Is Ovarian Tissue Transplantation Safe in Patients with Central Nervous System Primitive Neuroectodermal Tumors?
Abstract
:1. Introduction
2. Experimental Section
2.1. Patients
2.2. Thawing of Frozen Ovarian Strips
2.3. Xenografting
2.4. Histological Analysis
2.5. Immunohistochemical Analysis
2.6. Sample Storage, RNA Extraction and Reverse Transcription
2.7. Droplet Digital PCR
2.8. Defining the Limit of Blank and Limit of Detection
2.9. Next-Generation Sequencing
2.10. Statistical Analysis
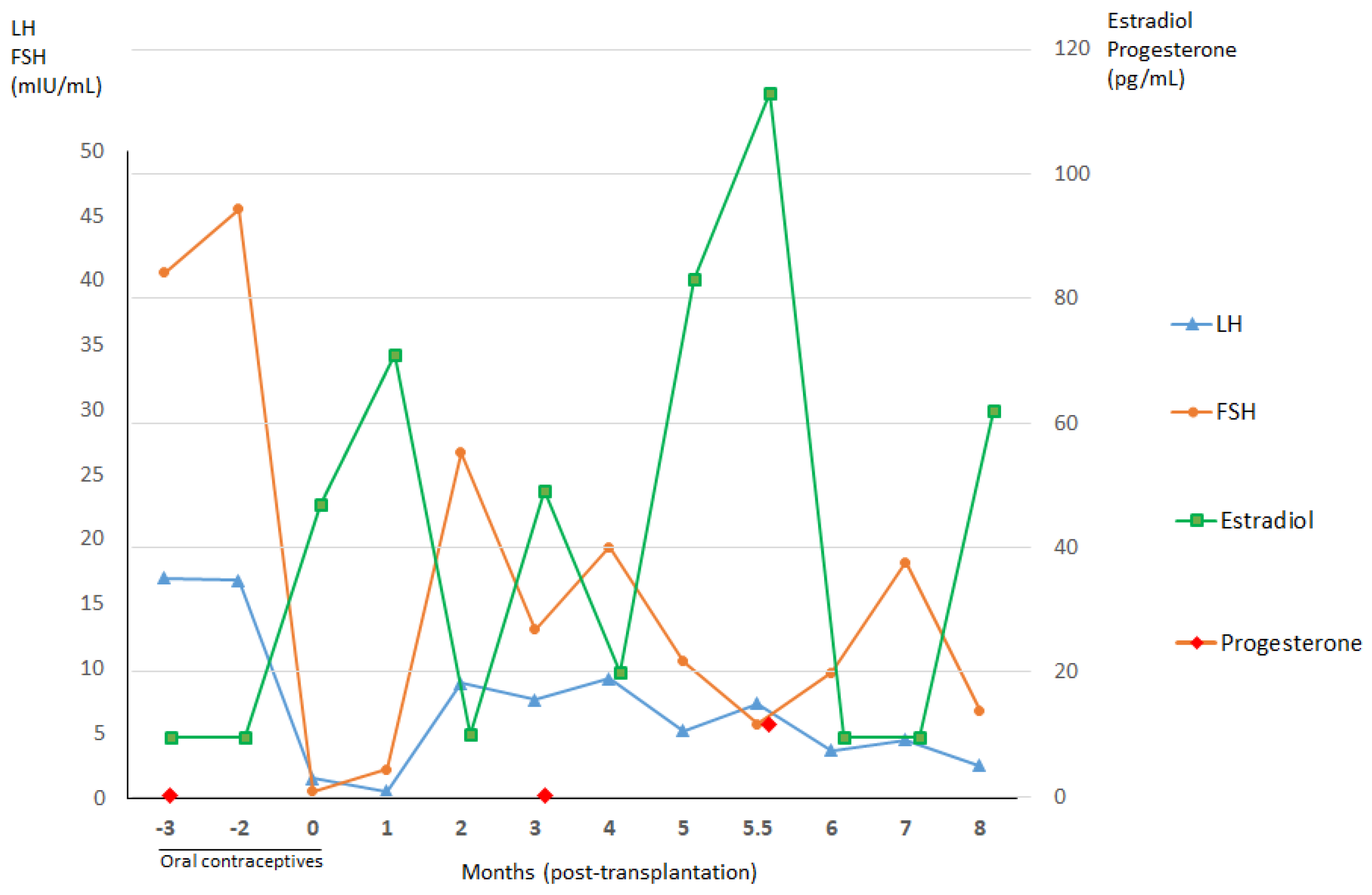
3. Results
3.1. Patient Information
3.1.1. Patient 1
3.1.2. Patient 2
3.1.3. Patient 3
3.2. Histology and Immunohistochemistry
3.3. Droplet Digital PCR
3.4. Next-Generation Sequencing
3.5. Xenotransplantation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Donnez, J.; Dolmans, M. Fertility preservation in women. Nat. Rev. Endocrinol. 2013, 9, 735–749. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M. Fertility Preservation in Women. N. Engl. J. Med. 2017, 377, 1657–1665. [Google Scholar] [CrossRef]
- Donnez, J.; Martinez-Madrid, B.; Jadoul, P.; Van Langendonckt, A.; Demylle, D.; Dolmans, M. Ovarian tissue cryopreservation and transplantation: A review. Hum. Reprod. Update 2006, 12, 519–535. [Google Scholar] [CrossRef]
- Donnez, J.; Squifflet, J.; Jadoul, P.; Demylle, D.; Cheron, A.; Van Langendonckt, A.; Dolmans, M. Pregnancy and live birth after autotransplantation of frozen-thawed ovarian tissue in a patient with metastatic disease undergoing chemotherapy and hematopoietic stem cell transplantation. Fertil. Steril. 2011, 95, 1787.e1–1787.e4. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Dolmans, M.; Pellicer, A.; Diaz-Garcia, C.; Sanchez Serrano, M.; Schmidt, K.; Ernst, E.; Luyckx, V.; Andersen, C.Y. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: A review of 60 cases of reimplantation. Fertil. Steril. 2013, 99, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S. Assessment of long term endocrine function after transplantation of frozen-thawed human ovarian tissue to the heterotopic site: 10 year longitudinal follow-up study. J. Assist. Reprod. Genet. 2012, 29, 489–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolmans, M.M. Trials and tribulations of in vitro fertilization after ovarian tissue transplantation. Fertil. Steril. 2019, 112, 817–818. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.M.; Luyckx, V.; Donnez, J.; Andersen, C.Y.; Greve, T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil. Steril. 2013, 99, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.M.; Masciangelo, R. Risk of transplanting malignant cells in cryopreserved ovarian tissue. Minerva Ginecol. 2018, 70, 436–443. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Iwahara, Y.; Donnez, J.; Soares, M.; Vaerman, J.L.; Amorim, C.A.; Poirel, H. Evaluation of minimal disseminated disease in cryopreserved ovarian tissue from bone and soft tissue sarcoma patients. Hum. Reprod. 2016, 31, 2292–2302. [Google Scholar] [CrossRef] [Green Version]
- Dolmans, M.M.; Marinescu, C.; Saussoy, P.; Van Langendonckt, A.; Amorim, C.; Donnez, J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood 2010, 116, 2908–2914. [Google Scholar] [CrossRef] [PubMed]
- Masciangelo, R.; Bosisio, C.; Donnez, J.; Amorim, C.A.; Dolmans, M.M. Safety of ovarian tissue transplantation in patients with borderline ovarian tumors. Hum. Reprod. 2018, 33, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, R.; Vicenti, R.; Magnani, V.; Pasquinelli, G.; Macciocca, M.; Parazza, I.; Paradisi, R.; Battaglia, C.; Venturoli, S. Cryopreservation of ovarian tissue in breast cancer patients: 10 years of experience. Future Oncol. 2012, 8, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Gellert, S.E.; Pors, S.E.; Kristensen, S.G.; Bay-Bjørn, A.M.; Ernst, E.; Yding Andersen, C. Transplantation of frozen-thawed ovarian tissue: An update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J. Assist. Reprod. Genet. 2018, 35, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Xu, K.; Khine, K.T.; Ooi, Y.C.; Quinsey, C.S. A systematic review of shunt-related extraneural metastases of primary central nervous system tumors. Clin. Neurol. Neurosurg. 2018, 174, 239–243. [Google Scholar] [CrossRef]
- Nisolle, M.; Casanas-Roux, F.; Qu, J.; Motta, P.; Donnez, J. Histologic and ultrastructural evaluation of fresh and frozen-thawed human ovarian xenografts in nude mice. Fertil. Steril. 2000, 74, 122–129. [Google Scholar] [CrossRef]
- Lombard, C.A.; Fabre, A.; Ambroise, J.; Ravau, J.; André, F.; Jazouli, N.; Najimi, M.; Stéphenne, X.; Smets, F.; Vaerman, J.L.; et al. Detection of Human Microchimerism following Allogeneic Cell Transplantation Using Droplet Digital PCR. Stem Cells Int. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- National Committee for Clinical Laboratory Standards. Protocols for Determination of Limits of Detection and Limits of Quantitation; Approved Guideline; NCCLS Document EP17-A; NCCLS: Wayne, PA, USA, 2004; Volume 24, p. 80. [Google Scholar]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 29 (Suppl. S1), S49–S52. [Google Scholar]
- Andersen, C.; Silber, S.; Bergholdt, S.; Jorgensen, J.; Ernst, E. Long-term duration of function of ovarian tissue transplants: Case reports. Reprod. Biomed. Online 2012, 25, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Meirow, D.; Ra’anani, H.; Shapira, M.; Brenghausen, M.; Derech Chaim, S.; Aviel-Ronen, S.; Amariglio, N.; Schiff, E.; Orvieto, R.; Dor, J. Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil. Steril. 2016, 106, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Smoll, N.R.; Drummond, K.J. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J. Clin. Neurosci. 2012, 19, 1541–1544. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picard, D.; Miller, S.; Hawkins, C.E.; Bouffet, E.; Rogers, H.A.; Chan, T.S.Y.; Kim, S.-K.; Ra, Y.-S.; Fangusaro, J.; Korshunov, A.; et al. Markers of survival and metastatic potential in childhood CNS primitive neuro-ectodermal brain tumours: An integrative genomic analysis. Lancet Oncol. 2012, 13, 838–848. [Google Scholar] [CrossRef] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.G.; Lee, D.Y.; Paek, S.H.; Chi, J.G.; Choe, G.; Jung, H.-W. Supratentorial primitive neuroectodermal tumors in adults. J. Neurooncol. 2002, 60, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Pizer, B.L.; Weston, C.L.; Robinson, K.J.; Ellison, D.W.; Ironside, J.; Saran, F.; Lashford, L.S.; Tait, D.; Lucraft, H.; Walker, D.A.; et al. Analysis of patients with supratentorial primitive neuro-ectodermal tumours entered into the SIOP/UKCCSG PNET 3 study. Eur. J. Cancer 2006, 42, 1120–1128. [Google Scholar] [CrossRef]
- Perreault, S.; Lober, R.M.; Carret, A.-S.; Zhang, G.; Hershon, L.; Décarie, J.-C.; Yeom, K.; Vogel, H.; Fisher, P.G.; Partap, S. Relapse patterns in pediatric embryonal central nervous system tumors. J. Neurooncol. 2013, 115, 209–215. [Google Scholar] [CrossRef]
- Biswas, A.; Mallick, S.; Purkait, S.; Roy, S.; Sarkar, C.; Bakhshi, S.; Singh, M.; Julka, P.K.; Rath, G.K. Treatment outcome and patterns of failure in patients of non-pineal supratentorial primitive neuroectodermal tumor: Review of literature and clinical experience form a regional cancer center in north India. Acta Neurochir. 2015, 157, 1251–1266. [Google Scholar] [CrossRef]
- Stensvold, E.; Krossnes, B.K.; Lundar, T.; Due-Tønnessen, B.J.; Frič, R.; Due-Tønnessen, P.; Bechensteen, A.G.; Myklebust, T.Å.; Johannesen, T.B.; Brandal, P. Outcome for children treated for medulloblastoma and supratentorial primitive neuroectodermal tumor (CNS-PNET)—A retrospective analysis spanning 40 years of treatment. Acta Oncol. 2017, 56, 698–705. [Google Scholar] [CrossRef] [Green Version]
- Jakacki, R.I.; Burger, P.C.; Kocak, M.; Boyett, J.M.; Goldwein, J.; Mehta, M.; Packer, R.J.; Tarbell, N.J.; Pollack, I.F. Outcome and prognostic factors for children with supratentorial primitive neuroectodermal tumors treated with carboplatin during radiotherapy: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2015, 62, 776–783. [Google Scholar] [CrossRef] [Green Version]
- Matar, N.; Bahri, K.; Kallel, J.; Boubaker, A.; Jemel, H. Extraneural Supratentorial Primitive Neuroectodermal Tumor Metastasis: An Adult Case Report. IJNS 2017, 6, 210–212. [Google Scholar] [CrossRef] [Green Version]
- Terheggen, F.; Troost, D.; Majoie, C.B.; Leenstra, S.; Richel, D.J. Local recurrence and distant metastasis of supratentorial primitive neuro-ectodermal tumor in an adult patient successfully treated with intensive induction chemotherapy and maintenance temozolomide. J. Neurooncol. 2007, 82, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Varan, A.; Sarı, N.; Akalan, N.; Söylemezoğlu, F.; Akyüz, C.; Kutluk, T.; Büyükpamukçu, M. Extraneural metastasis in intracranial tumors in children: The experience of a single center. J. Neurooncol. 2006, 79, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Rickert, C.H. Extraneural metastases of paediatric brain tumours. Acta Neuropathol. 2003, 105, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, S.; Fukuda, T.; Miyamoto, S.; Yoshioka, J.; Shirahama, S.; Saito, T.; Tsukamoto, N. Peripheral primitive neuroectodermal tumor of the ovary confirmed by CD99 immunostaining, karyotypic analysis, and RT-PCR for EWS/FLI-1 chimeric mRNA. Am. J. Surg. Pathol. 1998, 22, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.; Lin, M.; Shen, J.; Wang, S.; Jong, Y.; Chien, C. Analysis of chromosome abnormalities by comparative genomic hybridization in malignant peripheral primitive neuroectodermal tumor of the ovary. Gynecol. Oncol. 2004, 92, 752–760. [Google Scholar] [CrossRef]
- Demirtas, E.; Guven, S.; Guven, E.S.G.; Baykal, C.; Ayhan, A. Two successful spontaneous pregnancies in a patient with a primary primitive neuroectodermal tumor of the ovary. Fertil. Steril. 2004, 81, 679–681. [Google Scholar] [CrossRef]
- Kim, K.J.; Jang, B.W.; Lee, S.K.; Kim, B.K.; Nam, S.L. A case of peripheral primitive neuroectodermal tumor of the ovary. Int. J. Gynecol. Cancer 2004, 14, 370–372. [Google Scholar] [CrossRef]
- Ateser, G.; Yildiz, O.; Leblebici, C.; Mandel, N.M.; Unal, F.; Turna, H.; Arikan, I.; Colcaki, D. Metastatic primitive neuroectodermal tumor of the ovary in pregnancy. Int. J. Gynecol. Cancer 2007, 17, 266–269. [Google Scholar] [CrossRef]
- Anfinan, N.M.; Sait, K.H.; Al-Maghrabi, J.A. Primitive neuroectodermal tumor of the ovary. Saudi Med. J. 2008, 29, 444–446. [Google Scholar]
- Ostwal, V.; Rekhi, B.; Noronha, V.; Basak, R.; Desai, S.B.; Maheshwari, A.; Prabhash, K. Primitive neuroectodermal tumor of ovary in a young lady, confirmed with molecular and cytogenetic results—A rare case report with a diagnostic and therapeutic challenge. Pathol. Oncol. Res. 2012, 18, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Horng, H.; Lai, C.; Chang, W.; Su, W.; Yen, M.; Wang, P. Peripheral primitive neuroectodermal tumor of the ovary with torsion. Gynecol. Minim. Invasive Ther. 2013, 2, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Lotz, L.; Dittrich, R.; Hoffmann, I.; Beckmann, M.W. Ovarian Tissue Transplantation: Experience from Germany and Worldwide Efficacy. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119867357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastings, L.; Beerendonk, C.C.M.; Westphal, J.R.; Massuger, L.F.A.G.; Kaal, S.E.J.; van Leeuwen, F.E.; Braat, D.D.M.; Peek, R. Autotransplantation of cryopreserved ovarian tissue in cancer survivors and the risk of reintroducing malignancy: A systematic review. Hum. Reprod. Update 2013, 19, 483–506. [Google Scholar] [CrossRef] [Green Version]
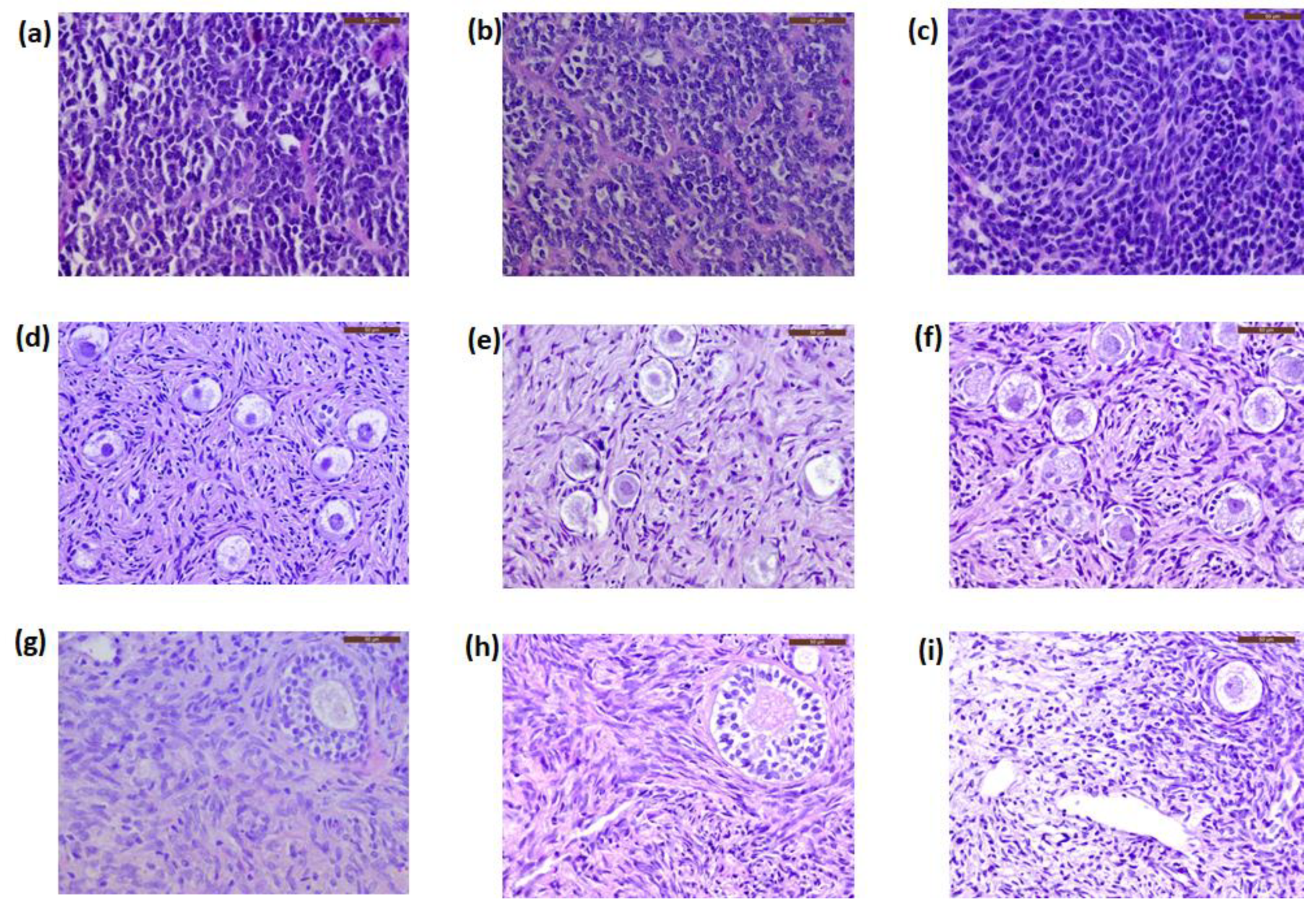
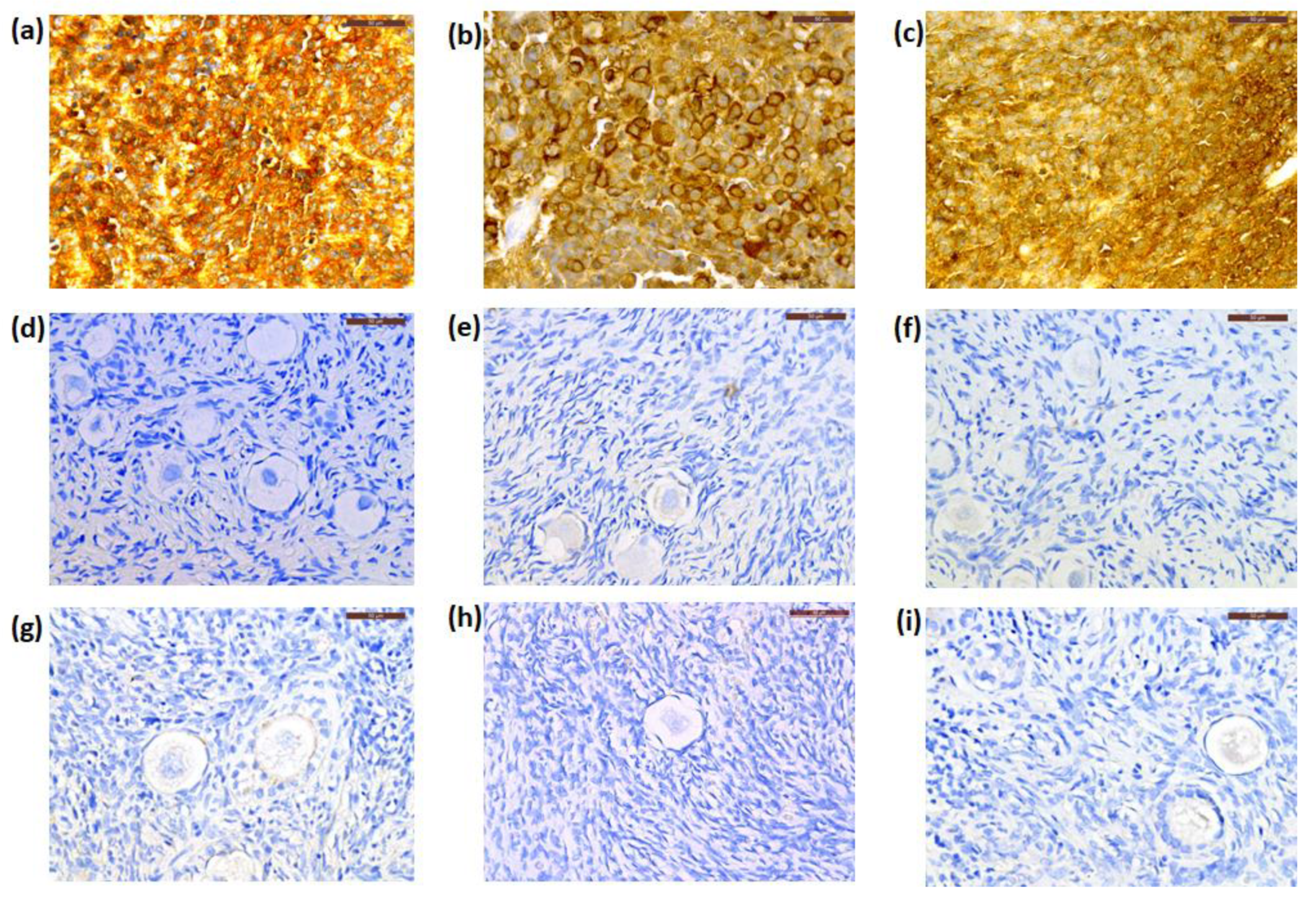

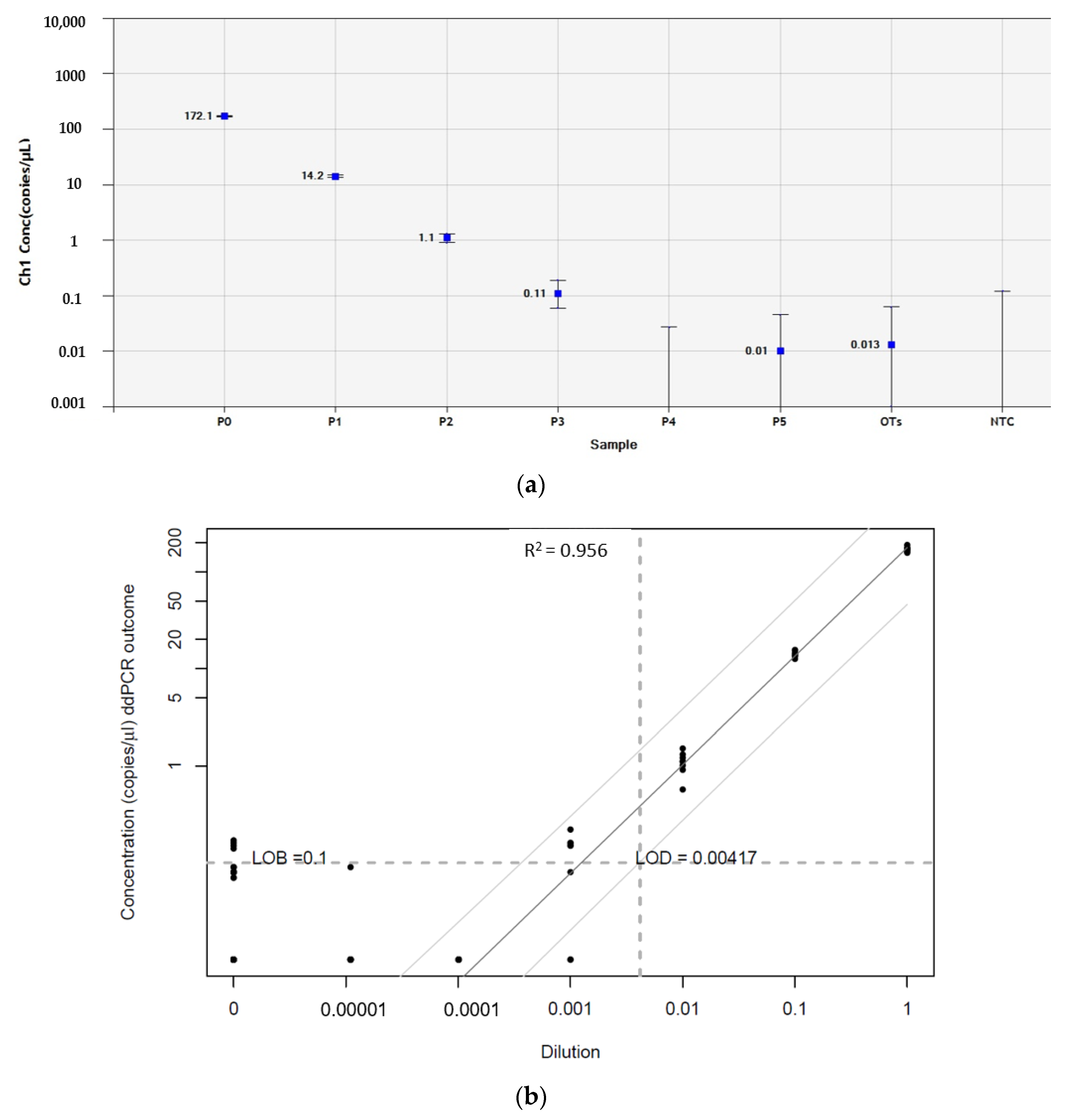

| Patient n° | Age * (years) | Extraneural Metastasis | Relapse | Alive or Deceased | Cancer Cells in Histology | Immunohistochemistry | Concentration of GFAP Transcripts by RT-ddPCR (Copies/µL) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient CNS Tumors | Cryopreserved OT | Xenografted OT | Cryopreserved OT | Xenografted OT | ||||||
| 1 | 17 | Yes, lungs | Yes | Deceased | No | GFAP (−), NSE (+) | NSE negative | NSE negative | 0 | 0 |
| 2 | 4 | No | No | Deceased | No | GFAP (+), NSE (+) | Both negative | Both negative | 0 | 0 |
| 3 | 9 | No | No | Alive | No | GFAP (+), NSE (+) | Both negative | Both negative | 0 | 0 |
| Authors | Patient Age (Years) | FIGO Stage | Treatment | Complications | Follow-Up |
|---|---|---|---|---|---|
| Kawauchi et al. (1998) [36] | 29 | II | TAH + BSO + omentectomy + PALA + chemotherapy | NA | 11 months DOD |
| Chow et al. (2004) [37] | 13 | IV | Debulking + chemotherapy 2nd debulking + chemotherapy + radiotherapy | NA | 17 months DOD |
| Demirtas et al. (2004) [38] | 25 | IC | LSO + omentectomy + PLA + chemotherapy 2nd look laparotomy | Pelvic abscess after 2nd look laparotomy | 2 years 2 births NED |
| Kim et al. (2004) [39] | 18 | IIIC | RSO + omentectomy + PLA + PALA + chemotherapy + radiotherapy | Bowel obstruction | 10 months DOD |
| Ateser et al. (2007) [40] | 28 | IV | TAH + LSO + omentectomy + chemotherapy + radiotherapy | Neutropenia | 13 months DOD |
| Anfinan et al. (2008) [41] | 31 | IIIC | TAH + BSO + omentectomy + chemotherapy | NA | 15 months DOD |
| Ostwal et al. (2012) [42] | 28 | NA | LSO + chemotherapy + radical excision upon recurrence | NA | 18 months DOD |
| Huang et al. (2013) [43] | 28 | IA | LSO + omentectomy + PLA + chemotherapy | NA | 28 months NED |
Publisher‘s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.Y.T.; Camboni, A.; Masciangelo, R.; Donnez, J.; Dolmans, M.-M. Is Ovarian Tissue Transplantation Safe in Patients with Central Nervous System Primitive Neuroectodermal Tumors? J. Clin. Med. 2020, 9, 4101. https://doi.org/10.3390/jcm9124101
Nguyen TYT, Camboni A, Masciangelo R, Donnez J, Dolmans M-M. Is Ovarian Tissue Transplantation Safe in Patients with Central Nervous System Primitive Neuroectodermal Tumors? Journal of Clinical Medicine. 2020; 9(12):4101. https://doi.org/10.3390/jcm9124101
Chicago/Turabian StyleNguyen, Thu Yen Thi, Alessandra Camboni, Rossella Masciangelo, Jacques Donnez, and Marie-Madeleine Dolmans. 2020. "Is Ovarian Tissue Transplantation Safe in Patients with Central Nervous System Primitive Neuroectodermal Tumors?" Journal of Clinical Medicine 9, no. 12: 4101. https://doi.org/10.3390/jcm9124101






