Prevalence of Bacteria of Genus Actinomyces in Persistent Extraradicular Lesions—Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria and Research Methodology
- Include all those studies that have identified Actinomyces on the external radiculatum surface of the dental roots in teeth with persistent lesions previously treated by means of endodontic therapy;
- Include all those articles that have identified bacteria in persistent endodontic lesions after retreatment with extraradicular involvement;
- Include all articles that have analyzed the presence of Actinomyces infections in endodontic lesions (secondary outcome);
- The exclusion criteria are to exclude all those studies and articles that deal only with case reports and reviews;
- Include articles performed on a population larger than twenty teeth;
- Exclude all those studies that did not search for the presence of Actinomyces in the endodontic setting and that do not report data on the prevalence or incidence of Actinomyces.
2.2. Screening Methodology
- (1)
- Primary outcome: number of teeth with the presence of a persistent extraradicular infection in which the presence of Actinomyces has been ascertained;
- (2)
- Secondary outcome: number of teeth with endodontic infection in which the presence of Actinomyces has been ascertained;
- (3)
- Tertiary outcome difference in the prevalence of bacteria of the genus Actinomyces between primary endodontic infections and secondary endodontic infections.
2.3. Risk of Bias Assessment and Planned Methods for Analysis
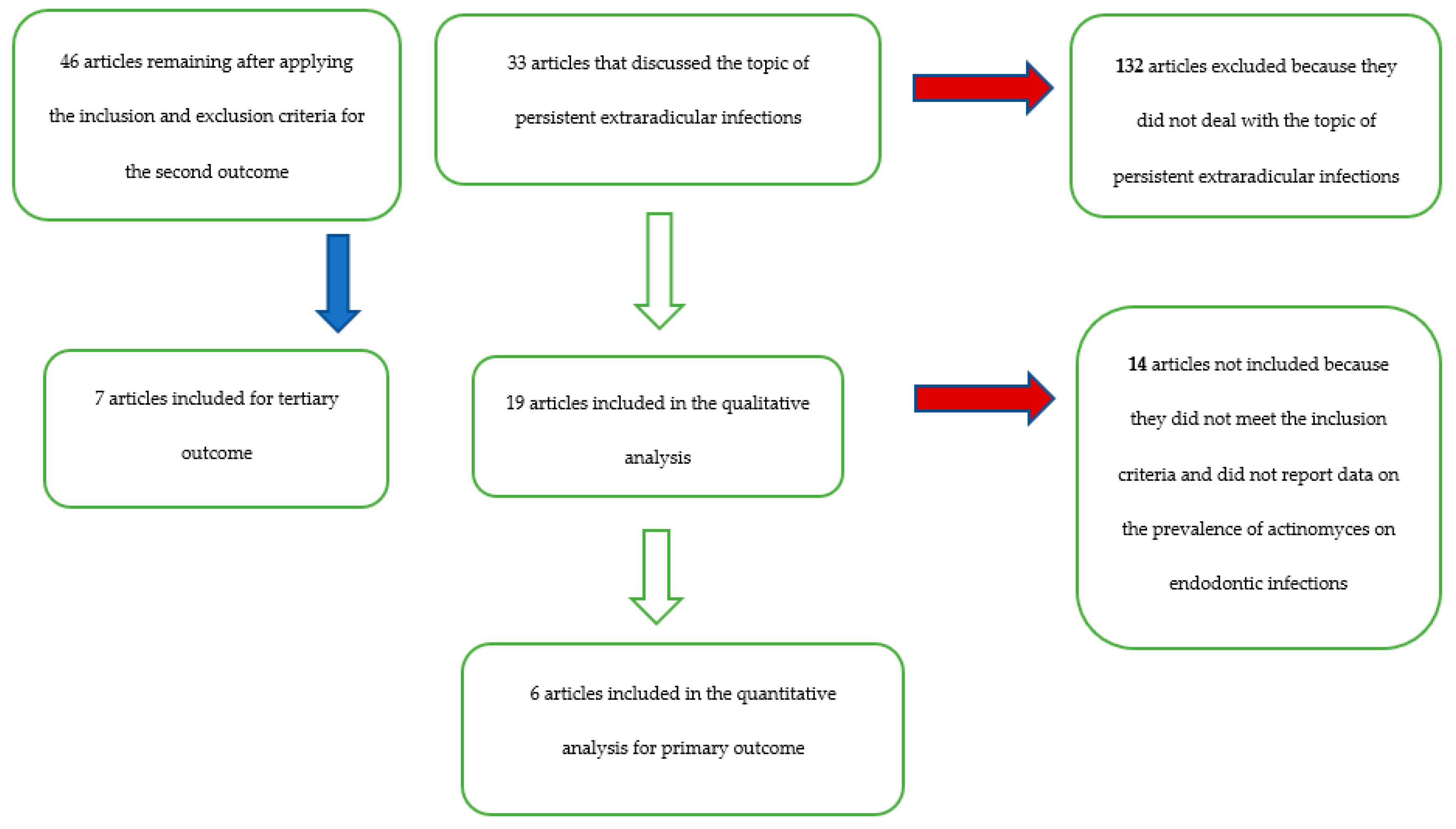
3. Results
- 46 articles for the secondary outcome: Pourhajibagher et al. 2018 [24], Lysakowska et al. 2016 [25], Halbauer et al. 2013 [26], Signoretti et al. 2013 [23], Niazi et al. 2010 [27], Fujii et al. 2009 [28], Vianna et al. 2007 [29], Chavez de Paz et al. 2005 [30], Gomes et al. 2004 [31], Claesson et al. 2017 [12], Rolph et al. 2001 [32], Sundqvist et al. 1998 [33], Vigil et al. 1997 [34], Sjogren et al. 1997 [35], Gomes et al. 1996 [36], Debelian et al. 1995 [37], Fukushima et al. 1990 [38], Qi et al. 2016 [39], Fernandes et al. 2014 [40], Tennert et al. 2014 [41], Chugal et al. 2011 [42], Ledezma-Rasillo et al. 2010 [43], Zhang et al. 2010 [22], Mindere et al. 2010 [44], Cogulu et al. 2008 [45], Chu et al. 2005 [46], Chavez de Paz et al. 2004 [47], Siqueira et al. 2004 [48], Hirshberg et al. 2003 [21], Tang et al. 2003 [49], Xia et al. 2003 [50], Pinheiro et al. 2003 [51], Siqueira et al. 2002 [52], Peters et al. 2002 [53], Sunde et al. 2002 [20], Siqueira et al. 2002 [54], Ercan et al. 2006 [55], Molander et al. 1998 [56], Ruviere et al. 2008 [57], Sundqvist et al. 1992 [58], Brauner and Conrads 1995 [59], Assed et al. 1996 [60], Hancock et al. 2001 [61], Esteves et al. 2017 [19], Persoon et al. 2017 [18];
3.1. Study Characteristics and Data Extraction
3.2. Risk of Bias
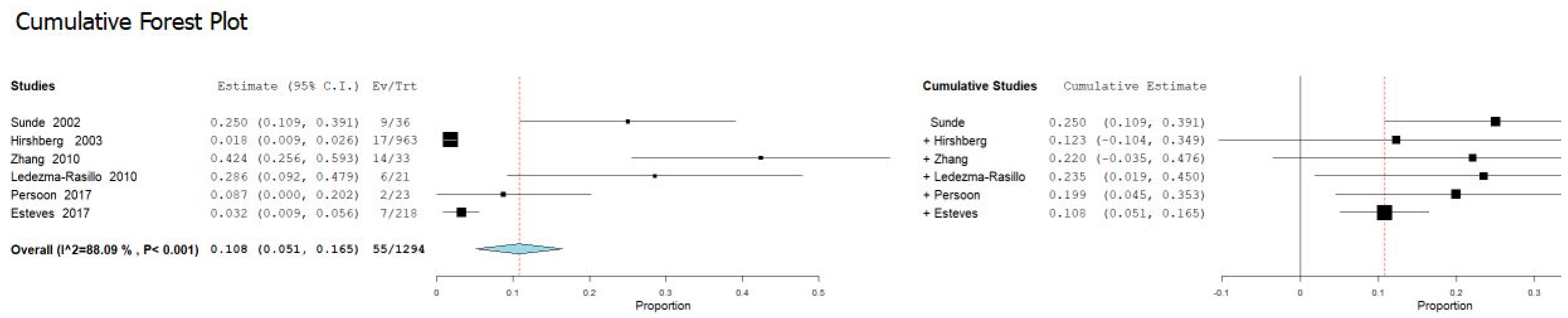
3.3. Meta-Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vishwanath, V.; Rao, H.M. Gutta-percha in endodontics—A comprehensive review of material science. J. Conserv. Dent. 2019, 22, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Troiano, G.; Perrone, D.; Dioguardi, M.; Buonavoglia, A.; Ardito, F.; Lo Muzio, L. In vitro evaluation of the cytotoxic activity of three epoxy resin-based endodontic sealers. Dent. Mater. J. 2018, 37, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, M.; Gioia, G.D.; Illuzzi, G.; Laneve, E.; Cocco, A.; Troiano, G. Endodontic irrigants: Different methods to improve efficacy and related problems. Eur. J. Dent. 2018, 12, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, M.; Di Gioia, G.; Illuzzi, G.; Arena, C.; Caponio, V.C.A.; Caloro, G.A.; Zhurakivska, K.; Adipietro, I.; Troiano, G.; Lo Muzio, L. Inspection of the Microbiota in Endodontic Lesions. Dent. J. 2019, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.S.; Rodrigues, V.A.A.; Furtado, W.T.; Gueiros, S.; Pereira, G.S.; Avila-Campos, M.J. Microbial analysis of root canal and periradicular lesion associated to teeth with endodontic failure. Anaerobe 2017, 48, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Rocas, I.N. Polymerase chain reaction detection of Propionibacterium propionicus and Actinomyces radicidentis in primary and persistent endodontic infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 96, 215–222. [Google Scholar] [CrossRef]
- Urs, A.B.; Singh, H.; Nunia, K.; Mohanty, S.; Gupta, S. Post endodontic Aspergillosis in an immunocompetent individual. J. Clin. Exp. Dent. 2015, 7, e535–e539. [Google Scholar] [CrossRef]
- Ricucci, D.; Lopes, W.S.P.; Loghin, S.; Rocas, I.N.; Siqueira, J.F., Jr. Large Bacterial Floc Causing an Independent Extraradicular Infection and Posttreatment Apical Periodontitis: A Case Report. J. Endod. 2018, 44, 1308–1316. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira, J.F., Jr.; Lopes, W.S.; Vieira, A.R.; Rocas, I.N. Extraradicular infection as the cause of persistent symptoms: A case series. J. Endod. 2015, 41, 265–273. [Google Scholar] [CrossRef]
- Ricucci, D.; Candeiro, G.T.; Bugea, C.; Siqueira, J.F., Jr. Complex Apical Intraradicular Infection and Extraradicular Mineralized Biofilms as the Cause of Wet Canals and Treatment Failure: Report of 2 Cases. J. Endod. 2016, 42, 509–515. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira, J.F., Jr. Apical actinomycosis as a continuum of intraradicular and extraradicular infection: Case report and critical review on its involvement with treatment failure. J. Endod. 2008, 34, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Claesson, R.; Sjogren, U.; Esberg, A.; Brundin, M.; Granlund, M. Actinomyces radicidentis and Actinomyces haliotis, coccoid Actinomyces species isolated from the human oral cavity. Anaerobe 2017, 48, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Sousa, B.C.; Gomes, F.A.; Ferreira, C.M.; Rocha, M.; Barros, E.B.; Albuquerque, D.S. Persistent extra-radicular bacterial biofilm in endodontically treated human teeth: Scanning electron microscopy analysis after apical surgery. Microsc. Res. Tech. 2017, 80, 662–667. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.; Jiang, Y.; Liang, J. Imaging of extraradicular biofilm using combined scanning electron microscopy and stereomicroscopy. Microsc. Res. Tech. 2013, 76, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Prada, I.; Mico-Munoz, P.; Giner-Lluesma, T.; Mico-Martinez, P.; Collado-Castellano, N.; Manzano-Saiz, A. Influence of microbiology on endodontic failure. Literature review. Med. OralPatol. Oral Y Cir. Bucal 2019, 24, e364–e372. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef]
- Persoon, I.F.; Buijs, M.J.; Ozok, A.R.; Crielaard, W.; Krom, B.P.; Zaura, E.; Brandt, B.W. The mycobiome of root canal infections is correlated to the bacteriome. Clin. Oral Investig. 2017, 21, 1871–1881. [Google Scholar] [CrossRef]
- Esteves, L.S.; Henriques, A.C.G.; Silva, C.; Cangussu, M.C.T.; Ramos, E.A.G.; Estrela, C.; Santos, J.N.D. Actinomycosis is not Frequent in the Periapex But is a Persistent Lesion. Braz. Dent. J. 2017, 28, 688–693. [Google Scholar] [CrossRef]
- Sunde, P.T.; Olsen, I.; Debelian, G.J.; Tronstad, L. Microbiota of periapical lesions refractory to endodontic therapy. J. Endod. 2002, 28, 304–310. [Google Scholar] [CrossRef]
- Hirshberg, A.; Tsesis, I.; Metzger, Z.; Kaplan, I. Periapical actinomycosis: A clinicopathologic study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 95, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Q.Q.; Zhang, C.F.; Soo, I. Identification of dominant pathogens in periapical lesions associated with persistent apical periodontitis. Chin. J. Dent. Res. 2010, 13, 115–121. [Google Scholar] [PubMed]
- Signoretti, F.G.; Gomes, B.P.; Montagner, F.; Jacinto, R.C. Investigation of cultivable bacteria isolated from longstanding retreatment-resistant lesions of teeth with apical periodontitis. J. Endod. 2013, 39, 1240–1244. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Bahador, A. An in vivo evaluation of microbial diversity before and after the photo-activated disinfection in primary endodontic infections: Traditional phenotypic and molecular approaches. Photodiagnosis Photodyn. Ther. 2018, 22, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lysakowska, M.E.; Ciebiada-Adamiec, A.; Sienkiewicz, M.; Sokolowski, J.; Banaszek, K. The cultivable microbiota of primary and secondary infected root canals, their susceptibility to antibiotics and association with the signs and symptoms of infection. Int. Endod. J. 2016, 49, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Halbauer, K.; Prskalo, K.; Jankovic, B.; Tarle, Z.; Panduric, V.; Kalenic, S. Efficacy of ozone on microorganisms in the tooth root canal. Coll. Antropol. 2013, 37, 101–107. [Google Scholar]
- Niazi, S.A.; Clarke, D.; Do, T.; Gilbert, S.C.; Mannocci, F.; Beighton, D. Propionibacterium acnes and Staphylococcus epidermidis isolated from refractory endodontic lesions are opportunistic pathogens. J. Clin. Microbiol. 2010, 48, 3859–3869. [Google Scholar] [CrossRef]
- Fujii, R.; Saito, Y.; Tokura, Y.; Nakagawa, K.I.; Okuda, K.; Ishihara, K. Characterization of bacterial flora in persistent apical periodontitis lesions. Oral Microbiol. Immunol. 2009, 24, 502–505. [Google Scholar] [CrossRef]
- Vianna, M.E.; Horz, H.P.; Conrads, G.; Zaia, A.A.; Souza-Filho, F.J.; Gomes, B.P. Effect of root canal procedures on endotoxins and endodontic pathogens. Oral Microbiol. Immunol. 2007, 22, 411–418. [Google Scholar] [CrossRef]
- Chavez de Paz, L.; Svensater, G.; Dahlen, G.; Bergenholtz, G. Streptococci from root canals in teeth with apical periodontitis receiving endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 232–241. [Google Scholar] [CrossRef]
- Gomes, B.P.; Pinheiro, E.T.; Gade-Neto, C.R.; Sousa, E.L.; Ferraz, C.C.; Zaia, A.A.; Teixeira, F.B.; Souza-Filho, F.J. Microbiological examination of infected dental root canals. Oral Microbiol. Immunol. 2004, 19, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Rolph, H.J.; Lennon, A.; Riggio, M.P.; Saunders, W.P.; MacKenzie, D.; Coldero, L.; Bagg, J. Molecular identification of microorganisms from endodontic infections. J. Clin. Microbiol. 2001, 39, 3282–3289. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist, G.; Figdor, D.; Persson, S.; Sjogren, U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 86–93. [Google Scholar] [CrossRef]
- Vigil, G.V.; Wayman, B.E.; Dazey, S.E.; Fowler, C.B.; Bradley, D.V., Jr. Identification and antibiotic sensitivity of bacteria isolated from periapical lesions. J. Endod. 1997, 23, 110–114. [Google Scholar] [CrossRef]
- Sjogren, U.; Figdor, D.; Persson, S.; Sundqvist, G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int. Endod. J. 1997, 30, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.P.; Lilley, J.D.; Drucker, D.B. Clinical significance of dental root canal microflora. J. Dent. 1996, 24, 47–55. [Google Scholar] [CrossRef]
- Debelian, G.J.; Olsen, I.; Tronstad, L. Bacteremia in conjunction with endodontic therapy. Endod. Dent. Traumatol. 1995, 11, 142–149. [Google Scholar] [CrossRef]
- Fukushima, H.; Yamamoto, K.; Hirohata, K.; Sagawa, H.; Leung, K.P.; Walker, C.B. Localization and identification of root canal bacteria in clinically asymptomatic periapical pathosis. J. Endod. 1990, 16, 534–538. [Google Scholar] [CrossRef]
- Qi, Z.; Cao, H.; Jiang, H.; Zhao, J.; Tang, Z. Combinations of bacterial species associated with symptomatic endodontic infections in a Chinese population. Int. Endod. J. 2016, 49, 17–25. [Google Scholar] [CrossRef]
- Fernandes Cdo, C.; Rechenberg, D.K.; Zehnder, M.; Belibasakis, G.N. Identification of Synergistetes in endodontic infections. Microb. Pathog. 2014, 73, 1–6. [Google Scholar] [CrossRef][Green Version]
- Tennert, C.; Fuhrmann, M.; Wittmer, A.; Karygianni, L.; Altenburger, M.J.; Pelz, K.; Hellwig, E.; Al-Ahmad, A. New bacterial composition in primary and persistent/secondary endodontic infections with respect to clinical and radiographic findings. J. Endod. 2014, 40, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Chugal, N.; Wang, J.K.; Wang, R.; He, X.; Kang, M.; Li, J.; Zhou, X.; Shi, W.; Lux, R. Molecular characterization of the microbial flora residing at the apical portion of infected root canals of human teeth. J. Endod. 2011, 37, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Ledezma-Rasillo, G.; Flores-Reyes, H.; Gonzalez-Amaro, A.M.; Garrocho-Rangel, A.; Ruiz-Rodriguez Mdel, S.; Pozos-Guillen, A.J. Identification of cultivable microorganisms from primary teeth with necrotic pulps. J. Clin. Pediatr. Dent. 2010, 34, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Mindere, A.; Kundzina, R.; Nikolajeva, V.; Eze, D.; Petrina, Z. Microflora of root filled teeth with apical periodontitis in Latvian patients. Stomatologija 2010, 12, 116–121. [Google Scholar]
- Cogulu, D.; Uzel, A.; Oncag, O.; Eronat, C. PCR-based identification of selected pathogens associated with endodontic infections in deciduous and permanent teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, 443–449. [Google Scholar] [CrossRef]
- Chu, F.C.; Tsang, C.S.; Chow, T.W.; Samaranayake, L.P. Identification of cultivable microorganisms from primary endodontic infections with exposed and unexposed pulp space. J. Endod. 2005, 31, 424–429. [Google Scholar] [CrossRef]
- Chavez de Paz, L.E.; Molander, A.; Dahlen, G. Gram-positive rods prevailing in teeth with apical periodontitis undergoing root canal treatment. Int. Endod. J. 2004, 37, 579–587. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rocas, I.N. Polymerase chain reaction-based analysis of microorganisms associated with failed endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 85–94. [Google Scholar] [CrossRef]
- Tang, G.; Samaranayake, L.P.; Yip, H.K.; Chu, F.C.; Tsang, P.C.; Cheung, B.P. Direct detection of Actinomyces spp. from infected root canals in a Chinese population: A study using PCR-based, oligonucleotide-DNA hybridization technique. J. Dent. 2003, 31, 559–568. [Google Scholar] [CrossRef]
- Xia, T.; Baumgartner, J.C. Occurrence of Actinomyces in infections of endodontic origin. J. Endod. 2003, 29, 549–552. [Google Scholar] [CrossRef]
- Pinheiro, E.T.; Gomes, B.P.; Ferraz, C.C.; Teixeira, F.B.; Zaia, A.A.; Souza Filho, F.J. Evaluation of root canal microorganisms isolated from teeth with endodontic failure and their antimicrobial susceptibility. Oral Microbiol. Immunol. 2003, 18, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F.; Rocas, I.N.; Moraes, S.R.; Santos, K.R. Direct amplification of rRNA gene sequences for identification of selected oral pathogens in root canal infections. Int. Endod. J. 2002, 35, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.B.; Wesselink, P.R.; van Winkelhoff, A.J. Combinations of bacterial species in endodontic infections. Int. Endod. J. 2002, 35, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Rocas, I.N.; Souto, R.; de Uzeda, M.; Colombo, A.P. Actinomyces species, streptococci, and Enterococcus faecalis in primary root canal infections. J. Endod. 2002, 28, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Ercan, E.; Dalli, M.; Yavuz, İ.; Özekinci, T. Investigation of Microorganisms in Infected Dental Root Canals. Biotechnol. Biotechnol. Equip. 2014, 20, 166–172. [Google Scholar] [CrossRef]
- Molander, A.; Reit, C.; Dahlen, G.; Kvist, T. Microbiological status of root-filled teeth with apical periodontitis. Int. Endod. J. 1998, 31, 1–7. [Google Scholar] [CrossRef]
- Ruviere, D.B.; Leonardo, M.R.; da Silva, L.A.; Ito, I.Y.; Nelson-Filho, P. Assessment of the microbiota in root canals of human primary teeth by checkerboard DNA-DNA hybridization. J. Dent. Child. 2007, 74, 118–123. [Google Scholar]
- Sundqvist, G. Associations between microbial species in dental root canal infections. Oral Microbiol. Immunol. 1992, 7, 257–262. [Google Scholar] [CrossRef]
- Brauner, A.W.; Conrads, G. Studies into the microbial spectrum of apical periodontitis. Int. Endod. J. 1995, 28, 244–248. [Google Scholar] [CrossRef]
- Assed, S.; Ito, I.Y.; Leonardo, M.R.; Silva, L.A.; Lopatin, D.E. Anaerobic microorganisms in root canals of human teeth with chronic apical periodontitis detected by indirect immunofluorescence. Endod. Dent. Traumatol. 1996, 12, 66–69. [Google Scholar] [CrossRef]
- Hancock, H.H.; Sigurdsson, A.; Trope, M.; Moiseiwitsch, J. Bacteria isolated after unsuccessful endodontic treatment in a North American population. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 91, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Collaboration. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: Hoboken, NJ, USA, 2008. [Google Scholar]
- Sundqvist, G.; Johansson, E.; Sjogren, U. Prevalence of black-pigmented bacteroides species in root canal infections. J. Endod. 1989, 15, 13–19. [Google Scholar] [CrossRef]
- Henry, N.R.; Hinze, J.D. Broncholithiasis secondary to pulmonary actinomycosis. Respir. Care 2014, 59, e27–e30. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial resistance in biofilm-associated bacteria. Future Microbiol. 2015, 10, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, M.; Troiano, G.; Laino, L.; Lo Russo, L.; Giannatempo, G.; Lauritano, F.; Cicciu, M.; Lo Muzio, L. ProTaper and WaveOne systems three-dimensional comparison of device parameters after the shaping technique. A micro-CT study on simulated root canals. Int. J. Clin. Exp. Med. 2015, 8, 17830–17834. [Google Scholar] [PubMed]
- Lubomski, M.; Dalgliesh, J.; Lee, K.; Damodaran, O.; McKew, G.; Reddel, S. Actinomyces cavernous sinus infection: A case and systematic literature review. Pr. Neurol 2018, 18, 373–377. [Google Scholar] [CrossRef]
- Boyanova, L.; Kolarov, R.; Mateva, L.; Markovska, R.; Mitov, I. Actinomycosis: A frequently forgotten disease. Future Microbiol. 2015, 10, 613–628. [Google Scholar] [CrossRef]
- Yoo, Y.J.; Perinpanayagam, H.; Oh, S.; Kim, A.R.; Han, S.H.; Kum, K.Y. Endodontic biofilms: Contemporary and future treatment options. Restor. Dent. Endod. 2019, 44, e7. [Google Scholar] [CrossRef]
- Sakko, M.; Tjaderhane, L.; Rautemaa-Richardson, R. Microbiology of Root Canal Infections. Prim. Dent. J. 2016, 5, 84–89. [Google Scholar] [CrossRef]
- Fouad, A.F. Microbiological Aspects of Traumatic Injuries. J. Endod. 2019, 45, S39–S48. [Google Scholar] [CrossRef]
- Zhang, C.; Du, J.; Peng, Z. Correlation between Enterococcus faecalis and Persistent Intraradicular Infection Compared with Primary Intraradicular Infection: A Systematic Review. J. Endod. 2015, 41, 1207–1213. [Google Scholar] [CrossRef]
- Manoil, D.; Al-Manei, K.; Belibasakis, G.N. A Systematic Review of the Root Canal Microbiota Associated with Apical Periodontitis: Lessons from Next-Generation Sequencing. Proteom. Clin. Appl. 2020. [Google Scholar] [CrossRef]





| Database-Provider | Keywords | Search Details | Number of Records | Number of Records) after Restriction by Year of Publication (Last 40 Years) | Number of Remaining Articles Related to the Topic of Bacteria in Endodontic Infections | Articles after Removing Overlapping Articles | Number of Articles Remaining after Applying the Inclusion and Exclusion Criteria for the Secondary Outcome | Number of Articles Included for Tertiary Outcome (Difference in the Prevalence of Bacteria of the Genus Actinomices Between Primary Endodontic Infections and Secondary Endodontic Infections) | Number of Remaining Articles Pertaining to the Topic of Persistent Extraradicular Infections | Number of Articles Focusing on the Role of Actinomycetes a on Extraradicular Persistent Lesions | Number of Articles Included for the Primary Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pub-med | persistent endodontic infections OR persistent intraradicular infection OR persistent extraradicular infection | (persistent (All Fields) AND endodontic (All Fields) AND (“infection” (MeSH Terms) OR “infection” (All Fields) OR “infections” (All Fields))) OR (persistent (All Fields) AND intraradicular (All Fields) AND (infection” (MeSH Terms) OR “infection” (All Fields))) OR (persistent (All Fields) AND extraradicular (All Fields) AND (“infection” (MeSH Terms) OR “infection” (All Fields))) | 160 | 158 | 42 | ||||||
| Pub-med | Actinomyces AND endodontic OR actinomycetes AND endodontic OR Endodontic failure | (“actinomyces” (MeSH Terms) OR “actinomyces” (All Fields)) AND endodontic (All Fields) OR (“actinobacteria” (MeSH Terms) OR “actinobacteria” (All Fields) OR “actinomycetes” (All Fields)) AND endodontic (All Fields) OR (Endodontic (All Fields) AND failure (All Fields)) | 1870 | 1814 | 111 | ||||||
| Pub-med | “Periapical actinomycosis” | “Periapical actinomycosis” (All Fields) | 26 | 11 | |||||||
| Scopus | persistent intraradicular infection | TITLE-ABS-KEY (persistent AND intraradicular AND infection) | 23 | 23 | 14 | ||||||
| Scopus | persistent extraradicular infection | TITLE-ABS-KEY (persistent AND extravascular AND infection) | 18 | 18 | 15 | ||||||
| Scopus | Actinomyces AND endodontic | TITLE-ABS-KEY (actinomyces AND endodontic) | 143 | 136 | 27 | ||||||
| Total records | 2240 | 2160 | 209 | 165 | 46 | 7 | 33 | 19 | 6 |
| Reviewer 2 | Reviewer 2 | Reviewer 2 | |||
|---|---|---|---|---|---|
| Include | Exclude | Unsure | Total | ||
| Reviewer 1 | Include | 6 | 0 | 0 | 6 |
| Reviewer 1 | Exclude | 3 | 22 | 2 | 27 |
| Reviewer 1 | Unsure | 0 | 0 | 0 | 0 |
| Total | 9 | 22 | 2 | 33 |
| Author, Date, Journal | Actinomyces | Type of Tooth or Root, of Dental Treatment, or Endodontic Pathology | Number of Teeth or Channels or Periapical Tissue in Which the Presence of Actinomycetes Has Been Identified | Total Number of Teeth or Channels or Periapical Tissue in Which the Presence of Actinomycetes Was Investigated | Identification Method of Bacterial Species | ||||
|---|---|---|---|---|---|---|---|---|---|
| [24] Pourhajibagher et al. 2018 Photodiagnosis and photodynamic therapy | A. naeslundii | 12 | root canal samples | 12/36 | 12 | 36 | culture | ||
| [25] Lysakowska et al. 2016 International endodontic journal | A. naeslundii | 0/19 | primary endodontic infections | 1/19 | 4 | 47 | culture | ||
| 2/28 | |||||||||
| A. meyeri | 1/19 | secondary treatment | 3/28 | ||||||
| 1/28 | |||||||||
| [26] Halbauer et al. 2013 Coll Antropol | A. meyeri | 1/23 | chronical apical periodontitis (n = 17 untreated teeth) | 17 | 1 | 23 | culture | ||
| chronical apical periodontitis (n = 6 retreatments) | 6 | ||||||||
| [23] Signoretti et al. 2013 Journal of endodontics | A. naeslundii | 2/13 | persistent apical lesions associated with well-performed endodontic retreatment (n = 13 cyst n = 7 granuloma) | 20 | 5 | 20 | culture | ||
| 3/7 | |||||||||
| A. meyeri | 1/13 | ||||||||
| 1/7 | |||||||||
| [27] Niazi et al. 2010 Journal of endodontics | A. gerencseriae | 1/20 | 20 refractory endodontic lesions (5/9 with abscesses and 6/11 without abscesses) | 20 | 11 | 20 | PCR | ||
| A. massiliensis | 1/20 | ||||||||
| A. meyeri | 1/20 | ||||||||
| A. radicidentis | 1/20 | ||||||||
| A. israelii | 1/20 | ||||||||
| Actinomyces sp. | 7/20 | ||||||||
| [28] Fujii et al. 2009 Oral microbiology and immunology | A. israelii | 2/16 | infection lesions with apical periodontitis 20 (16 without sinus tract, 5 with sinus tract) | 2/20 | 2 | 20 | PCR | ||
| 0/5 | |||||||||
| [29] Vianna et al. 2007 Oral microbiology and immunology | A. naeslundii | 6/24 | human necrotic root canals | 6/24 | 6 | 24 | PCR | ||
| [30] Chavez de Paz et al. 2005 Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics | A. israelii | 1/100 | teeth with apical periodontitis | 4/100 | 4 | 100 | PCR | ||
| A. meyerii | 2/100 | ||||||||
| A. naeslundii | 2/100 | ||||||||
| A. odontolyticus | 4/100 | ||||||||
| Actinomyces spp | 1/100 | ||||||||
| [31] Gomes et al. 2004 Oral microbiology and immunology | Actinomyces meyerii | 3/0 | 41 primary infection | 3/60 | 3 | 60 | PCR | ||
| 0/19 | 19 endodontic failure | ||||||||
| [12] Claesson et al. 2017 Anaerobe | A. radicidentis | 16/926 | root canal samples | 17 | 926 | PCR | |||
| A. haliotis | 1/926 | ||||||||
| [32] Rolph et al. 2001 Journal of clinical microbiology | A. naeslundii | 2/15 | 2/15 primary endodontic infections | 3 | 41 | culture | |||
| 0/26 | |||||||||
| A. viscosus | 1/15 | ||||||||
| 0/26 | 1/26 refractory cases of endodontic infections | ||||||||
| A. israelii | 0/15 | ||||||||
| 1/26 | |||||||||
| [33] Sundqvist et al. 1998 Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics | A. israelii | 3/54 | 54 teeth with failed endodontic treatment | 3 | 54 | culture | |||
| [34] Vigil et al. 1997 Journal of endodontics | A. odontolyticus | 1/28 | 28 refractory endodontic cases requiring surgical intervention | 1 | 28 | culture | |||
| [35] Sjogren et al. 1997 International endodontic journal | A. naeslundii | 1/20 | 20 apical periodontitis | 2 | 20 | culture | |||
| A. odontolyticus | 1/20 | ||||||||
| A. israelii | 2/20 | ||||||||
| [36] Gomes et al. 1996 J dental | A. naeslundii | 2/70 | necrotic pulp | 5 | 70 | culture | |||
| A. viscosus | 3/70 | ||||||||
| A. israelii | 4/70 | ||||||||
| A. meyeri | 5/70 | ||||||||
| [37] Debelian et al. 1995 Endodontics & dental traumatology | A. israelii | 5/26 | 26 teeth with asymptomatic apical periodontitis | 5 | 26 | culture | |||
| A. meyeri | 1/26 | ||||||||
| A. naeslundii | 2/26 | ||||||||
| A. odontolyticus | 1/26 | ||||||||
| [38] Fukushima et al. 1990 Journal of endodontics | A. israelii | 2/21 | 21 untreated cases | 4 | 21 | culture | |||
| A. viscosus | 2/21 | ||||||||
| A. meyeri | 1/21 | ||||||||
| A. naeslundii | 1/21 | ||||||||
| [39] Qi et al. 2016 Int Endod J | A. naeslundii | 14/90 | primary endodontic infections | 14 | 90 | PCR | |||
| A. israelii | 2/90 | ||||||||
| A. viscosus | 0/90 | ||||||||
| [40] Fernandes et al. 2014 Microb Pathog | Actinomyces spp | irreversible pulpitis (0–27) pulp necrotic teeth with apical periodontitis (4–33) | 4/60 | 14 | 81 | PCR | |||
| apical periodontitis associated with a root-filled tooth (10–21) | 10/21 | ||||||||
| Tennert et al. 2014 [41] | A. viscosus | 1/11 | primary infection | 2/11 | 2 | 22 | PCR | ||
| 0/11 | |||||||||
| A. naeslundii | 1/11 | secondary/persistent infection | 0/11 | ||||||
| 0/11 | |||||||||
| [42] Chugal et al. 2011 J Endod | Actinomyces spp. | 11/19 | primary endodontic infections | 11/19 | 16 | 29 | PCR | ||
| 5/10 | secondary infections | 5/10 | |||||||
| [43] Ledezma-Rasillo et al. 2010 J Clin Pediatr Dent | A. israelii | 4/21 | primary teeth with necrotic pulps | 6 | 21 | culture | |||
| A. naeslundii | 2/21 | ||||||||
| [22] Zhang et al. 2010 Chin J Dent Res | A. israelii (21%) | persistent apical periodontitis | 14 | 33 | PCR | ||||
| A. viscosus (42%) | |||||||||
| [44] Mindere et al. 2010 Stomatologija | A. odontolyticus | 1/33 | root-filled teeth with asymptomatic persisting periapical lesions | 4 | 33 | culture | |||
| A. israelii | 1/33 | ||||||||
| A. viscosus | 2/33 | ||||||||
| [45] Cogulu et al. 2008 Oral Surg Oral Med Oral Pathol Oral Radiol Endod | A. israelii | acute apical periodontitis (deciduous 20, permanent 22) | 0 | 145 | PCR | ||||
| chronic apical periodontitis (deciduous 35, permanent 28) | |||||||||
| exacerbated apical periodontitis (deciduous 24, permanent 16) | |||||||||
| [46] Chu et al. 2005 J Endod | A. israelii (7%) (14%) | 3/45 | primary endodontic infections with exposed 45; primary endodontic infections with unexposed 43 | 14 | 88 | culture | |||
| 6/43 | |||||||||
| A. meyeri (13%) (19%) | 6/45 | ||||||||
| 8/43 | |||||||||
| A. odontolyticus (11%) (19%) | 5/45 | ||||||||
| 8/43 | |||||||||
| [47] Chavez de Paz et al. 2004 Int Endod J | A. israelli | 2/23 | apical periodontitis | 23 | 139 | PCR | |||
| A. meyeri | 7/23 | ||||||||
| A. naeslundii | 3/23 | ||||||||
| A. odontolyticus | 6/23 | ||||||||
| A. radicidentis | 0/23 | ||||||||
| A. viscosus | 0/23 | ||||||||
| Actinomyces spp. | 1/23 | ||||||||
| [48] Siqueira et al. 2004 Oral Surg Oral Med Oral Pathol Oral Radiol Endod | A. israelii | 0/22 | root-filled teeth with persistent periradicular lesions | 1 | 22 | PCR | |||
| A. radicidentis | 1/22 | ||||||||
| [21] Hirshberg et al. 2003 Oral Surg Oral Med Oral Pathol Oral Radiol Endod | Actinomyces spp | persistent periapical lesions | 17 | 963 | histology | ||||
| [49] Tang et al. 2003 J Dent | odontolyticus (31.3%) | 10/32 | primary root canal infections | 16 | 32 | PCR | |||
| A. meyeri (9.4%) | 3/32 | ||||||||
| A. naeslundii (9.4%) | 3/32 | ||||||||
| A. israelii (6.3%) | 2/32 | ||||||||
| A. gerencseriae (3.1%) | 1/32 | ||||||||
| [50] Xia et al. 2003 J Endod | A. israelii (23.7%) | 31/129 | primary root canal infections (41/51) | 72 | 129 | PCR | |||
| A. naeslundii (8.5%) | 11/129 | abscesses (22/48) | |||||||
| A. viscosus (32.1%) | 41/129 | cellulitis (9/31) | |||||||
| [51] Pinheiro et al. 2003 Oral Microbiol Immunol | A. naeslundii | 2/30 | teeth with endodontic failure | 4 | 30 | PCR | |||
| A. odontolyticus | 1/30 | ||||||||
| A. viscosus | 1/30 | ||||||||
| [52] Siqueira et al. 2002 Int Endod J | A. israelii | root canal infections, necrotic pulps | 2 | 40 | PCR | ||||
| [53] Peters et al. 2002 Int Endod J | Actinomyces spp. | 3/58 | primary endodontic infections | 11 | 58 | culture | |||
| A. odontolyticus | 11/58 | ||||||||
| A. meyeri | 6/58 | ||||||||
| [20] Sunde et al. 2002 J Endod | A. israelii | 6/36 | periapical lesions refractory to endodontic therapy | 9 | 36 | culture | |||
| A. meyeri | 3/36 | ||||||||
| A. viscosus | 7/36 | ||||||||
| Actinomyces species | 1/36 | ||||||||
| A. naeslundii | 5/36 | ||||||||
| [54] Siqueira et al. 2002 J Endod | A. gerencseriae | 4/53 | primary root; canal infections | 7 | 53 | PCR | |||
| A. israelli | 2/53 | ||||||||
| A. naeslundii | 0/53 | ||||||||
| A. odontolyticus | 1/53 | ||||||||
| [55] Ercan et al. 2006 Biotechnol. & Biotechnol. Eq. | A. odontolyticus | 4/61 | 61 had necrotic pulp tissues (primary infection) | 6/61 | 14 | 100 | culture | ||
| 4/39 | |||||||||
| A. meyeri | 2/61 | ||||||||
| 0/39 | 39 had a history failed endodontic treatment (secondary infection) | 8/39 | |||||||
| A. naeslundii | 0/61 | ||||||||
| 4/39 | |||||||||
| [56] Molander et al. 1998 Int Endod J | Actinomyces spp. | 100 root-filled teeth with radiographically verified apical periodontitis (n = 2) | 2 | 120 | culture | ||||
| 20 root-filled teeth without signs of apical periodontitis | |||||||||
| [57] Ruviere et al. 2008 J Dent Child (Chic) | A. viscosus | 0/55 | 55 root canals of primary teeth with irreversible pulpitis | 16 | 106 | PCR | |||
| 0/51 | |||||||||
| A. naeslundii genospecies 1 | 0/55 | ||||||||
| 2/51 | |||||||||
| A. odontolyticus | 3/55 | ||||||||
| 10/51 | 51 root canals of primary teeth with necrotic pulp and apical periodontitis | ||||||||
| A. israelii | 0/55 | ||||||||
| 10/51 | |||||||||
| A. gerencseriae | 2/55 | ||||||||
| 10/51 | |||||||||
| [58] Sundqvist et al. 1992 Oral Microbiol Immunol | Actinomyces sp., ‘1 | 1/65 | nonvital teeth with periapical lesions | 7 | 65 | culture | |||
| A. israelii | 7/65 | ||||||||
| A. meyeri | 1/65 | ||||||||
| A. naeslundii | 3/65 | ||||||||
| A. odonlotyticus | 1/65 | ||||||||
| A. viscosus | 1/65 | ||||||||
| [59] Brauner and Conrads 1995 Int Endod J | Actiuomyccs spp. | 5/19 | 19 root canal (n = 6) | 8 | 43 | culture PCR | |||
| 2/24 | |||||||||
| A. israelii | 1/19 | 24 periapical granuloma (n = 2) | |||||||
| 0/24 | |||||||||
| [60] Assed et al. 1996 Endod Dent Traumatol | A. viscosiis | chronic apical periodontitis | 14 | 25 | immunofluorescence | ||||
| [61] Hancock et al. 2001 Oral Surg Oral Med Oral Pathol Oral Radiol Endod | Actinomyces spp. | chronic apical periodontitis in teeth with endodontic failure | 9 | 54 | culture | ||||
| [19] Esteves et al. 2017 Braz Dent J | Actinomyces | persistent periapical lesions (cysts, granulomas or abscess) | 7 | 218 | histology | ||||
| [18] Persoon et al. 2017 Clin Oral Investig | Actinomyces | apical periodontitis and refrained from endodontic treatment | 2 | 23 | PCR | ||||
| [63] Sundqvist et al. 1989 J Endod | A. israelii | 1/72 | necrotic pulps and apical periodontitis | 5 | 72 | culture | |||
| A. meyerii | 2/72 | ||||||||
| A. viscosus | 1/72 | ||||||||
| A. odontolyticus | 1/72 | ||||||||
| Author, Date, Journal | Species | Primary Endodontic Infections | Secondary/Persistent Infection | ||
|---|---|---|---|---|---|
| event | total | event | total | ||
| [42] Chugal et al. 2011 J Endod | Actinomyces spp. | 11 | 19 | 5 | 10 |
| tot | 11 | 19 | 5 | 10 | |
| [55] Ercan et al. 2006 Biotechnol. & Biotechnol. Eq. | A. odontolyticus | 4 | 61 | 4 | 39 |
| A. naeslundii | 0 | 61 | 4 | 39 | |
| A. meyeri | 2 | 61 | 0 | 39 | |
| tot | 6 | 61 | 8 | 39 | |
| [41] Tennert et al. 2014 J Endod | A. viscosus | 1 | 11 | 0 | 11 |
| A. naeslundii | 1 | 11 | 0 | 11 | |
| tot | 2 | 11 | 0 | 11 | |
| [40] Fernandes et al. 2014 Microb Pathog | Actinomyces spp. | 4 | 60 | 10 | 21 |
| tot | 4 | 60 | 10 | 21 | |
| [32] Rolph et al. 2001 Journal of clinical microbiology | A. naeslundii | 2 | 15 | 0 | 26 |
| A. israelii | 0 | 15 | 1 | 26 | |
| A. viscosus | 1 | 15 | 0 | 26 | |
| tot | 2 | 15 | 1 | 26 | |
| [31] Gomes et al. 2004 Oral microbiology and immunology | Actinomyces meyerii | 3 | 41 | 0 | 19 |
| tot | 3 | 41 | 0 | 19 | |
| [25] Lysakowska et al. 2016 International endodontic journal | A. naeslundii | 0 | 19 | 2 | 28 |
| A. meyeri | 1 | 19 | 1 | 28 | |
| tot | 1 | 19 | 3 | 28 | |
| Selection | Comparability | Exposure | Score | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Definition of Cases | Representativeness of Cases | Selection of Controls | Definition of Controls | Comparability of Cases and Controls on the Basis of the Design or Analysis | Ascertainment of Exposure | Same Method of Ascertainment for Cases and Controls | Non-Response Rate | |
| [24] Pourhajibagher et al. 2018 Photodiagnosis and photodynamic therapy | 3 | 3 | 0 | 0 | 0 | 3 | 3 | 0 | 12 |
| [12] Claesson et al. 2017 Anaerobe | 2 | 3 | 0 | 0 | 0 | 3 | 3 | 0 | 11 |
| [19] Esteves et al. 2017 Braz Dent J | 3 | 3 | 0 | 0 | 3 | 0 | 0 | 0 | 9 |
| [18] Persoon et al. 2017 Clin Oral Investig | 3 | 3 | 0 | 0 | 1 | 3 | 1 | 0 | 11 |
| [25] Lysakowska et al. 2016 International endodontic journal | 3 | 3 | 3 | 3 | 2 | 2 | 3 | 0 | 19 |
| [39] Qi et al. 2016 Int Endod J | 3 | 3 | 0 | 0 | 0 | 3 | 3 | 0 | 12 |
| [40] Fernandes et al. 2014 Microb Pathog | 3 | 3 | 2 | 3 | 2 | 3 | 3 | 0 | 19 |
| [41] Tennert et al. 2014 Journal of endodontics | 2 | 3 | 3 | 2 | 2 | 3 | 3 | 0 | 18 |
| [26] Halbauer et al. 2013 Coll Antropol | 1 | 3 | 3 | 1 | 2 | 3 | 3 | 0 | 16 |
| [23] Signoretti et al. 2013 Journal of endodontics | 2 | 1 | 2 | 2 | 2 | 3 | 3 | 0 | 15 |
| [42] Chugal et al. 2011 Journal of endodontics | 2 | 2 | 1 | 2 | 2 | 3 | 3 | 0 | 15 |
| [22] Zhang et al. 2010 Chin J Dent Res | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 14 |
| [43] Ledezma-Rasillo et al. 2010 The Journal of clinical pediatric dentistry | 3 | 1 | 2 | 2 | 2 | 2 | 3 | 0 | 15 |
| [44] Mindere et al. 2010 Stomatologija | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 0 | 8 |
| [45] Cogulu et al. 2008 Oral Surg Oral Med Oral Pathol Oral Radiol Endod | 3 | 3 | 0 | 0 | 0 | 3 | 3 | 0 | 12 |
| [27] Niazi et al. 2010 Journal of endodontics | 3 | 1 | 3 | 3 | 2 | 1 | 3 | 0 | 16 |
| [28] Fujii et al. 2009 Oral microbiology and immunology | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 0 | 8 |
| [57] Ruviere et al. 2008 J Dent Child (Chic) | 3 | 3 | 2 | 2 | 2 | 3 | 3 | 0 | 18 |
| [29] Vianna et al. 2007 Oral microbiology and immunology | 3 | 2 | 3 | 2 | 2 | 2 | 3 | 0 | 17 |
| [55] Ercan et al. 2006 Biotechnol. & Biotechnol. Eq. | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 0 | 20 |
| [46] Chu et al. 2005 Journal of endodontics | 3 | 2 | 3 | 3 | 3 | 2 | 3 | 0 | 19 |
| [30] Chavez de Paz et al. 2005 Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 0 | 20 |
| [31] Gomes et al. 2004 Oral microbiology and immunology | 2 | 2 | 2 | 3 | 3 | 3 | 3 | 0 | 21 |
| [47] Chavez de Paz et al. 2004 International endodontic journal | 3 | 3 | 3 | 3 | 3 | 2 | 3 | 0 | 20 |
| [48] Siqueira et al. 2004 Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics | 3 | 1 | 0 | 0 | 0 | 3 | 2 | 0 | 9 |
| [21] Hirshberg et al. 2003 Oral Surg Oral Med Oral Pathol Oral Radiol Endod | 3 | 2 | 0 | 0 | 0 | 2 | 2 | 0 | 9 |
| [49] Tang et al. 2003 J Dent | 3 | 3 | 0 | 0 | 0 | 3 | 1 | 0 | 10 |
| [50] Xia et al. 2003 J Endod | 3 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 9 |
| [51] Pinheiro et al. 2003 Oral Microbiol Immunol | 3 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 9 |
| [52] Siqueira et al. 2002 Int Endod J | 2 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 8 |
| [53] Peters et al. 2002 Int Endod J | 2 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 8 |
| [20] Sunde et al. 2002 Journal of endodontics | 2 | 2 | 2 | 2 | 3 | 2 | 3 | 0 | 16 |
| [54] Siqueira et al. 2002 J Endod | 3 | 3 | 1 | 0 | 0 | 0 | 3 | 0 | 10 |
| [61] Hancock et al. 2001 Oral Surg Oral Med Oral Pathol Oral Radiol Endod | 3 | 3 | 2 | 0 | 0 | 0 | 3 | 0 | 11 |
| [32] Rolph et al. 2001 Journal of clinical microbiology | 3 | 3 | 3 | 3 | 3 | 2 | 3 | 0 | 20 |
| [33] Sundqvist et al. 1998 Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 0 | 15 |
| [56] Molander et al. 1998 International endodontic journal | 3 | 3 | 0 | 0 | 0 | 2 | 3 | 0 | 11 |
| [34] Vigil et al. 1997 Journal of endodontics | 3 | 2 | 0 | 0 | 0 | 3 | 2 | 0 | 10 |
| [35] Sjogren et al. 1997 International endodontic journal | 2 | 2 | 2 | 2 | 3 | 2 | 3 | 0 | 16 |
| [36] Gomes et al. 1996 J dental | 3 | 2 | 0 | 0 | 0 | 2 | 2 | 0 | 9 |
| [60] Assed et al. 1996 Endod Dent Traumatol | 2 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 8 |
| [59] Brauner et al. 1995 International endodontic journal | 3 | 2 | 0 | 0 | 0 | 2 | 2 | 0 | 9 |
| [37] Debelian et al. 1995 Endodontics & dental traumatology | 2 | 2 | 2 | 2 | 3 | 2 | 2 | 0 | 15 |
| [58] Sundqvist et al.1992 Oral microbiology and immunology | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 8 |
| [38] Fukushima et al. 1990 Journal of endodontics | 2 | 1 | 2 | 0 | 0 | 0 | 2 | 0 | 7 |
| [63] Sundqvist et al. 1989 Journal of endodontics | 3 | 3 | 3 | 0 | 0 | 0 | 2 | 0 | 11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dioguardi, M.; Crincoli, V.; Laino, L.; Alovisi, M.; Sovereto, D.; Lo Muzio, L.; Troiano, G. Prevalence of Bacteria of Genus Actinomyces in Persistent Extraradicular Lesions—Systematic Review. J. Clin. Med. 2020, 9, 457. https://doi.org/10.3390/jcm9020457
Dioguardi M, Crincoli V, Laino L, Alovisi M, Sovereto D, Lo Muzio L, Troiano G. Prevalence of Bacteria of Genus Actinomyces in Persistent Extraradicular Lesions—Systematic Review. Journal of Clinical Medicine. 2020; 9(2):457. https://doi.org/10.3390/jcm9020457
Chicago/Turabian StyleDioguardi, Mario, Vito Crincoli, Luigi Laino, Mario Alovisi, Diego Sovereto, Lorenzo Lo Muzio, and Giuseppe Troiano. 2020. "Prevalence of Bacteria of Genus Actinomyces in Persistent Extraradicular Lesions—Systematic Review" Journal of Clinical Medicine 9, no. 2: 457. https://doi.org/10.3390/jcm9020457
APA StyleDioguardi, M., Crincoli, V., Laino, L., Alovisi, M., Sovereto, D., Lo Muzio, L., & Troiano, G. (2020). Prevalence of Bacteria of Genus Actinomyces in Persistent Extraradicular Lesions—Systematic Review. Journal of Clinical Medicine, 9(2), 457. https://doi.org/10.3390/jcm9020457










