Comparison between Micro-Computed Tomography and Cone-Beam Computed Tomography in the Assessment of Bone Quality and a Long-Term Volumetric Study of the Augmented Sinus Grafted with an Albumin Impregnated Allograft
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- History of systemic diseases that alter bone metabolism (osteoporosis, diabetes mellitus),
- History of medication known to alter bone remodeling (bisphosphonates, RANK ligand inhibitor monoclonal antibodies, corticosteroids),
- History of uncontrolled medical or psychiatric disorders,
- Inflammations of the paranasal sinuses or the alveolar process,
- History of tumors or irradiation therapy in the head and neck region,
- Unwillingness to return for follow-ups,
- Pregnancy,
- Smoking,
- Inability to perform proper oral hygiene.
2.2. Albumin Impregnated Allograft Preparation
2.3. Surgical Interventions
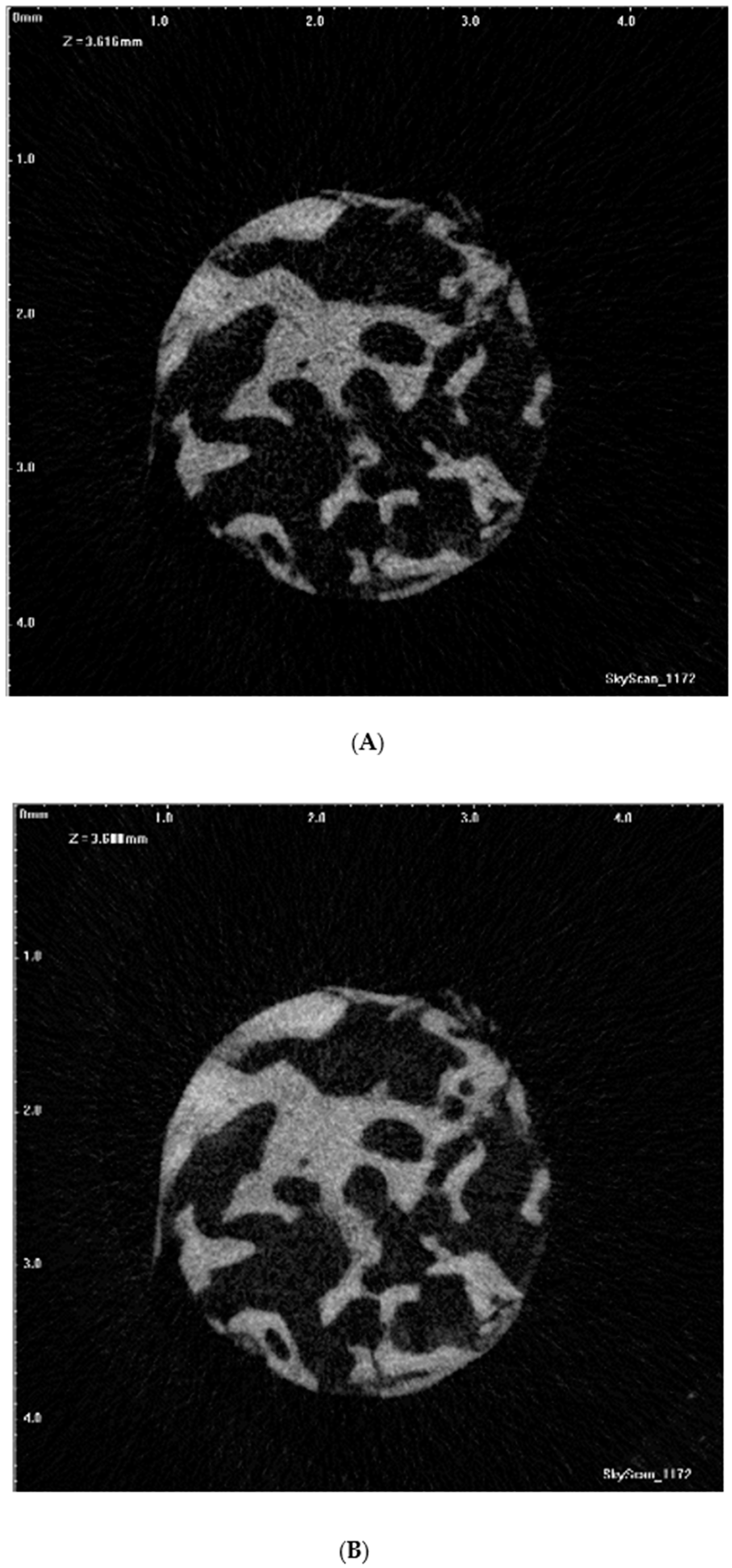
2.4. Micro-CT Scanning
2.5. Micro-CT Imaging Analysis
2.6. CBCT Imaging
2.7. Statistical Analysis
3. Results
3.1. Correlation of the Micromorphometric Data
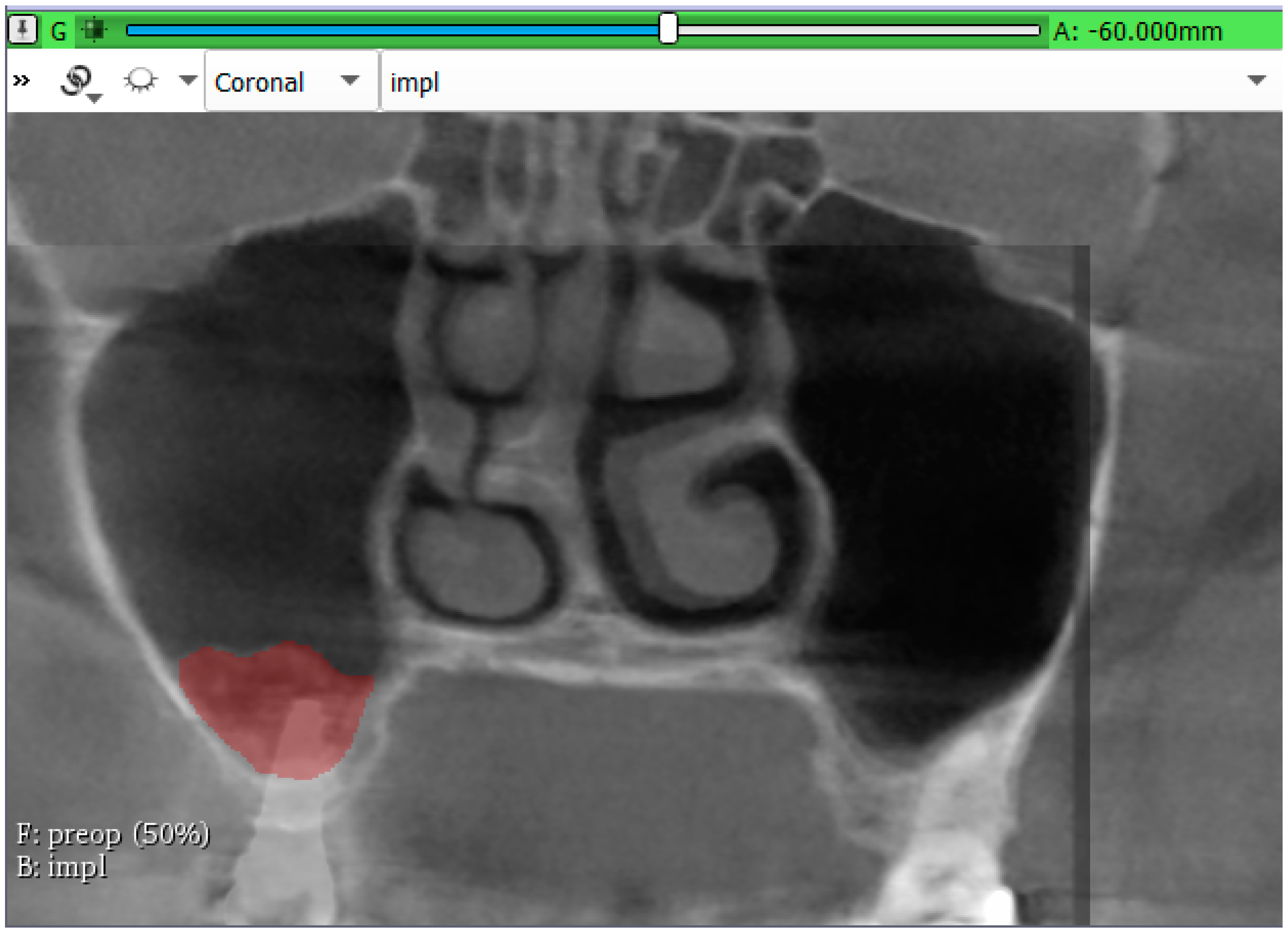
3.2. Volumetric Assessment of the Augmented Sinuses
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, L.R.; Ko, N.Y.; Chen, K.H. Medical treatment for osteoporosis: From molecular to clinical opinions. Int. J. Mol. Sci. 2019, 20, 2213. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Cardaropoli, G.; Araujo, M.; Lindhe, J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J. Clin. Periodontol. 2003, 30, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, V.; Engel, O.; Reyes, M.; Shahim, K.; Nolte, L.P.; Buser, D. Ridge alterations post-Extraction in the esthetic zone: A 3d analysis with cbct. J. Dent. Res. 2013, 92, 195s–201s. [Google Scholar] [CrossRef]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-Tooth extraction: A clinical and radiographic 12-Month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Johnson, K. A study of the dimensional changes occurring in the maxilla following tooth extraction. Aust. Dent. J. 1969, 14, 241–244. [Google Scholar] [CrossRef]
- Lana, J.P.; Carneiro, P.M.; Machado Vde, C.; de Souza, P.E.; Manzi, F.R.; Horta, M.C. Anatomic variations and lesions of the maxillary sinus detected in cone beam computed tomography for dental implants. Clin. Oral Implant. Res. 2012, 23, 1398–1403. [Google Scholar] [CrossRef]
- Schuh, E.; Schmiedl, R.; Vogel, G. [anatomic limits of endosseous dental implantation]. Z Stomatol. 1984, 81, 81–90. [Google Scholar]
- Tatum, O.H., Jr.; Lebowitz, M.S.; Tatum, C.A.; Borgner, R.A. Sinus augmentation. Rationale, development, long-term results. N.Y. State Dent. J. 1993, 59, 43–48. [Google Scholar]
- Liu, R.; Yan, M.; Chen, S.; Huang, W.; Wu, D.; Chen, J. Effectiveness of platelet-Rich fibrin as an adjunctive material to bone graft in maxillary sinus augmentation: A meta-Analysis of randomized controlled trails. Biomed. Res. Int. 2019, 2019, 7267062. [Google Scholar] [CrossRef]
- Yamada, M.; Egusa, H. Current bone substitutes for implant dentistry. J. Prosthodont. Res. 2018, 62, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Tan, W.C.; Zwahlen, M.; Lang, N.P. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. J. Clin. Periodontol. 2008, 35, 216–240. [Google Scholar] [CrossRef] [PubMed]
- Handschel, J.; Simonowska, M.; Naujoks, C.; Depprich, R.A.; Ommerborn, M.A.; Meyer, U.; Kubler, N.R. A histomorphometric meta-Analysis of sinus elevation with various grafting materials. Head Face Med. 2009, 5, 12. [Google Scholar] [CrossRef]
- Horvathy, D.B.; Vacz, G.; Szabo, T.; Szigyarto, I.C.; Toro, I.; Vamos, B.; Hornyak, I.; Renner, K.; Klara, T.; Szabo, B.T.; et al. Serum albumin coating of demineralized bone matrix results in stronger new bone formation. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 126–132. [Google Scholar] [CrossRef]
- Horvathy, D.B.; Vacz, G.; Toro, I.; Szabo, T.; May, Z.; Duarte, M.; Hornyak, I.; Szabo, B.T.; Dobo-Nagy, C.; Doros, A.; et al. Remineralization of demineralized bone matrix in critical size cranial defects in rats: A 6-Month follow-Up study. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1336–1342. [Google Scholar] [CrossRef]
- Schandl, K.; Horvathy, D.B.; Doros, A.; Majzik, E.; Schwarz, C.M.; Csonge, L.; Abkarovits, G.; Bucsi, L.; Lacza, Z. Bone-Albumin filling decreases donor site morbidity and enhances bone formation after anterior cruciate ligament reconstruction with bone-patellar tendon-Bone autografts. Int. Orthop. 2016, 40, 2097–2104. [Google Scholar] [CrossRef]
- Kubler, N.R.; Will, C.; Depprich, R.; Betz, T.; Reinhart, E.; Bill, J.S.; Reuther, J.F. [comparative studies of sinus floor elevation with autologous or allogeneic bone tissue]. Mund. Kiefer. Gesichtschir. 1999, 3 (Suppl. 1), S53–S60. [Google Scholar]
- Scarano, A.; Degidi, M.; Iezzi, G.; Pecora, G.; Piattelli, M.; Orsini, G.; Caputi, S.; Perrotti, V.; Mangano, C.; Piattelli, A. Maxillary sinus augmentation with different biomaterials: A comparative histologic and histomorphometric study in man. Implant. Dent. 2006, 15, 197–207. [Google Scholar] [CrossRef]
- Jensen, O.T.; Shulman, L.B.; Block, M.S.; Iacono, V.J. Report of the sinus consensus conference of 1996. Int. J. Oral Maxillofac. Implant. 1998, 13, 11–45. [Google Scholar]
- Nyström, E.; Legrell, P.E.; Forssell, A.; Kahnberg, K.E. Combined use of bone grafts and implants in the severely resorbed maxilla. Postoperative evaluation by computed tomography. Int. J. Oral Maxillofac. Surg. 1995, 24, 20–25. [Google Scholar] [CrossRef]
- Hatano, N.; Shimizu, Y.; Ooya, K. A clinical long-Term radiographic evaluation of graft height changes after maxillary sinus floor augmentation with a 2:1 autogenous bone/xenograft mixture and simultaneous placement of dental implants. Clin. Oral Implant. Res. 2004, 15, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, R.; Jacobs, R.; Singer, S.R.; Mupparapu, M. Cbct-Based bone quality assessment: Are hounsfield units applicable? Dentomaxillofac. Radiol. 2015, 44, 20140238. [Google Scholar] [CrossRef] [PubMed]
- Merheb, J.; Van Assche, N.; Coucke, W.; Jacobs, R.; Naert, I.; Quirynen, M. Relationship between cortical bone thickness or computerized tomography-derived bone density values and implant stability. Clin. Oral Implant. Res. 2010, 21, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E. Density of bone: Effect on treatment plans, surgical approach, healing, and progressive boen loading. Int. J. Oral Implantol. 1990, 6, 23–31. [Google Scholar]
- Chappard, D.; Retailleau-Gaborit, N.; Legrand, E.; Basle, M.F.; Audran, M. Comparison insight bone measurements by histomorphometry and microct. J. Bone Miner. Res. 2005, 20, 1177–1184. [Google Scholar] [CrossRef]
- Muller, R.; Van Campenhout, H.; Van Damme, B.; Van Der Perre, G.; Dequeker, J.; Hildebrand, T.; Ruegsegger, P. Morphometric analysis of human bone biopsies: A quantitative structural comparison of histological sections and micro-Computed tomography. Bone 1998, 23, 59–66. [Google Scholar] [CrossRef]
- Ludlow, J.B.; Timothy, R.; Walker, C.; Hunter, R.; Benavides, E.; Samuelson, D.B.; Scheske, M.J. Effective dose of dental cbct-A meta analysis of published data and additional data for nine cbct units. Dentomaxillofac. Radiol. 2015, 44, 20140197. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.Y.; DoDo, H.; Yousef, H.; Firestone, A.R.; Chaudhry, J.; Johnston, W.M.; Lee, D.J.; Emam, H.A.; Kim, D.G. Efficacy of cone-Beam computed tomography in evaluating bone quality for optimum implant treatment planning. Implant Dent. 2017, 26, 405–411. [Google Scholar] [CrossRef]
- Schulze, R.; Heil, U.; Gross, D.; Bruellmann, D.D.; Dranischnikow, E.; Schwanecke, U.; Schoemer, E. Artefacts in cbct: A review. Dentomaxillofac. Radiol. 2011, 40, 265–273. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, R.; Monje, F. Is micro-Computed tomography reliable to determine the microstructure of the maxillary alveolar bone? Clin. Oral Implant. Res. 2013, 24, 730–737. [Google Scholar] [CrossRef]
- Hua, Y.; Nackaerts, O.; Duyck, J.; Maes, F.; Jacobs, R. Bone quality assessment based on cone beam computed tomography imaging. Clin. Oral Implant. Res. 2009, 20, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Parsa, A.; Hassan, B.; van der Stelt, P.; Aartman, I.H.; Wismeijer, D. Accuracy of trabecular bone microstructural measurement at planned dental implant sites using cone-Beam ct datasets. Clin. Oral Implant. Res. 2014, 25, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Yi, W.J.; Heo, M.S.; Lee, S.S.; Choi, S.C.; Huh, K.H. Three-Dimensional evaluation of human jaw bone microarchitecture: Correlation between the microarchitectural parameters of cone beam computed tomography and micro-Computer tomography. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Monje, F.; Gonzalez-Garcia, R.; Galindo-Moreno, P.; Rodriguez-Salvanes, F.; Wang, H.L. Comparison between microcomputed tomography and cone-Beam computed tomography radiologic bone to assess atrophic posterior maxilla density and microarchitecture. Clin. Oral Implant. Res. 2014, 25, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Parsa, A.; Ibrahim, N.; Hassan, B.; van der Stelt, P.; Wismeijer, D. Bone quality evaluation at dental implant site using multislice ct, micro-ct, and cone beam ct. Clin. Oral Implant. Res. 2015, 26, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, R.; Sessirisombat, S.; Panmekiate, S. Mandibular bone structure analysis using cone beam computed tomography vs primary implant stability: An ex vivo study. Int. J. Oral Maxillofac. Implant. 2017, 32, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Soardi, C.M.; Zaffe, D.; Motroni, A.; Wang, H.L. Quantitative comparison of cone beam computed tomography and microradiography in the evaluation of bone density after maxillary sinus augmentation: A preliminary study. Clin. Implant Dent. Relat. Res. 2014, 16, 557–564. [Google Scholar] [CrossRef][Green Version]
- Van Dessel, J.; Huang, Y.; Depypere, M.; Rubira-Bullen, I.; Maes, F.; Jacobs, R. A comparative evaluation of cone beam ct and micro-ct on trabecular bone structures in the human mandible. Dentomaxillofac. Radiol. 2013, 42, 20130145. [Google Scholar] [CrossRef]
- Van Dessel, J.; Nicolielo, L.F.; Huang, Y.; Coudyzer, W.; Salmon, B.; Lambrichts, I.; Jacobs, R. Accuracy and reliability of different cone beam computed tomography (cbct) devices for structural analysis of alveolar bone in comparison with multislice ct and micro-ct. Eur. J. Oral Implantol. 2017, 10, 95–105. [Google Scholar]
- Marton, K.; Tamas, S.B.; Orsolya, N.; Bela, C.; Ferenc, D.; Peter, N.; Csaba, D.N.; Lajos, C.; Zsombor, L.; Eitan, M.; et al. Microarchitecture of the augmented bone following sinus elevation with an albumin impregnated demineralized freeze-Dried bone allograft (bonealbumin) versus anorganic bovine bone mineral: A randomized prospective clinical, histomorphometric, and micro-Computed tomography study. Materials 2018, 11, 202. [Google Scholar] [CrossRef]
- Feldkamp, L.A.; Goldstein, S.A.; Parfitt, A.M.; Jesion, G.; Kleerekoper, M. The direct examination of three-dimensional bone architecture in vitro by computed tomography. J. Bone Miner. Res. 1989, 4, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Kivovics, M.; Szabo, B.T.; Nemeth, O.; Tari, N.; Dori, F.; Nagy, P.; Dobo-Nagy, C.; Szabo, G. Microarchitectural study of the augmented bone following ridge preservation with a porcine xenograft and a collagen membrane: Preliminary report of a prospective clinical, histological, and micro-Computed tomography analysis. Int. J. Oral Maxillofac. Surg. 2017, 46, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, N.; Çetin, S.; Bilecenoğlu, B.; Altan, A.; Akbulut, S.; Ocak, M.; Orhan, K. The micro-ct evaluation of enamel-cement thickness, abrasion, and mineral density in teeth in the postmortem interval (pmi): New parameters for the determination of pmi. Int. J. Legal Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, H.J.; Boyce, R.W.; Nyengaard, J.R.; Odgaard, A. The conneulor: Unbiased estimation of connectivity using physical disectors under projection. Bone 1993, 14, 217–222. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Garcia, R.; Monje, F. The reliability of cone-Beam computed tomography to assess bone density at dental implant recipient sites: A histomorphometric analysis by micro-ct. Clin. Oral Implant. Res. 2013, 24, 871–879. [Google Scholar] [CrossRef]
- Bover-Ramos, F.; Vina-Almunia, J.; Cervera-Ballester, J.; Penarrocha-Diago, M.; Garcia-Mira, B. Accuracy of implant placement with computer-Guided surgery: A systematic review and meta-Analysis comparing cadaver, clinical, and in vitro studies. Int. J. Oral Maxillofac. Implant. 2018, 33, 101–115. [Google Scholar] [CrossRef]
- Schneider, D.; Marquardt, P.; Zwahlen, M.; Jung, R.E. A systematic review on the accuracy and the clinical outcome of computer-Guided template-Based implant dentistry. Clin. Oral Implant. Res. 2009, 20 (Suppl. 4), 73–86. [Google Scholar] [CrossRef]
- Szabó, B.T.; Dobó-Nagy, C. Modulation transfer function evaluation of cone beam computed and microcomputed tomography by using slanted edge phantom. Med. Imaging Process Technol. 2019. [Google Scholar] [CrossRef]

| Abbreviation | Variable | Description | Standard Unit |
|---|---|---|---|
| BV/TV | Bone volume fraction | The relative volume of calcified tissue in the selected volume of interest (VOI). | % |
| Tb.Th | Trabecular thickness | Mean thickness of trabeculae, assessed using direct 3D methods. | µm |
| Tb.Sp | Trabecular separation | Mean distance between trabeculae, assessed using direct 3D methods. | µm |
| Po.V(op) | The volume of open pore space | The total volume of all open pores within the VOI is reported. An open pore is defined as any space located within a solid object or between solid objects, which has any connection in 3D to space outside the object or objects. | µm3 |
| Po(op) | Open porosity (percent) | Percent of open porosity is the volume of open pores as a percent of the total VOI volume. | % |
| Po.V(tot) | The total volume of pore space | The total volume of all open and closed pores within the VOI is reported. | µm3 |
| Po(top) | Total porosity (percent) | Percent of total porosity is the volume of total pores as a percent of the total VOI volume. | % |
| Unit | Mean | Minimum | Maximum | ||
|---|---|---|---|---|---|
| BV/TV | CBCT | % | 81.289 | 37.872 | 98.423 |
| micro-CT | % | 12.251 | 4.548 | 21.879 | |
| Tb.Th | CBCT | µm | 1823.571 | 1122.201 | 2257.021 |
| micro-CT | µm | 148.936 | 115.679 | 194.131 | |
| Tb.Sp | CBCT | µm | 846.649 | 500.000 | 1767.296 |
| micro-CT | µm | 875.978 | 332.867 | 1588.054 | |
| PoV(op) | CBCT | mm3 | 8.986 | 0.811 | 27.270 |
| micro-CT | mm3 | 45.826 | 10.322 | 85.207 | |
| Po(op) | CBCT | % | 18.691 | 1.569 | 62.128 |
| micro-CT | % | 87.744 | 78.112 | 95.451 | |
| PoV(tot) | CBCT | mm3 | 8.994 | 0.811 | 27.270 |
| micro-CT | mm3 | 45.828 | 10.324 | 85.207 | |
| Po(tot) | CBCT | % | 18.711 | 1.577 | 62.128 |
| micro-CT | % | 87.749 | 78.121 | 95.452 | |
| Micromorphometric Parameter | R-Value | p-Value |
|---|---|---|
| BV/TV | 0.550 | 0.034 * |
| Tb.Th | −0.550 | 0.034 * |
| Tb.Sp | 0.613 | 0.015 * |
| PoV(op) | 0.575 | 0.025 * |
| Po(op) | 0.539 | 0.038 * |
| PoV(tot) | 0.575 | 0.025 * |
| Po(tot) | 0.550 | 0.034 * |
| Unit | Mean | Minimum | Maximum | |
|---|---|---|---|---|
| Bone gain after 6-months | mm3 | 1623.32 | 815.79 | 2330.43 |
| Bone gain after 3-years | mm3 | 981.19 | 371.89 | 1403.69 |
| Volume reduction | % | 39.28 | 11.88 | 61.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kivovics, M.; Szabó, B.T.; Németh, O.; Iványi, D.; Trimmel, B.; Szmirnova, I.; Orhan, K.; Mijiritsky, E.; Szabó, G.; Dobó-Nagy, C. Comparison between Micro-Computed Tomography and Cone-Beam Computed Tomography in the Assessment of Bone Quality and a Long-Term Volumetric Study of the Augmented Sinus Grafted with an Albumin Impregnated Allograft. J. Clin. Med. 2020, 9, 303. https://doi.org/10.3390/jcm9020303
Kivovics M, Szabó BT, Németh O, Iványi D, Trimmel B, Szmirnova I, Orhan K, Mijiritsky E, Szabó G, Dobó-Nagy C. Comparison between Micro-Computed Tomography and Cone-Beam Computed Tomography in the Assessment of Bone Quality and a Long-Term Volumetric Study of the Augmented Sinus Grafted with an Albumin Impregnated Allograft. Journal of Clinical Medicine. 2020; 9(2):303. https://doi.org/10.3390/jcm9020303
Chicago/Turabian StyleKivovics, Márton, Bence Tamás Szabó, Orsolya Németh, Dóra Iványi, Bálint Trimmel, Ilona Szmirnova, Kaan Orhan, Eitan Mijiritsky, György Szabó, and Csaba Dobó-Nagy. 2020. "Comparison between Micro-Computed Tomography and Cone-Beam Computed Tomography in the Assessment of Bone Quality and a Long-Term Volumetric Study of the Augmented Sinus Grafted with an Albumin Impregnated Allograft" Journal of Clinical Medicine 9, no. 2: 303. https://doi.org/10.3390/jcm9020303
APA StyleKivovics, M., Szabó, B. T., Németh, O., Iványi, D., Trimmel, B., Szmirnova, I., Orhan, K., Mijiritsky, E., Szabó, G., & Dobó-Nagy, C. (2020). Comparison between Micro-Computed Tomography and Cone-Beam Computed Tomography in the Assessment of Bone Quality and a Long-Term Volumetric Study of the Augmented Sinus Grafted with an Albumin Impregnated Allograft. Journal of Clinical Medicine, 9(2), 303. https://doi.org/10.3390/jcm9020303









