Sex Differences in Left Ventricular Remodeling and Outcomes in Chronic Aortic Regurgitation
Abstract
:1. Introduction
2. Experimental Section
2.1. Setting and Study Design
2.2. Echocardiography
2.3. Cardiac Magnetic Resonance Imaging
2.4. Follow-Up and Outcomes
2.5. Statistical Analysis
3. Results
3.1. Clinical Data
3.2. Echocardiographic Results
3.3. Cardiovascular Magnetic Resonance Imaging Results
3.4. Left Ventricular Size and Regurgitation Fraction
3.5. Association of Severe AR and LV Size
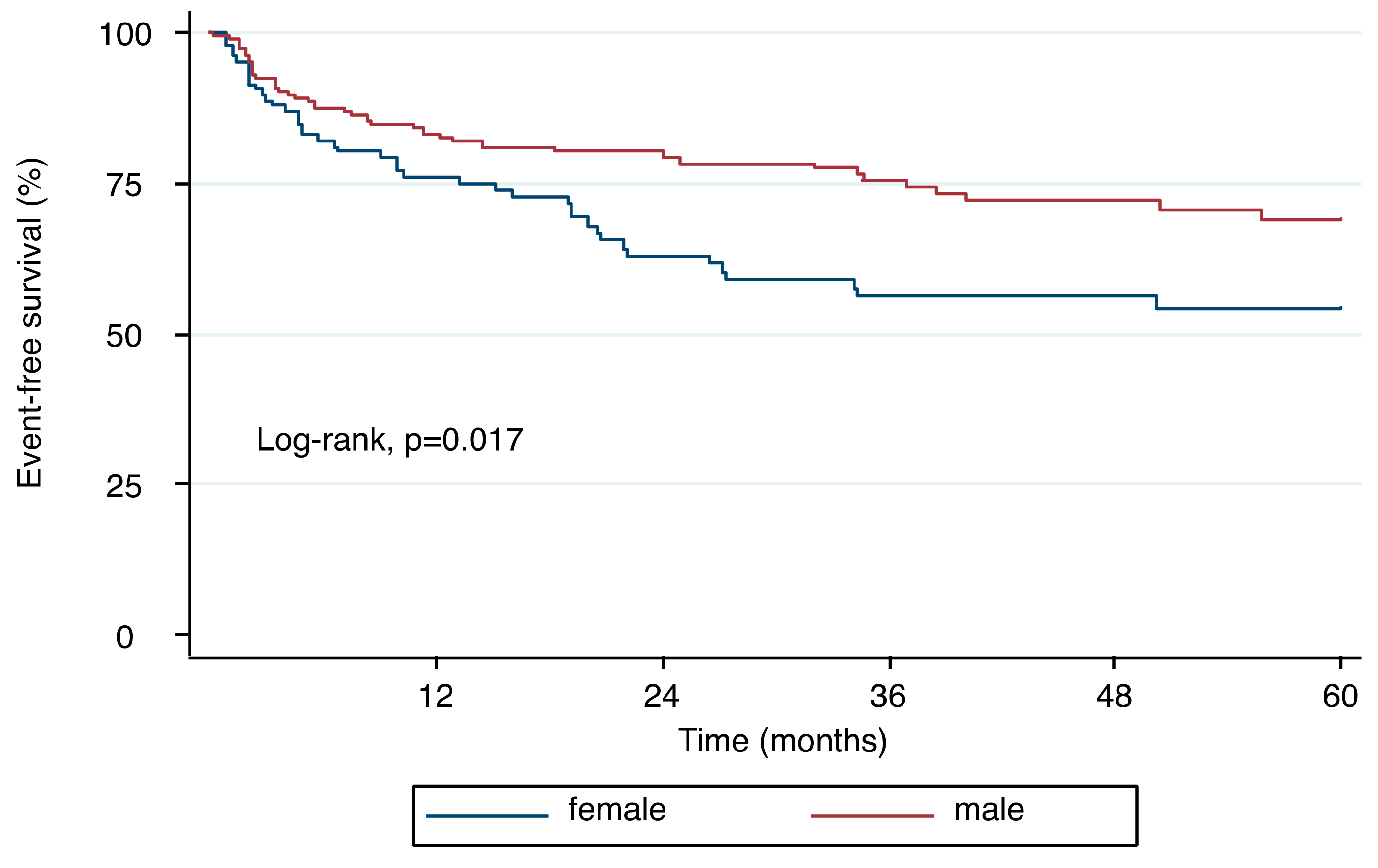
3.6. Outcomes
4. Discussion
4.1. Left Ventricular Remodeling in Aortic Regurgitation
4.2. Sex-Specific Outcome Differences in Chronic Aortic Regurgitation
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bonow, R.O.; Lakatos, E.; Maron, B.J.; Epstein, S.E. Serial long-term assessment of the natural history of asymptomatic patients with chronic aortic regurgitation and normal left ventricular systolic function. Circulation 1991, 84, 1625–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Scientific Document Committee of the European Association of Cardiovascular, I. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, A.; Bolen, M.; Grimm, R.A.; Rodriguez, L.L.; Thomas, J.D.; Marwick, T.H.; Investigators, A.R.C. Development of a consensus document to improve multireader concordance and accuracy of aortic regurgitation severity grading by echocardiography versus cardiac magnetic resonance imaging. Am. J. Cardiol. 2012, 110, 709–714. [Google Scholar] [CrossRef]
- Chatzimavroudis, G.P.; Oshinski, J.N.; Franch, R.H.; Pettigrew, R.I.; Walker, P.G.; Yoganathan, A.P. Quantification of the aortic regurgitant volume with magnetic resonance phase velocity mapping: A clinical investigation of the importance of imaging slice location. J. Heart Valve Dis. 1998, 7, 94–101. [Google Scholar]
- Grothues, F.; Smith, G.C.; Moon, J.C.; Bellenger, N.G.; Collins, P.; Klein, H.U.; Pennell, D.J. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am. J. Cardiol. 2002, 90, 29–34. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, e57–e185. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Munoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease: The Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2017. [Google Scholar] [CrossRef]
- Globits, S.; Frank, H.; Mayr, H.; Neuhold, A.; Glogar, D. Quantitative assessment of aortic regurgitation by magnetic resonance imaging. Eur. Heart J. 1992, 13, 78–83. [Google Scholar] [CrossRef]
- Kozerke, S.; Schwitter, J.; Pedersen, E.M.; Boesiger, P. Aortic and mitral regurgitation: Quantification using moving slice velocity mapping. J. Magn. Reson. Imaging JMRI 2001, 14, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Gelfand, E.V.; Hughes, S.; Hauser, T.H.; Yeon, S.B.; Goepfert, L.; Kissinger, K.V.; Rofsky, N.M.; Manning, W.J. Severity of mitral and aortic regurgitation as assessed by cardiovascular magnetic resonance: Optimizing correlation with Doppler echocardiography. J. Cardiovasc. Magn. Reson. 2006, 8, 503–507. [Google Scholar] [CrossRef]
- Myerson, S.G.; d’Arcy, J.; Mohiaddin, R.; Greenwood, J.P.; Karamitsos, T.D.; Francis, J.M.; Banning, A.P.; Christiansen, J.P.; Neubauer, S. Aortic regurgitation quantification using cardiovascular magnetic resonance: Association with clinical outcome. Circulation 2012, 126, 1452–1460. [Google Scholar] [CrossRef] [Green Version]
- Kammerlander, A.A.; Wiesinger, M.; Duca, F.; Aschauer, S.; Binder, C.; Zotter Tufaro, C.; Nitsche, C.; Badre-Eslam, R.; Schonbauer, R.; Bartko, P.; et al. Diagnostic and Prognostic Utility of Cardiac Magnetic Resonance Imaging in Aortic Regurgitation. JACC Cardiovasc. Imaging 2019, 12, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 16, 233–271. [Google Scholar] [CrossRef] [Green Version]
- Pfaffenberger, S.; Bartko, P.; Graf, A.; Pernicka, E.; Babayev, J.; Lolic, E.; Bonderman, D.; Baumgartner, H.; Maurer, G.; Mascherbauer, J. Size matters! Impact of age, sex, height, and weight on the normal heart size. Circ. Cardiovasc. Imaging 2013, 6, 1073–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, C.M.; Barkhausen, J.; Flamm, S.D.; Kim, R.J.; Nagel, E.; Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: Board of trustees task force on standardized protocols. J. Cardiovasc. Magn. Reson. 2008, 10, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriel, R.S.; Renapurkar, R.; Bolen, M.A.; Verhaert, D.; Leiber, M.; Flamm, S.D.; Griffin, B.P.; Desai, M.Y. Comparison of severity of aortic regurgitation by cardiovascular magnetic resonance versus transthoracic echocardiography. Am. J. Cardiol. 2011, 108, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Kawel-Boehm, N.; Maceira, A.; Valsangiacomo-Buechel, E.R.; Vogel-Claussen, J.; Turkbey, E.B.; Williams, R.; Plein, S.; Tee, M.; Eng, J.; Bluemke, D.A. Normal values for cardiovascular magnetic resonance in adults and children. J. Cardiovasc. Magn. Reson. 2015, 17, 29. [Google Scholar] [CrossRef] [Green Version]
- Petersen, S.E.; Aung, N.; Sanghvi, M.M.; Zemrak, F.; Fung, K.; Paiva, J.M.; Francis, J.M.; Khanji, M.Y.; Lukaschuk, E.; Lee, A.M.; et al. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J. Cardiovasc. Magn. Reson. 2017, 19, 18. [Google Scholar] [CrossRef] [Green Version]
- Ratto, E.; Viazzi, F.; Bonino, B.; Gonnella, A.; Garneri, D.; Parodi, E.L.; Bezante, G.P.; Derchi, L.E.; Leoncini, G.; Pontremoli, R. Left ventricular dilatation and subclinical renal damage in primary hypertension. J. Hypertens 2015, 33, 605–611, discussion 611. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 2017, 70, 776–803. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.D.; Brower, G.L.; Janicki, J.S. Gender differences in cardiac remodeling secondary to chronic volume overload. J. Card. Fail. 2002, 8, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Dobson, L.E.; Fairbairn, T.A.; Musa, T.A.; Uddin, A.; Mundie, C.A.; Swoboda, P.P.; Ripley, D.P.; McDiarmid, A.K.; Erhayiem, B.; Garg, P.; et al. Sex-related differences in left ventricular remodeling in severe aortic stenosis and reverse remodeling after aortic valve replacement: A cardiovascular magnetic resonance study. Am. Heart J. 2016, 175, 101–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donaldson, C.; Eder, S.; Baker, C.; Aronovitz, M.J.; Weiss, A.D.; Hall-Porter, M.; Wang, F.; Ackerman, A.; Karas, R.H.; Molkentin, J.D.; et al. Estrogen attenuates left ventricular and cardiomyocyte hypertrophy by an estrogen receptor-dependent pathway that increases calcineurin degradation. Circ. Res. 2009, 104, 265–275. [Google Scholar] [CrossRef]
- Mantovani, F.; Clavel, M.A.; Michelena, H.I.; Suri, R.M.; Schaff, H.V.; Enriquez-Sarano, M. Comprehensive Imaging in Women With Organic Mitral Regurgitation: Implications for Clinical Outcome. JACC Cardiovasc. Imaging 2016, 9, 388–396. [Google Scholar] [CrossRef]
- Dujardin, K.S.; Enriquez-Sarano, M.; Schaff, H.V.; Bailey, K.R.; Seward, J.B.; Tajik, A.J. Mortality and morbidity of aortic regurgitation in clinical practice. A long-term follow-up study. Circulation 1999, 99, 1851–1857. [Google Scholar] [CrossRef] [Green Version]
- Tarasoutchi, F.; Grinberg, M.; Spina, G.S.; Sampaio, R.O.; Cardoso, L.; Rossi, E.G.; Pomerantzeff, P.; Laurindo, F.; da Luz, P.L.; Ramires, J.A. Ten-year clinical laboratory follow-up after application of a symptom-based therapeutic strategy to patients with severe chronic aortic regurgitation of predominant rheumatic etiology. J. Am. Coll. Cardiol. 2003, 41, 1316–1324. [Google Scholar] [CrossRef] [Green Version]
- Corti, R.; Binggeli, C.; Turina, M.; Jenni, R.; Luscher, T.F.; Turina, J. Predictors of long-term survival after valve replacement for chronic aortic regurgitation; is M-mode echocardiography sufficient? Eur. Heart J. 2001, 22, 866–873. [Google Scholar] [CrossRef] [Green Version]
- Tornos, P.; Sambola, A.; Permanyer-Miralda, G.; Evangelista, A.; Gomez, Z.; Soler-Soler, J. Long-term outcome of surgically treated aortic regurgitation: Influence of guideline adherence toward early surgery. J. Am. Coll. Cardiol. 2006, 47, 1012–1017. [Google Scholar] [CrossRef] [Green Version]
- Klodas, E.; Enriquez-Sarano, M.; Tajik, A.J.; Mullany, C.J.; Bailey, K.R.; Seward, J.B. Surgery for aortic regurgitation in women. Contrasting indications and outcomes compared with men. Circulation 1996, 94, 2472–2478. [Google Scholar] [CrossRef]
- Yang, L.T.; Michelena, H.I.; Scott, C.G.; Enriquez-Sarano, M.; Pislaru, S.V.; Schaff, H.V.; Pellikka, P.A. Outcomes in Chronic Hemodynamically Significant Aortic Regurgitation and Limitations of Current Guidelines. J. Am. Coll. Cardiol. 2019, 73, 1741–1752. [Google Scholar] [CrossRef] [PubMed]




| All Patients n = 270 | Women n = 109 (40.4%) | Men n = 161 (59.6%) | p-Value | |
|---|---|---|---|---|
| Age (years) | 60 ± 21 | 64 ± 20 | 57.3 ± 21.0 | 0.01 * |
| BMI (kg/m2) | 26.3 ± 4.6 | 26.5 ± 5.4 | 26.2 ± 4.1 | 0.84 |
| BSA (m2) | 1.90 ± 1.26 | 1.77 ± 0.21 | 1.99 ± 0.19 | <0.001 * |
| Hypertension (%) | 65.4 | 68.0 | 63.3 | 0.46 |
| Atrial fibrillation (%) | 28.1 | 27.5 | 28.6 | 0.85 |
| Diabetes (%) | 13.6 | 18.6 | 9.5 | 0.046 * |
| Hyperlipidemia (%) | 39.5 | 31.7 | 45.7 | 0.03 * |
| CAD (%) | 27.5 | 23.5 | 30.7 | 0.23 |
| Previous PCI (%) | 10.0 | 4.9 | 14.2 | 0.02 * |
| Previous CABG (%) | 7.9 | 7.8 | 7.9 | 0.99 |
| Previous MI (%) | 9.6 | 5.9 | 12.6 | 0.09 |
| Previous stroke (%) | 3.9 | 4.3 | 3.5 | 0.75 |
| Hematocrit (g/dL) | 39.4 ± 5.1 | 37.1 ± 3.9 | 41.0 ± 5.2 | <0.001 * |
| eGFR (mL/min/1.73 m2) | 66.1 ± 25.9 | 64.1 ± 26.8 | 67.5 ± 25.3 | 0.20 |
| Bicuspid valve (%) | 20.5 | 7.0 | 29.0 | <0.001 * |
| All Patients (n = 270) | Women (n = 109, 40.4%) | Men (n = 161, 59.6%) | p-Value | |
|---|---|---|---|---|
| ECHOCARDIOGRAPHIC PARAMETERS | ||||
| LA diameter (mm) | 56.5 ± 10.4 | 55.3 ± 9.5 | 57.2 ± 11.0 | 0.24 |
| RA diameter (mm) | 55.3 ± 10.0 | 54.6 ± 9.7 | 55.8 ± 10.2 | 0.75 |
| LVEDD (mm) | 49.0 ± 9.0 | 44.3 ± 6.5 | 52.1 ± 9.1 | <0.001 * |
| LVESD (mm) | 41.3 ± 9.7 | 36.2 ± 7.0 | 44.6 ± 9.8 | <0.001 * |
| LVEDV (mL) | 134.2 ± 60.2 | 99.5 ± 32.7 | 157.7 ± 63.2 | <0.001 * |
| LVEDV/BSA (mL/m2) | 70.1 ± 29.1 | 56.7 ± 18.5 | 79.2 ± 31.4 | <0.001 * |
| LVESV (mL) | 62.5 ± 37.9 | 42.6 ± 19.8 | 75.9 ± 41.3 | <0.001 * |
| LVESV/BSA (mL/m2) | 32.6 ± 18.9 | 24.3 ± 11.4 | 38.2 ± 20.8 | <0.001 * |
| LVEF (%) | 55.6 ± 11.8 | 58.9 ± 10.4 | 53.3 ± 12.1 | <0.001 * |
| RVEDD (mm) | 34.4 ± 7.1 | 33.7 ± 6.3 | 34.9 ± 7.5 | <0.001 * |
| RV FAC (%) | 44.8 ± 11.0 | 44.5 ± 11.0 | 45.1 ± 11.1 | 0.13 |
| TAPSE (mm) | 21.0 ± 6.0 | 19.7 ± 5.7 | 21.8 ± 6.1 | 0.72 |
| IVS (mm) | 13.4 ± 3.0 | 12.8 ± 2.8 | 13.9 ± 3.1 | 0.02 * |
| Peak TR velocity (m/s) | 3.1 ± 0.6 | 3.1 ± 0.6 | 3.0 ± 0.7 | 0.002 * |
| Valvular lesions | ||||
| AR ≥ moderate (%) | 59.6 | 51.4 | 65.2 | 0.02 * |
| AS ≥ moderate (%) | 27.0 | 31.2 | 24.2 | 0.21 |
| MR ≥ moderate (%) ‡ | 23.3 | 26.6 | 21.1 | 0.30 |
| TR ≥ moderate (%) | 21.5 | 31.2 | 14.9 | 0.001 * |
| CMR PARAMETERS | ||||
| LA diameter (mm) | 61.6 ± 11.1 | 60.3 ± 9.4 | 62.5 ± 12.0 | 0.31 |
| LA volume (mL) | 89.4 ± 48.0 | 81.8 ± 4.0 | 94.5 ± 51.5 | 0.06 |
| RA diameter (mm) | 61.6 ± 9.6 | 59.8 ± 8.2 | 62.9 ± 10.2 | 0.01 * |
| RA volume (mL) | 93.6 ± 66.0 | 75.0 ± 37.9 | 106.3 ± 77.3 | <0.001 |
| IVS (mm) | 12.5 ± 3.2 | 11.8 ± 3.2 | 12.9 ± 3.2 | 0.001 * |
| LV mass (g) | 157.7 ± 59.1 | 128.7 ± 34.7 | 188.9 ± 64.3 | <0.001 * |
| LV mass/BSA (g/m2) | 83.7 ± 28.6 | 72.7 ± 22.2 | 95.4 ± 30.2 | <0.001 * |
| LVEF (%) | 57.4 ± 13.7 | 62.9 ± 12.8 | 53.7 ± 13.1 | <0.001 * |
| LVEDV (mL) | 181.2 ± 73.6 | 133.6 ± 38.9 | 213.5 ± 74.1 | <0.001 * |
| LVEDV/BSA (mL/m2) | 94.7 ± 35.3 | 76.1 ± 22.0 | 107.3 ± 37.0 | <0.001 * |
| LVESV (mL) | 82.4 ± 53.3 | 52.2 ± 31.1 | 102.8 ± 55.4 | <0.001 * |
| LVESV/BSA (mL/m2) | 42.9 ± 26.6 | 29.8 ± 17.5 | 51.8 ± 28.0 | <0.001 * |
| Cardiac output (L/min) | 6.5 ± 2.3 | 5.5 ± 1.3 | 7.2 ± 2.5 | <0.001 * |
| RVEF (%) | 52.0 ± 12.4 | 54.3 ± 11.8 | 50.5 ± 12.6 | 0.02 * |
| RVEDV (mL) | 155.0 ± 55.1 | 129.6 ± 40.6 | 172.3 ± 57.0 | <0.001 * |
| AR quantification parameters | ||||
| RegV (mL) | 19.6 ± 24.1 | 9.3 ± 13.8 | 26.0 ± 26.8 | <0.001 * |
| RegF (%) | 18.1 ± 18.0 | 12.9 ± 15.1 | 21.6 ± 19.0 | <0.001 * |
| RegF ≥ 27% | 27.8 | 15.6 | 35.4 | <0.001 * |
| RegF ≥ 30% | 24.1 | 15.6 | 29.8 | 0.01 * |
| RegF ≥ 33% | 20.7 | 13.8 | 25.5 | 0.02 * |
| LV Size Parameter | OR (95%CI) Per 1SD | p-Value | OR (95%CI) Per 1SD | p-Value |
|---|---|---|---|---|
| Crude | Adjusted for Age | |||
| MEN | ||||
| Echo LVEDD | 3.80 [2.29–6.30] | <0.001 | 4.47 [2.61–7.66] | <0.001 |
| Echo LVEDV | 3.50 [2.12–5.53] | <0.001 | 4.26 [2.57–7.05] | <0.001 |
| Echo LVEDV/BSA | 3.93 [2.42–6.39] | <0.001 | 4.83 [2.81–8.31] | <0.001 |
| CMR LVEDV | 3.55 [2.20–5.73] | <0.001 | 3.74 [2.30–6.08] | <0.001 |
| CMR LVEDV/BSA | 4.04 [2.42–6.73] | <0.001 | 4.17 [2.49–6.97] | <0.001 |
| Women | ||||
| Echo LVEDD | 2.30 [1.33–3.98] | 0.003 | 2.48 [1.40–4.38] | 0.002 |
| Echo LVEDV | 2.12 [1.25–3.61] | 0.005 | 2.26 [1.31–3.93] | 0.004 |
| Echo LVEDV/BSA | 2.09 [1.23–3.53] | 0.006 | 2.25 [1.30–3.89] | 0.004 |
| CMR LVEDV | 2.63 [1.53–4.51] | <0.001 | 2.91 [1.65–5.14] | <0.001 |
| CMR LVEDV/BSA | 2.53 [1.49–4.28] | 0.001 | 2.74 [1.57–4.80] | <0.001 |
| Crude HR (95% CI) | p-Value | |
|---|---|---|
| Age | 1.04 (1.03–1.60) | <0.001 |
| Female Sex | 1.69 (1.09–2.61) | 0.018 |
| Body Mass Index (kg/m2) | 1.01 (0.96–1.06) | 0.776 |
| Hypertension | 2.10 (1.26–3.49) | 0.004 |
| Atrial Fibrillation | 1.82 (1.16–2.86) | 0.009 |
| Diabetes | 1.86 (1.07–3.22) | 0.027 |
| Hyperlipidemia | 1.56 (0.99–2.43) | 0.051 |
| Coronary Artery Disease | 2.36 (1.50–3.69) | <0.001 |
| Previous stroke | 0.76 (0.19–3.11) | 0.704 |
| Hematocrit | 0.91 (0.87–0.95) | <0.001 |
| eGFR (mL/min/1.73 m2) | 0.98 (0.97–0.99) | <0.001 |
| Bicuspid aortic valve | 0.12 (0.03–0.50) | 0.003 |
| CMR LVEDV/BSA (1-SD, mL/m2) | 1.03 (0.812–1.30) | 0.830 |
| CMR RVEDV/BSA (1-SD, ml/m2) | 1.11 (0.89–1.39) | 0.353 |
| CMR LVEF (%) | 0.97 (0.95–0.99) | <0.001 |
| CMR RVEF (%) | 0.97 (0.96–0.99) | <0.001 |
| CMR RegF (%) | 1.02 (1.01–1.03) | 0.004 |
| CMR RegV (mL) | 1.01 (0.99–1.02) | 0.273 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kammerlander, A.A.; Donà, C.; Nitsche, C.; Koschutnik, M.; Zafar, A.; Eslami, P.; Duca, F.; Aschauer, S.; Schönbauer, R.; Beitzke, D.; et al. Sex Differences in Left Ventricular Remodeling and Outcomes in Chronic Aortic Regurgitation. J. Clin. Med. 2020, 9, 4100. https://doi.org/10.3390/jcm9124100
Kammerlander AA, Donà C, Nitsche C, Koschutnik M, Zafar A, Eslami P, Duca F, Aschauer S, Schönbauer R, Beitzke D, et al. Sex Differences in Left Ventricular Remodeling and Outcomes in Chronic Aortic Regurgitation. Journal of Clinical Medicine. 2020; 9(12):4100. https://doi.org/10.3390/jcm9124100
Chicago/Turabian StyleKammerlander, Andreas A., Carolina Donà, Christian Nitsche, Matthias Koschutnik, Amna Zafar, Parastou Eslami, Franz Duca, Stefan Aschauer, Robert Schönbauer, Dietrich Beitzke, and et al. 2020. "Sex Differences in Left Ventricular Remodeling and Outcomes in Chronic Aortic Regurgitation" Journal of Clinical Medicine 9, no. 12: 4100. https://doi.org/10.3390/jcm9124100






