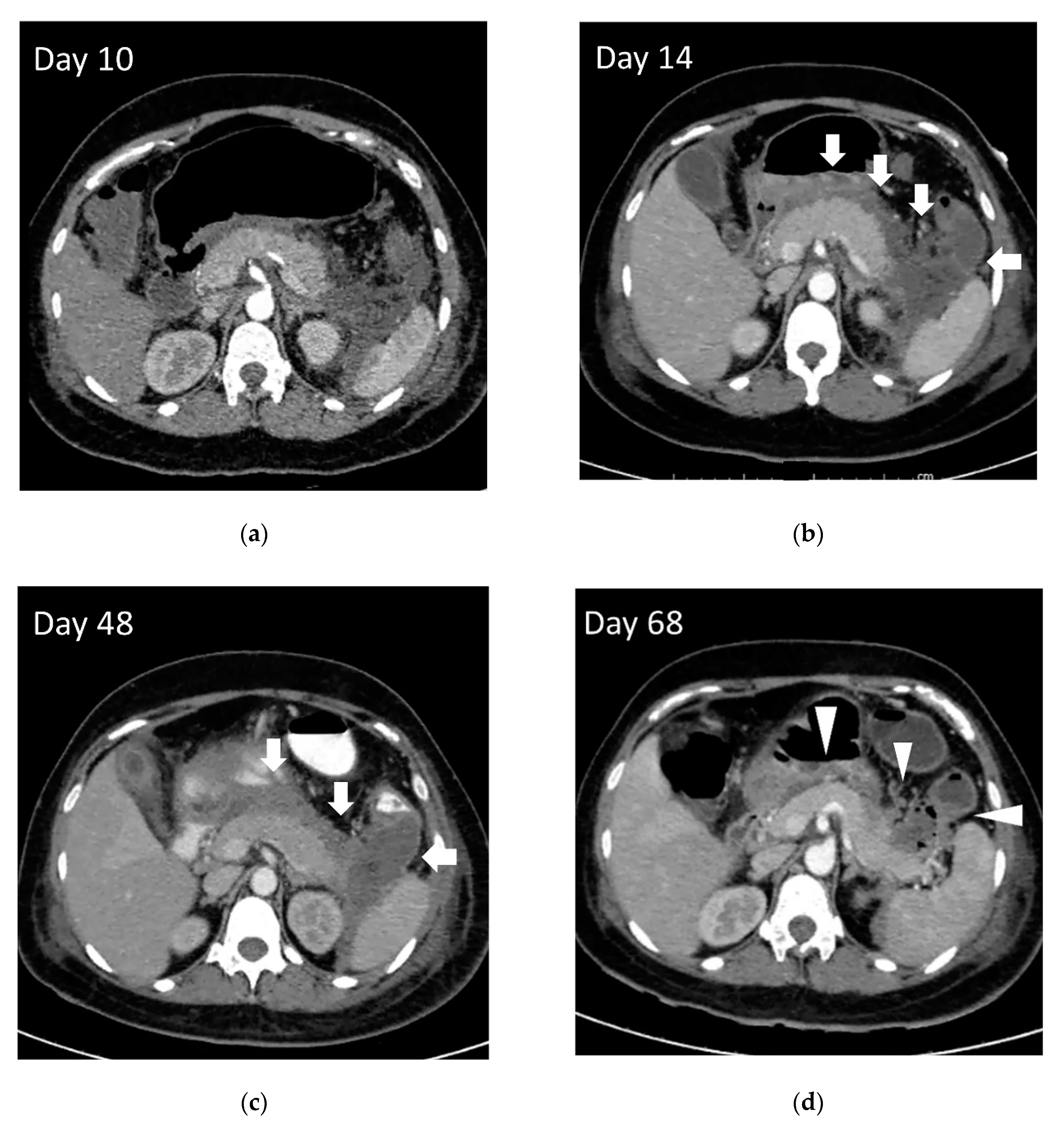


| General Indication for Necrosectomy | Endoscopic Transmural Necrosectomy Preferred | Percutaneous Necrosectomy Preferred |
|---|---|---|
| Suspected infection | Centrally located lesion | Paracolic gutter extension |
| Large amount of necrotic debris | well encapsulation by contrast-enhanced CT | Very early lesion (<2 weeks) or not fully encapsulated |
| Failed clinical improvement after initial drainage |
| Authors (Year) | Stents | Type of Study | Number of Patients | Outcome | Remarks |
|---|---|---|---|---|---|
| Mukai (2015) [45] | DPS versus LAMS (Axios® 15 mm, Nagi® 16 mm, Spaxus® 12 mm) | Retrospective | 70 | No difference in success but a shorter procedure time with LAMS | Nasocystic irrigation in all cases |
| Ang (2016) [46] | DPS versus Nagi® 16 mm) | Retrospective | 49 | DPS associated with higher need for secondary drainage | Both pancreatic pseudocyst and WON included |
| Bapaye (2017) [47] | DPS versus FCSEMS (Nagi®, 16 mm) | Retrospective | 133 | FCSEMS superior to DPS in terms of clinical success, number of necrosectomies, salvage surgeries, and length of hospital stay | Nasocystic irrigation in all cases |
| Siddiqui (2017) [48] | DPS versus FCSEMS (10 mm) versus LAMS (Axios® 10,15 mm) | Retrospective | 313 | FCSEMS and LAMs superior to DPS in efficacy. Fewer procedures are required in LAMS | More acute adverse events in LAMS but fewer stent occlusions or migrations |
| Abu Dayyeh (2018) [49] | DPS versus FCSEMS (Axios®, Nagi®, 15, 18, 20 mm) | Retrospective | 94 | FCSEMS decreases the need for repeated necrosectomy and procedure-related hemorrhage | |
| Law (2018) [50] | FCSEMS (10 mm) versus LAMS (Axios® 10, 15 mm) | Retrospective | 68 | Comparable efficacy and safety, but more revisions needed in LAMS | |
| Lang (2018) [43] | DPS versus LAMS (Axios® 10, 15 mm) | Retrospective | 103 | Increased complications (bleeding, occlusion) in LAMS | Both pancreatic pseudocyst and WON included |
| Mohan (2019) [40] | DPS versus LAMS | Meta-analysis | 9 studies (737 patients) of LAMS, 7 studies (527 patients) of DPS | Equal clinical outcomes and adverse events in DPS and LAMS | |
| Bang (2019) [39] | DPS versus LAMS (Axios® 15 mm) | RCT | 60 | No significant differences in treatment outcome | |
| Chen (2019) [41] | DPS versus LAMS | Retrospective | 189 | Higher clinical success, shorter procedure time, lower need for surgery, and lower rate of recurrence in LAMS | |
| Cho (2019) [51] | DPS versus LAMS (HANARO® 10 mm) | Retrospective | 28 | No difference in clinical success rate and complications | Pilot study. Included both pseudocyst and WON. New stent with antireflux and antimigration property |
| Kayal (2020) [42] | DPS versus FCSEMS tubular versus Axios® | Historical cohort | 58 | Higher clinical success in LAMS than FCSEMS and DPS (96.3% vs. 81.8% vs. 77.8%) | Both pancreatic pseudocyst and WON included |
| Zhu (2020) [52] | DPS versus LAMS (Microtech, 16 mm) | Retrospective | 84 | Better outcome using LAMS in cases with debris <20% | |
| Rana (2020) [44] | DPS versus LAMS (Nagi®, Plumber®, 14, 16 mm) | Retrospective | 166 | Similar technical success rate, complications, and resolution but shorter time to resolution in LAMS | |
| Ge (2020) [38] | DPS versus LAMS (Axios® 10, 15 mm) | Retrospective | 112 | LAMS associated with faster resolution, lower recurrence, and decreased requirement for surgery but higher adverse event rates (bleeding, perforation) | Additional DPS inserted through LAMS |
| Parsa (2020) [53] | LAMS (Axios®) 15 mm versus 20 mm | Retrospective | 306 | Comparable clinical success and safety but with fewer necrosectomies in larger LAMS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chantarojanasiri, T.; Ratanachu-Ek, T.; Isayama, H. When Should We Perform Endoscopic Drainage and Necrosectomy for Walled-Off Necrosis? J. Clin. Med. 2020, 9, 4072. https://doi.org/10.3390/jcm9124072
Chantarojanasiri T, Ratanachu-Ek T, Isayama H. When Should We Perform Endoscopic Drainage and Necrosectomy for Walled-Off Necrosis? Journal of Clinical Medicine. 2020; 9(12):4072. https://doi.org/10.3390/jcm9124072
Chicago/Turabian StyleChantarojanasiri, Tanyaporn, Thawee Ratanachu-Ek, and Hiroyuki Isayama. 2020. "When Should We Perform Endoscopic Drainage and Necrosectomy for Walled-Off Necrosis?" Journal of Clinical Medicine 9, no. 12: 4072. https://doi.org/10.3390/jcm9124072
APA StyleChantarojanasiri, T., Ratanachu-Ek, T., & Isayama, H. (2020). When Should We Perform Endoscopic Drainage and Necrosectomy for Walled-Off Necrosis? Journal of Clinical Medicine, 9(12), 4072. https://doi.org/10.3390/jcm9124072






