An Introduction to High Intensity Focused Ultrasound: Systematic Review on Principles, Devices, and Clinical Applications
Abstract
:1. Introduction
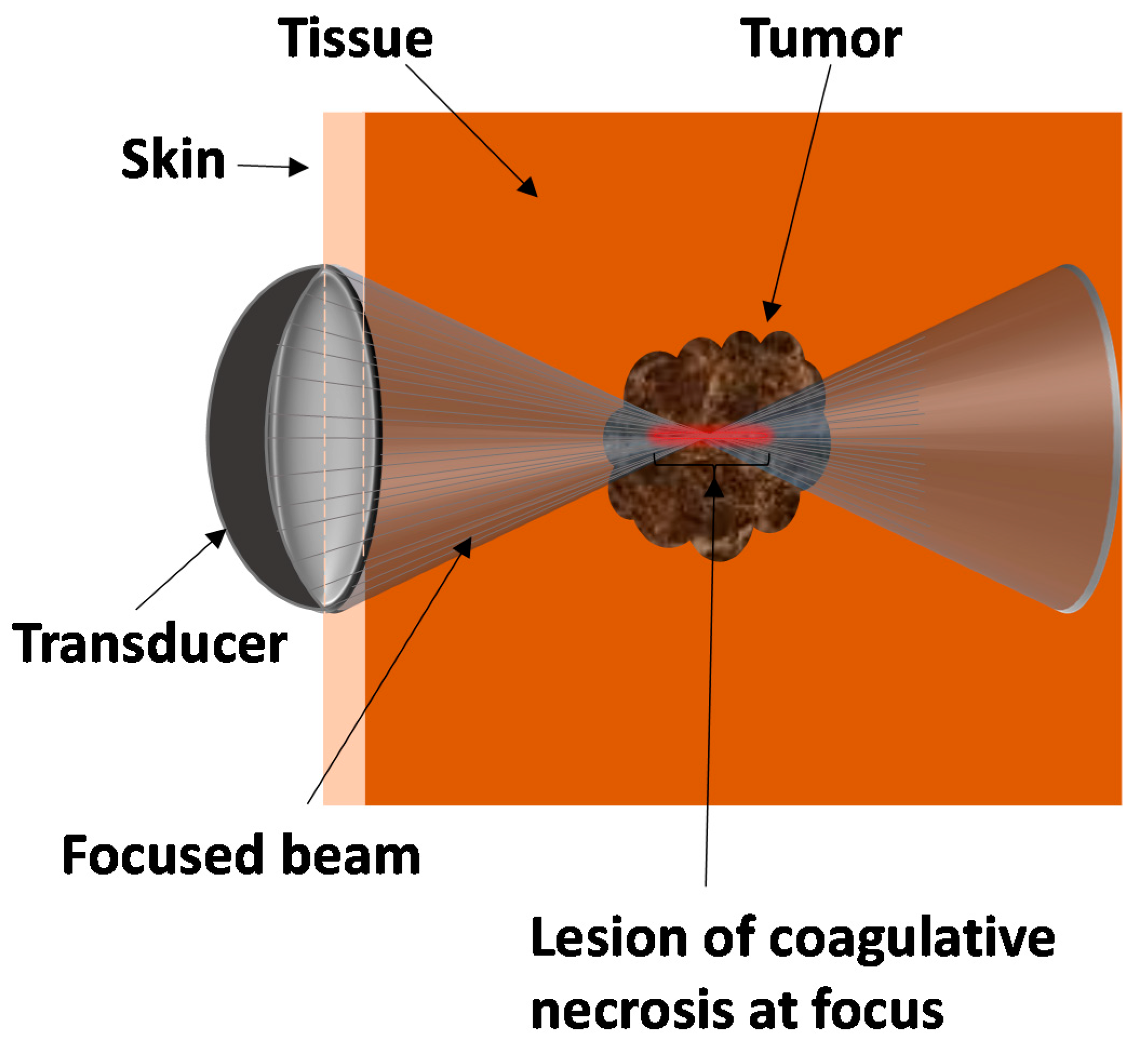
2. Principles behind HIFU
3. Ultrasound Beam Delivery System
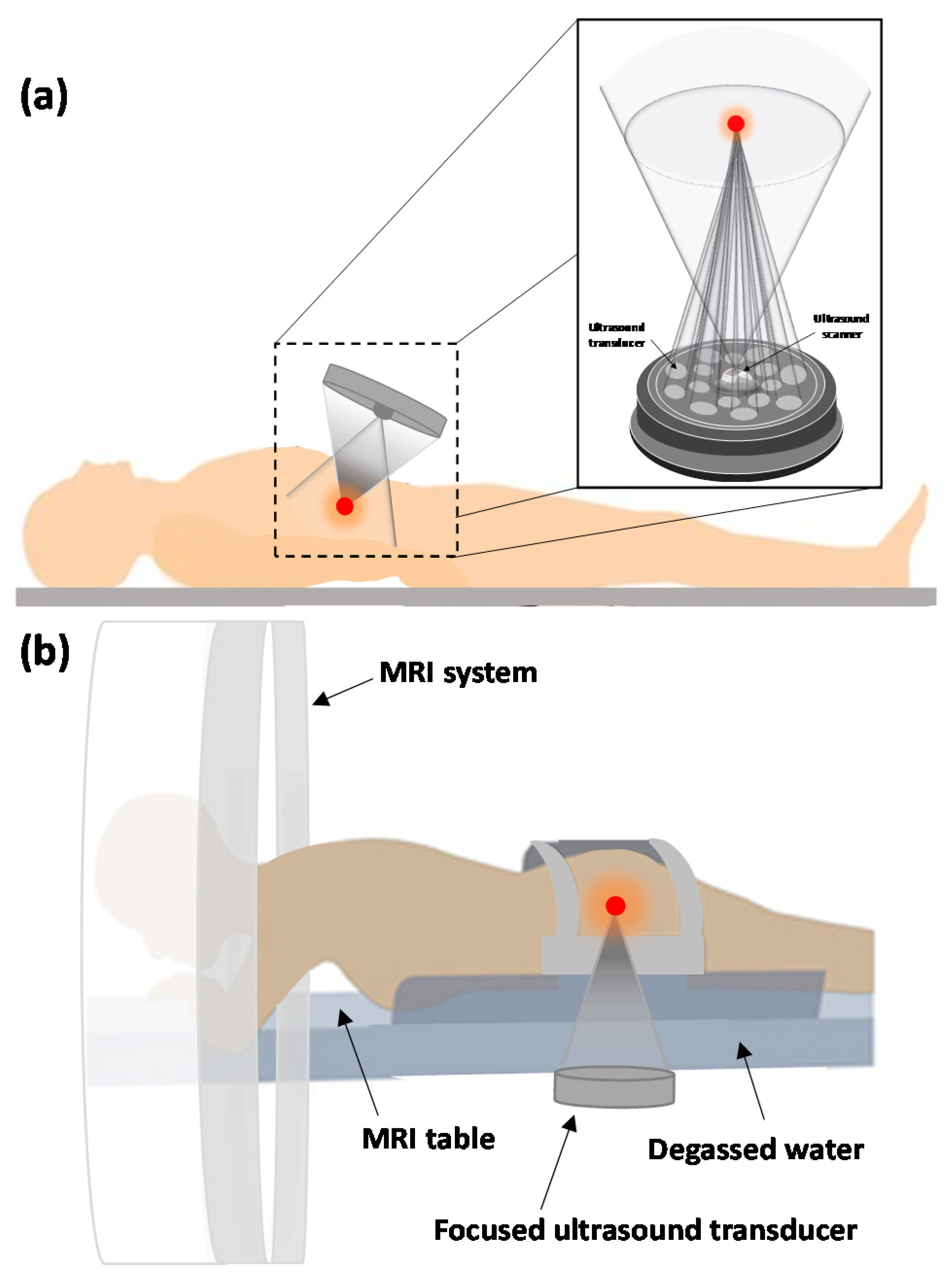
4. Ultrasound Guidance Modalities
4.1. MRI
4.2. Sonography
5. Accessibility of the Tissue to Ultrasound
6. HIFU Analysis
6.1. Benefits
6.2. Limitations and Complications
7. Clinical Applications of HIFU
7.1. Malignant Tumors
7.1.1. Liver
7.1.2. Breast
7.1.3. Prostate Cancer
7.1.4. Kidney
7.1.5. Esophagus
7.1.6. Pancreas
7.1.7. Brain
7.1.8. Bone
7.2. Benign Tumors and Conditions
7.2.1. Uterine Fibroids
7.2.2. Breast
7.2.3. Brain Disorders
7.2.4. Essential Tremor
7.2.5. Parkinson’s Disease
7.2.6. Chronic and Non-Malignant Pain
7.2.7. Benign Prostate Conditions
7.2.8. Thyroid
7.2.9. Brain
7.2.10. Imaging Guided HIFU
8. Potential Upcoming HIFU Clinical Applications and Techniques
8.1. Vessel Blockage by HIFU
8.2. Blood-Brain Barrier Disruption
8.3. Stroke and Thrombolysis
8.4. Abscesses
8.5. Emerging Focused Ultrasound Techniques
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lafon, C.; Melodelima, D.; Salomir, R.; Chapelon, J.Y. Interstitial devices for minimally invasive thermal ablation by high-intensity ultrasound. Int. J.Hyperth. 2007, 23, 153–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, J.F. The biophysical basis of biologic ultrasonic reactions with special reference to ultrasonic therapy. Arch. Phys. Med. Rehabil. 1953, 34, 139. [Google Scholar] [PubMed]
- Woo, J. A Short History of the Development of Ultrasound in Obstetrics and Gynecology. 2002. Available online: http://www.ob-ultrasound.net/history1.html (accessed on 14 May 2011).
- Fry, F.; Ades, H.; Fry, W. Production of reversible changes in the central nervous system by ultrasound. Science 1958, 127, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Jolesz, F.A. MRI-guided focused ultrasound surgery. Annu. Rev. Med. 2009, 60, 417–430. [Google Scholar] [CrossRef] [Green Version]
- Cline, H.E.; Hynynen, K.; Watkins, R.D.; Adams, W.J.; Schenck, J.F.; Ettinger, R.H.; Freund, W.R.; Vetro, J.P.; Jolesz, F.A. Focused US system for MR imaging-guided tumor ablation. Radiology 1995, 194, 731–737. [Google Scholar] [CrossRef]
- Hynynen, K.; Hynynen, K.; Damianou, C.; Darkazanli, A.; Unger, E.; Schenck, J.F. The feasibility of using MRI to monitor and guide noninvasive ultrasound surgery. Ultrasound Med. Biol. 1993, 19, 91–92. [Google Scholar] [CrossRef]
- Hynynen, K.; Darkazanli, A.; Unger, E.; Schenck, J. MRI-guided noninvasive ultrasound surgery. Med. Phys. 1993, 20, 107–115. [Google Scholar] [CrossRef]
- Zhou, Y.-F. High intensity focused ultrasound in clinical tumor ablation. World J. Clin. Oncol. 2011, 2, 8. [Google Scholar] [CrossRef]
- Ter Haar, G.; Rivens, I.; Chen, L.; Riddler, S. High intensity focused ultrasound for the treatment of rat tumours. Phys. Med. Biol. 1991, 36, 1495–1501. [Google Scholar] [CrossRef]
- Dewey, W.C. Arrhenius relationships from the molecule and cell to the clinic. Int. J. Hyperth. 2009, 25, 3–20. [Google Scholar] [CrossRef]
- Lagneaux, L.; de Meulenaer, E.C.; Delforge, A.; Dejeneffe, M.; Massy, M.; Moerman, C.; Hannecart, B.; Canivet, Y.; Lepeltier, M.F.; Bron, D. Ultrasonic low-energy treatment: A novel approach to induce apoptosis in human leukemic cells. Exp. Hematol. 2002, 30, 1293–1301. [Google Scholar] [CrossRef]
- Yagel, S. High-intensity focused ultrasound: A revolution in non-invasive ultrasound treatment? Ultrasound Obstet. Gynecol. 2004, 23, 216–217. [Google Scholar] [CrossRef] [PubMed]
- Jolesz, F.A.; McDannold, N. Current status and future potential of MRI-guided focused ultrasound surgery. J. Magn. Reson. Imaging 2008, 27, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Makin, I.R.; Mast, T.D.; Faidi, W.; Runk, M.M.; Barthe, P.G.; Slayton, M.H. Miniaturized ultrasound arrays for interstitial ablation and imaging. Ultrasound Med. Biol. 2005, 31, 1539–1550. [Google Scholar] [CrossRef]
- Salgaonkar, V.A.; Diederich, C.J. Catheter-based ultrasound technology for image-guided thermal therapy: Current technology and applications. Int. J. Hyperth. 2015, 31, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Haar, G.T.; Coussios, C. High intensity focused ultrasound: Physical principles and devices. Int. J. Hyperth. 2007, 23, 89–104. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.R.; Ballesteros, N.; Short, K.L.; Gathani, K.K.; Ankem, M.K. Extra corporeal shockwave lithotripsy resulting in skin burns—A report of two cases. Int. Braz J. Urol 2014, 40, 853–857. [Google Scholar] [CrossRef] [Green Version]
- Rangarajan, S.; Mirheydar, H.; Sur, R.L. Second-Degree Burn after Shock Wave Lithotripsy: An Unusual Complication. BJU Int. 2012. [Google Scholar] [CrossRef]
- Miller, D.L.; Smith, N.B.; Bailey, M.R.; Czarnota, G.J.; Hynynen, K.; Makin, I.R. Overview of therapeutic ultrasound applications and safety considerations. J. Ultrasound Med. 2012, 31, 623–634. [Google Scholar] [CrossRef] [Green Version]
- Hynynen, K.; Chung, A.H.; Colucci, V.; Jolesz, F.A. Potential adverse effects of high-intensity focused ultrasound exposure on blood vessels in vivo. Ultrasound Med. Biol. 1996, 22, 193–201. [Google Scholar] [CrossRef]
- Rove, K.O.; Sullivan, K.F.; Crawford, E.D. High-intensity focused ultrasound: Ready for primetime. Urol. Clin. North Am. 2010, 37, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Borchert, B.; Lawrenz, T.; Hansky, B.; Stellbrink, C. Lethal atrioesophageal fistula after pulmonary vein isolation using high-intensity focused ultrasound (HIFU). Heart Rhythm 2008, 5, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.E.; Cho, S.H.; Jang, J.H.; Han, J.-Y. High-intensity focused ultrasound ablation in hepatic and pancreatic cancer: Complications. Abdom. Imaging 2011, 36, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Chen, W.Z.; Bai, J.; Zou, J.Z.; Wang, Z.L.; Zhu, H.; Wang, Z.B. Pathological changes in human malignant carcinoma treated with high-intensity focused ultrasound. Ultrasound Med. Biol. 2001, 27, 1099–1106. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.B.; Chen, W.Z.; Wang, W.; Gui, Y.; Zhang, M.; Zheng, G.; Zhou, Y.; Xu, G.; Li, M.; et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: An overview. Ultrason. Sonochem. 2004, 11, 149–154. [Google Scholar] [CrossRef]
- Li, C.X.; Xu, G.L.; Jiang, Z.Y.; Li, J.J.; Luo, G.Y.; Shan, H.B.; Zhang, R.; Li, Y. Analysis of clinical effect of high-intensity focused ultrasound on liver cancer. World J. Gastroenterol. 2004, 10, 2201–2204. [Google Scholar] [CrossRef]
- Ter Haar, G. Acoustic surgery. Phys. Today 2001, 54, 29–34. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.B.; Chen, W.Z.; Zou, J.Z.; Bai, J.; Zhu, H.; Li, K.Q.; Jin, C.B.; Xie, F.L.; Su, H.B. Advanced hepatocellular carcinoma: Treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology 2005, 235, 659–667. [Google Scholar] [CrossRef]
- Huber, P.E.; Jenne, J.W.; Rastert, R.; Simiantonakis, I.; Sinn, H.-P.; Strittmatter, H.-J.; von Fournier, D.; Wannenmacher, M.F.; Debus, J. A new noninvasive approach in breast cancer therapy using magnetic resonance imaging-guided focused ultrasound surgery. Cancer Res. 2001, 61, 8441–8447. [Google Scholar]
- Furusawa, H.; Namba, K.; Nakahara, H.; Tanaka, C.; Yasuda, Y.; Hirabara, E.; Imahariyama, M.; Komaki, K. The evolving non-surgical ablation of breast cancer: MR guided focused ultrasound (MRgFUS). Breast Cancer 2007, 14, 55–58. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.B.; Cao, Y.D.; Zhu, X.Q.; Zhu, H.; Chen, W.Z.; Zou, J.Z. “Wide local ablation” of localized breast cancer using high intensity focused ultrasound. J. Surg. Oncol. 2007, 96, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, H.; Namba, K.; Thomsen, S.; Akiyama, F.; Bendet, A.; Tanaka, C.; Yasuda, Y.; Nakahara, H. Magnetic resonance–guided focused ultrasound surgery of breast cancer: Reliability and effectiveness. J. Am. Coll. Surg. 2006, 203, 54–63. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.-B.; Zhu, H.; Chen, W.-Z.; Zou, J.-Z.; Bai, J.; Li, K.-Q.; Jin, C.-B.; Xie, F.-L.; Su, H.-B. Extracorporeal high intensity focused ultrasound treatment for patients with breast cancer. Breast Cancer Res. Treat. 2005, 92, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Zippel, D.B.; Papa, M.Z. The use of MR imaging guided focused ultrasound in breast cancer patients; a preliminary phase one study and review. Breast Cancer 2005, 12, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, Z.-B.; Cao, Y.-D.; Chen, W.; Bai, J.; Zou, J.; Zhu, H. A randomised clinical trial of high-intensity focused ultrasound ablation for the treatment of patients with localised breast cancer. Br. J.Cancer 2003, 89, 2227–2233. [Google Scholar] [CrossRef] [PubMed]
- Gianfelice, D.; Khiat, A.; Amara, M.; Belblidia, A.; Boulanger, Y. MR imaging–guided focused us ablation of breast cancer: Histopathologic assessment of effectiveness—Initial experience 1. Radiology 2003, 227, 849–855. [Google Scholar] [CrossRef]
- Hynynen, K.; Pomeroy, O.; Smith, D.N.; Huber, P.E.; McDannold, N.J.; Kettenbach, J.; Baum, J.; Singer, S.; Jolesz, F.A. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: A feasibility study 1. Radiology 2001, 219, 176–185. [Google Scholar] [CrossRef]
- Peek MC, L.; Wu, F. High-intensity focused ultrasound in the treatment of breast tumours. Ecancermedicalscience 2018, 12, 794. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.; Zacharakis, E.; Dudderidge, T.; Armitage, J.; Scott, R.; Calleary, J.; Illing, R.; Kirkham, A.; Freeman, A.; Ogden, C. High-intensity-focused ultrasound in the treatment of primary prostate cancer: The first UK series. Br. J. Cancer 2009, 101, 19–26. [Google Scholar] [CrossRef]
- Chaussy, C.; Thuroff, S. The status of high-intensity focused ultrasound in the treatment of localized prostate cancer and the impact of a combined resection. Curr. Urol. Rep. 2003, 4, 248–252. [Google Scholar] [CrossRef]
- Beerlage, H.P.; Thuroff, S.; Debruyne, F.M.; Chaussy, C.; de la Rosette, J.J. Transrectal high-intensity focused ultrasound using the Ablatherm device in the treatment of localized prostate carcinoma. Urology 1999, 54, 273–277. [Google Scholar] [CrossRef]
- Gelet, A.; Chapelon, J.Y.; Bouvier, R.; Souchon, R.; Pangaud, C.; Abdelrahim, A.F.; Cathignol, D.; Dubernard, J.M. Treatment of prostate cancer with transrectal focused ultrasound: Early clinical experience. Eur. Urol. 1996, 29, 174–183. [Google Scholar] [PubMed]
- Chaussy, C.; Thuroff, S. High-intensity focused ultrasound in prostate cancer: Results after 3 years. Mol. Urol. 2000, 4, 179–182. [Google Scholar] [PubMed]
- Diederich, C.J.; Wootton, J.; Prakash, P.; Salgaonkar, V.; Juang, T.; Scott, S.; Chen, X.; Cunha, A.; Pouliot, J.; Hsu, I. Catheter-based ultrasound hyperthermia with HDR brachytherapy for treatment of locally advanced cancer of the prostate and cervix. In Energy-based Treatment of Tissue and Assessment VI; International Society for Optics and Photonics: San Francisco, CA, USA, 2011; p. 79010O. [Google Scholar]
- Ripert, T.; Azémar, M.-D.; Ménard, J.; Bayoud, Y.; Messaoudi, R.; Duval, F.; Staerman, F. Transrectal high-intensity focused ultrasound (HIFU) treatment of localized prostate cancer: Review of technical incidents and morbidity after 5 years of use. Prostate Cancer Prostatic Dis. 2010, 13, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Blana, A.; Walter, B.; Rogenhofer, S.; Wieland, W.F. High-intensity focused ultrasound for the treatment of localized prostate cancer: 5-year experience. Urology 2004, 63, 297–300. [Google Scholar] [CrossRef]
- Chaussy, C.; Thuroff, S.; Rebillard, X.; Gelet, A. Technology insight: High-intensity focused ultrasound for urologic cancers. Nat. Clin. Pract. Urol. 2005, 2, 191–198. [Google Scholar] [CrossRef]
- Rebillard, X.; Gelet, A.; Davin, J.L.; Soulie, M.; Prapotnich, D.; Cathelineau, X.; Rozet, F.; Vallancien, G. Transrectal high-intensity focused ultrasound in the treatment of localized prostate cancer. J. Endourol. 2005, 19, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Klingler, H.C.; Susani, M.; Seip, R.; Mauermann, J.; Sanghvi, N.; Marberger, M.J. A novel approach to energy ablative therapy of small renal tumours: Laparoscopic high-intensity focused ultrasound. Eur. Urol. 2008, 53, 810–818. [Google Scholar] [CrossRef]
- Illing, R.; Kennedy, J.; Wu, F.; Ter Haar, G.; Protheroe, A.; Friend, P.; Gleeson, F.; Cranston, D.; Phillips, R.; Middleton, M. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br. J. Cancer 2005, 93, 890–895. [Google Scholar] [CrossRef] [Green Version]
- KÖHRMANN, K.U.; Michel, M.S.; Gaa, J.; Marlinghaus, E.; Alken, P. High intensity focused ultrasound as noninvasive therapy for multilocal renal cell carcinoma: Case study and review of the literature. J. Urol. 2002, 167, 2397–2403. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.-B.; Chen, W.-Z.; Bai, J.; Zhu, H.; Qiao, T.-Y. Preliminary experience using high intensity focused ultrasound for the treatment of patients with advanced stage renal malignancy. J. Urol. 2003, 170, 2237–2240. [Google Scholar] [CrossRef] [Green Version]
- Arya, M.; Ahmed, H.U.; Scardino, P.; Emberton, M. Interventional Techniques in Uro-Oncology; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Napier, K.J.; Scheerer, M.; Misra, S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J. Gastrointest Oncol. 2014, 6, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Melodelima, D.; Prat, F.; Fritsch, J.; Theillere, Y.; Cathignol, D. Treatment of esophageal tumors using high intensity intraluminal ultrasound: First clinical results. J. Transl. Med. 2008, 6, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Wang, G.; Niu, S.; Yao, B.; Wang, X. The noninvasive treatment of 251 cases of advanced pancreatic cancer with focused ultrasound surgery. In Proceedings of the 2nd International Symposium on Therapeutic, Seattle, WA, USA, 29 July–1 August 2012; University of Washington: Seattle, WA, USA, 2002; pp. 51–56. [Google Scholar]
- Napoli, A.; Anzidei, M.; Marincola, B.C.; Brachetti, G.; Noce, V.; Boni, F.; Bertaccini, L.; Passariello, R.; Catalano, C. MR Imaging–guided Focused Ultrasound for Treatment of Bone Metastasis. Radiographics 2013, 33, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.L.; Hwang, J.H.; Huang, X.B.; Yao, S.S.; He, C.J.; Ge, X.H.; Ge, H.Y.; Wang, X.F. Early clinical experience using high intensity focused ultrasound for palliation of inoperable pancreatic cancer. JOP 2009, 10, 123–129. [Google Scholar]
- Izumi, M.; Ikeuchi, M.; Kawasaki, M.; Ushida, T.; Morio, K.; Namba, H.; Graven-Nielsen, T.; Ogawa, Y.; Tani, T. MR-guided focused ultrasound for the novel and innovative management of osteoarthritic knee pain. BMC Musculoskelet. Disord. 2013, 14, 267. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Moraga, J.; Valero-Altes, T.; Riquelme, A.M.; Isarria-Marcosy, M.I.; de la Torre, J.R. Body contouring by non-invasive transdermal focused ultrasound. Lasers Surg. Med. 2007, 39, 315–323. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, G.; Wang, D.; Yu, X.; Zhang, Y.; Zhu, J.; Ji, Y.; Zhong, B.; Zhao, W.; Yang, Z. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anti-Cancer Drugs 2010, 21, 447–452. [Google Scholar] [CrossRef]
- Lipsman, N.; Mainprize, T.G.; Schwartz, M.L.; Hynynen, K.; Lozano, A.M. Intracranial applications of magnetic resonance-guided focused ultrasound. Neurotherapeutics 2014, 11, 593–605. [Google Scholar] [CrossRef]
- McDannold, N.; Clement, G.T.; Black, P.; Jolesz, F.; Hynynen, K. Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: Initial findings in 3 patients. Neurosurgery 2010, 66, 323–332, discussion 332. [Google Scholar] [CrossRef] [Green Version]
- McDannold, N.; Vykhodtseva, N.; Jolesz, F.A.; Hynynen, K. MRI investigation of the threshold for thermally induced blood-brain barrier disruption and brain tissue damage in the rabbit brain. Magn. Reson. Med. 2004, 51, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Inbar, O.; Xu, Z.; Sheehan, J.P. Focused ultrasound-aided immunomodulation in glioblastoma multiforme: A therapeutic concept. J. Ther. Ultrasound 2016, 4, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberman, B.; Gianfelice, D.; Inbar, Y.; Beck, A.; Rabin, T.; Shabshin, N.; Chander, G.; Hengst, S.; Pfeffer, R.; Chechick, A.; et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: A multicenter study. Ann. Surg. Oncol. 2009, 16, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhu, H.; Zhang, L.; Li, K.; Su, H.; Jin, C.; Zhou, K.; Bai, J.; Wu, F.; Wang, Z. Primary bone malignancy: Effective treatment with high-intensity focused ultrasound ablation 1. Radiology 2010, 255, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, W.; Fan, W.; Huang, J.; Zhang, F.; Wu, P. Noninvasive treatment of malignant bone tumors using high-intensity focused ultrasound. Cancer 2010, 116, 3934–3942. [Google Scholar] [CrossRef]
- Temple, M.J.; Waspe, A.C.; Amaral, J.G.; Napoli, A.; LeBlang, S.; Ghanouni, P.; Bucknor, M.D.; Campbell, F.; Drake, J.M. Establishing a clinical service for the treatment of osteoid osteoma using magnetic resonance-guided focused ultrasound: Overview and guidelines. J. Ther. Ultrasound 2016, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Stewart, E.A. Uterine fibroids. Lancet 2001, 357, 293–298. [Google Scholar] [CrossRef]
- LeBlang, S.D.; Hoctor, K.; Steinberg, F.L. Leiomyoma shrinkage after MRI-guided focused ultrasound treatment: Report of 80 patients. AJR Am. J. Roentgenol. 2010, 194, 274–280. [Google Scholar] [CrossRef]
- Stewart, E.A.; Rabinovici, J.; Tempany, C.M.; Inbar, Y.; Regan, L.; Gostout, B. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil. Steril. 2006, 85, 22–29. [Google Scholar] [CrossRef]
- Lénárd, Z.M.; McDannold, N.J.; Fennessy, F.M.; Stewart, E.A.; Jolesz, F.A.; Hynynen, K.; Tempany, C.M. Uterine Leiomyomas: MR Imaging–guided Focused Ultrasound Surgery—Imaging Predictors of Success 1. Radiology 2008, 249, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Gorny, K.R.; Borah, B.J.; Brown, D.L.; Woodrum, D.A.; Stewart, E.A.; Hesley, G.K. Incidence of Additional Treatments in Women Treated with MR-Guided Focused US for Symptomatic Uterine Fibroids: Review of 138 Patients with an Average Follow-up of 2.8 Years. J. Vasc. Interv. Radiol. 2014, 25, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Nau, W.H., Jr.; Diederich, C.J.; Simko, J.; Juang, T.; Jacoby, A.; Burdette, E.C. Ultrasound Interstitial Thermal Therapy (USITT) for the Treatment of Uterine Myomas. In Thermal Treatment of Tissue: Energy Delivery and Assessment IV; International Society for Optics and Photonics: San Francisco, CA, USA, 2007; p. 64400F. [Google Scholar]
- Rueff, L.E.; Raman, S.S. Clinical and Technical Aspects of MR-Guided High Intensity Focused Ultrasound for Treatment of Symptomatic Uterine Fibroids. Semin. Interv. Radiol. 2013, 30, 347–353. [Google Scholar]
- Peek, M.C.; Ahmed, M.; Pinder, S.E.; Douek, M. A review of ablative techniques in the treatment of breast fibroadenomata. J. Ther. Ultrasound 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipsman, N.; Schwartz, M.L.; Huang, Y.; Lee, L.; Sankar, T.; Chapman, M.; Hynynen, K.; Lozano, A.M. MR-guided focused ultrasound thalamotomy for essential tremor: A proof-of-concept study. Lancet Neurol. 2013, 12, 462–468. [Google Scholar] [CrossRef]
- Elias, W.J.; Huss, D.; Voss, T.; Loomba, J.; Khaled, M.; Zadicario, E.; Frysinger, R.C.; Sperling, S.A.; Wylie, S.; Monteith, S.J.; et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N. Engl. J. Med. 2013, 369, 640–648. [Google Scholar] [CrossRef]
- Jordão, J.F.; Thévenot, E.; Markham-Coultes, K.; Scarcelli, T.; Weng, Y.-Q.; Xhima, K.; O’Reilly, M.; Huang, Y.; McLaurin, J.; Hynynen, K. Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp. Neurol. 2013, 248, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Monteith, S.J.; Kassell, N.F.; Goren, O.; Harnof, S. Transcranial MR-guided focused ultrasound sonothrombolysis in the treatment of intracerebral hemorrhage. Neurosurg. Focus 2013, 34, E14. [Google Scholar] [CrossRef]
- Martin, E.; Jeanmonod, D.; Morel, A.; Zadicario, E.; Werner, B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann. Neurol. 2009, 66, 858–861. [Google Scholar] [CrossRef] [Green Version]
- Jeanmonod, D.; Werner, B.; Morel, A.; Michels, L.; Zadicario, E.; Schiff, G.; Martin, E. Transcranial magnetic resonance imaging-guided focused ultrasound: Noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg. Focus 2012, 32, E1. [Google Scholar] [CrossRef] [Green Version]
- Burgess, A.; Huang, Y.; Waspe, A.C.; Ganguly, M.; Goertz, D.E.; Hynynen, K. High-intensity focused ultrasound (HIFU) for dissolution of clots in a rabbit model of embolic stroke. PLoS ONE 2012, 7, e42311. [Google Scholar] [CrossRef]
- Hölscher, T.; Ahadi, G.; Fisher, D.; Zadicario, E.; Voie, A. MR-guided focused ultrasound for acute stroke a rabbit model. Stroke 2013, 44, S58–S60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, C.; Hynynen, K.; Goertz, D. In vitro and in vivo high intensity focused ultrasound thrombolysis. Invest Radiol. 2012, 47, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkins, R.; Huang, Y.; Pajek, D.; Hynynen, K. Cavitation-based third ventriculostomy using MRI-guided focused ultrasound: Laboratory investigation. J. Neurosurg. 2013, 119, 1520. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.S.; Jung, H.H.; Kweon, E.J.; Zadicario, E.; Rachmilevitch, I.; Chang, J.W. Unilateral magnetic resonance guided focused ultrasound thalamotomy for essential tremor: Practices and clinicoradiological outcomes. J. Neurol. Neurosurg. Psychiatry 2015, 86, 257–264. [Google Scholar] [CrossRef]
- Lozano, A.M.; Lipsman, N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron 2013, 77, 406–424. [Google Scholar] [CrossRef] [Green Version]
- Magara, A.; Bühler, R.; Moser, D.; Kowalski, M.; Pourtehrani, P.; Jeanmonod, D. First experience with MR-guided focused ultrasound in the treatment of Parkinson’s disease. J. Ther. Ultrasound 2014, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cetas, J.S.; Saedi, T.; Burchiel, K.J. Destructive procedures for the treatment of nonmalignant pain: A structured literature review. J. Neurosurg. 2008, 109, 389–404. [Google Scholar] [CrossRef]
- Sullivan, L.D.; McLoughlin, M.G.; Goldenberg, L.G.; Gleave, M.E.; Marich, K.W. Early experience with high-intensity focused ultrasound for the treatment of benign prostatic hypertrophy. Br. J. Urol. 1997, 79, 172–176. [Google Scholar] [CrossRef]
- Madersbacher, S.; Schatzl, G.; Djavan, B.; Stulnig, T.; Marberger, M. Long-term outcome of transrectal high- intensity focused ultrasound therapy for benign prostatic hyperplasia. Eur. Urol. 2000, 37, 687–694. [Google Scholar] [CrossRef]
- Bergenfelz, A.; Jansson, S.; Kristoffersson, A.; Mårtensson, H.; Reihnér, E.; Wallin, G.; Lausen, I. Complications to thyroid surgery: Results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch. Surg. 2008, 393, 667–673. [Google Scholar] [CrossRef]
- Kovatcheva, R.D.; Vlahov, J.D.; Stoinov, J.I.; Zaletel, K. Benign Solid Thyroid Nodules: US-guided High-Intensity Focused Ultrasound Ablation—Initial Clinical Outcomes. Radiology 2015, 276, 597–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esnault, O.; Franc, B.; Ménégaux, F.; Rouxel, A.; De Kerviler, E.; Bourrier, P.; Lacoste, F.; Chapelon, J.Y.; Leenhardt, L. High-intensity focused ultrasound ablation of thyroid nodules: First human feasibility study. Thyroid 2011, 21, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Korkusuz, H.; Fehre, N.; Sennert, M.; Happel, C.; Grünwald, F. Volume reduction of benign thyroid nodules 3 months after a single treatment with high-intensity focused ultrasound (HIFU). J. Ther. Ultrasound 2015, 3, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leenhardt, L.; Rouxel, A.; Menegaux, F.; Esnault, O. An open-label, randomized, controlled study of the effectiveness and safety of a high intensity focused ultrasound device compared with observation in patients with non-malignant cold thyroid nodules. Endocr. Abstr. 2013, 32, 1013. [Google Scholar] [CrossRef] [Green Version]
- Korkusuz, H.; Fehre, N.; Sennert, M.; Happel, C.; Grünwald, F. Early assessment of high-intensity focused ultrasound treatment of benign thyroid nodules by scintigraphic means. J. Ther. Ultrasound 2014, 2, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovatcheva, R.D.; Zaletel, K. High-intensity focused ultrasound for thyroid nodule ablation: The evidence to date. Rep. Med Imaging 2017, 10, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Kovatcheva, R.D.; Vlahov, J.D.; Stoinov, J.I.; Zaletel, K. The effect of one and two sessions of US-guided high-intensity focused ultrasound (HIFU) treatment on thyroid nodule volume and thyroid function. Thyroid 2015, 25, S176–S177. [Google Scholar]
- Gliklich, R.E.; White, W.M.; Slayton, M.H.; Barthe, P.G.; Makin, I.R.S. Clinical pilot study of intense ultrasound therapy to deep dermal facial skin and subcutaneous tissues. Arch. Facial Plast. Surg. 2007, 9, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Wang, Z.-B.; Chen, W.-Z.; Zou, J.-Z.; Bai, J.; Zhu, H.; Li, K.-Q.; Xie, F.-L.; Jin, C.-B.; Su, H.-B. Extracorporeal focused ultrasound surgery for treatment of human solid carcinomas: Early Chinese clinical experience. Ultrasound Med. Biol. 2004, 30, 245–260. [Google Scholar] [CrossRef]
- Napoli, A.; Anzidei, M.; De Nunzio, C.; Cartocci, G.; Panebianco, V.; De Dominicis, C.; Catalano, C.; Petrucci, F.; Leonardo, C. Real-time magnetic resonance-guided high-intensity focused ultrasound focal therapy for localised prostate cancer: Preliminary experience. Eur. Urol. 2013, 63, 395–398. [Google Scholar] [CrossRef]
- Okada, A.; Murakami, T.; Mikami, K.; Onishi, H.; Tanigawa, N.; Marukawa, T.; Nakamura, H. A case of hepatocellular carcinoma treated by MR-guided focused ultrasound ablation with respiratory gating. Magn. Reson. Med Sci. 2006, 5, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y. Principles and Applications of Therapeutic Ultrasound in Healthcare; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Vykhodtseva, N.; McDannold, N.; Hynynen, K. Progress and problems in the application of focused ultrasound for blood-brain barrier disruption. Ultrasonics 2008, 48, 279–296. [Google Scholar] [CrossRef] [Green Version]
- Wei, K.-C.; Chu, P.-C.; Wang, H.-Y.J.; Huang, C.-Y.; Chen, P.-Y.; Tsai, H.-C.; Lu, Y.-J.; Lee, P.-Y.; Tseng, I.-C.; Feng, L.-Y. Focused ultrasound-induced blood–brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: A preclinical study. PLoS ONE 2013, 8, e58995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thevenot, E.; Jordao, J.F.; O’Reilly, M.A.; Markham, K.; Weng, Y.Q.; Foust, K.D.; Kaspar, B.K.; Hynynen, K.; Aubert, I. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum. Gene Ther. 2012, 23, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Samiotaki, G.; Konofagou, E.E. Dependence of the reversibility of focused- ultrasound-induced blood-brain barrier opening on pressure and pulse length in vivo. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Bonow, R.H.; Silber, J.R.; Enzmann, D.R.; Beauchamp, N.J.; Ellenbogen, R.G.; Mourad, P.D. Towards use of MRI-guided ultrasound for treating cerebral vasospasm. J. Ther. Ultrasound 2016, 4, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieck, B.; Bates, D.; Zhang, K.; Escott, N.; Mougenot, C.; Pichardo, S.; Curiel, L. Focused ultrasound treatment of abscesses induced by methicillin resistant Staphylococcus aureus: Feasibility study in a mouse model. Med. Phys. 2014, 41, 063301. [Google Scholar] [CrossRef]
- Smith, N.B. Applications of ultrasonic skin permeation in transdermal drug delivery. Expert Opin. Drug Deliv. 2008, 5, 1107–1120. [Google Scholar] [CrossRef]
- Pitt, W.G.; Husseini, G.A.; Staples, B.J. Ultrasonic drug delivery-a general review. Expert Opin. Drug Deliv. 2004, 1, 37–56. [Google Scholar] [CrossRef] [Green Version]
- Fan, C.-H.; Ting, C.-Y.; Lin, C.Y.; Chan, H.-L.; Chang, Y.-C.; Chen, Y.-Y.; Liu, H.-L.; Yeh, C.-K. Noninvasive, targeted, and non-viral ultrasound-mediated GDNF-plasmid delivery for treatment of Parkinson’s disease. Sci. Rep. 2016, 6, 19579. [Google Scholar] [CrossRef]
- Meng, Y.; Solomon, B.; Boutet, A.; Llinas, M.; Scantlebury, N.; Huang, Y.; Hynynen, K.; Hamani, C.; Fasano, A.; Lozano, A.M.; et al. Magnetic resonance-guided focused ultrasound thalamotomy for treatment of essential tremor: A 2-year outcome study. Mov. Disord. 2018, 33, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Zaaroor, M.; Sinai, A.; Goldsher, D.; Eran, A.; Nassar, M.; Schlesinger, I. Magnetic resonance-guided focused ultrasound thalamotomy for tremor: A report of 30 Parkinson’s disease and essential tremor cases. J. Neurosurg. 2018, 128, 202–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacopino, D.G.; Gagliardo, C.; Giugno, A.; Giammalva, G.R.; Napoli, A.; Maugeri, R.; Graziano, F.; Valentino, F.; Cosentino, G.; D’Amelio, M.; et al. Preliminary experience with a transcranial magnetic resonance-guided focused ultrasound surgery system integrated with a 1.5-T MRI unit in a series of patients with essential tremor and Parkinson’s disease. Neurosurg. Focus 2018, 44, E7. [Google Scholar] [CrossRef] [Green Version]
- Kubanek, J. Neuromodulation with transcranial focused ultrasound. Neurosurg. Focus 2018, 44, E14. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.H.; Chang, W.S.; Kim, S.J.; Kim, C.-H.; Chang, J.W. The Potential Usefulness of Magnetic Resonance Guided Focused Ultrasound for Obsessive Compulsive Disorders. J. Korean Neurosurg. Soc. 2018, 61, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izadifar, Z.; Izadifar, Z.; Chapman, D.; Babyn, P. An Introduction to High Intensity Focused Ultrasound: Systematic Review on Principles, Devices, and Clinical Applications. J. Clin. Med. 2020, 9, 460. https://doi.org/10.3390/jcm9020460
Izadifar Z, Izadifar Z, Chapman D, Babyn P. An Introduction to High Intensity Focused Ultrasound: Systematic Review on Principles, Devices, and Clinical Applications. Journal of Clinical Medicine. 2020; 9(2):460. https://doi.org/10.3390/jcm9020460
Chicago/Turabian StyleIzadifar, Zahra, Zohreh Izadifar, Dean Chapman, and Paul Babyn. 2020. "An Introduction to High Intensity Focused Ultrasound: Systematic Review on Principles, Devices, and Clinical Applications" Journal of Clinical Medicine 9, no. 2: 460. https://doi.org/10.3390/jcm9020460
APA StyleIzadifar, Z., Izadifar, Z., Chapman, D., & Babyn, P. (2020). An Introduction to High Intensity Focused Ultrasound: Systematic Review on Principles, Devices, and Clinical Applications. Journal of Clinical Medicine, 9(2), 460. https://doi.org/10.3390/jcm9020460



