Urinary Liver-Type Fatty-Acid-Binding Protein Predicts Long-Term Adverse Outcomes in Medical Cardiac Intensive Care Units
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Definitions and Calculations
2.3. Biomarker Measurements
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics and Outcomes
3.2. Prognostic Value of Urinary L-FABP
3.3. Discrimination and Reclassification of L-FABP for Adverse Outcomes
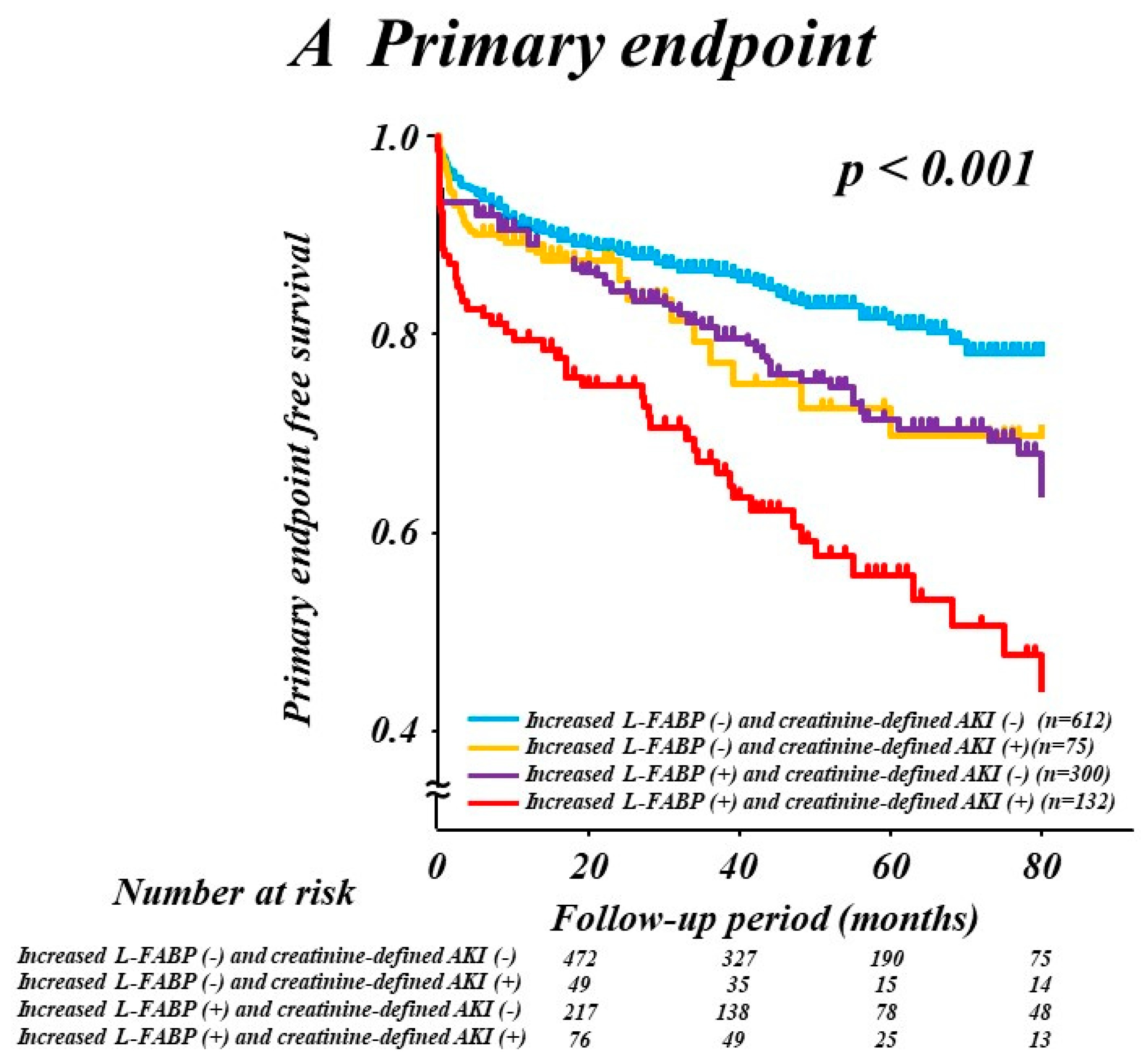
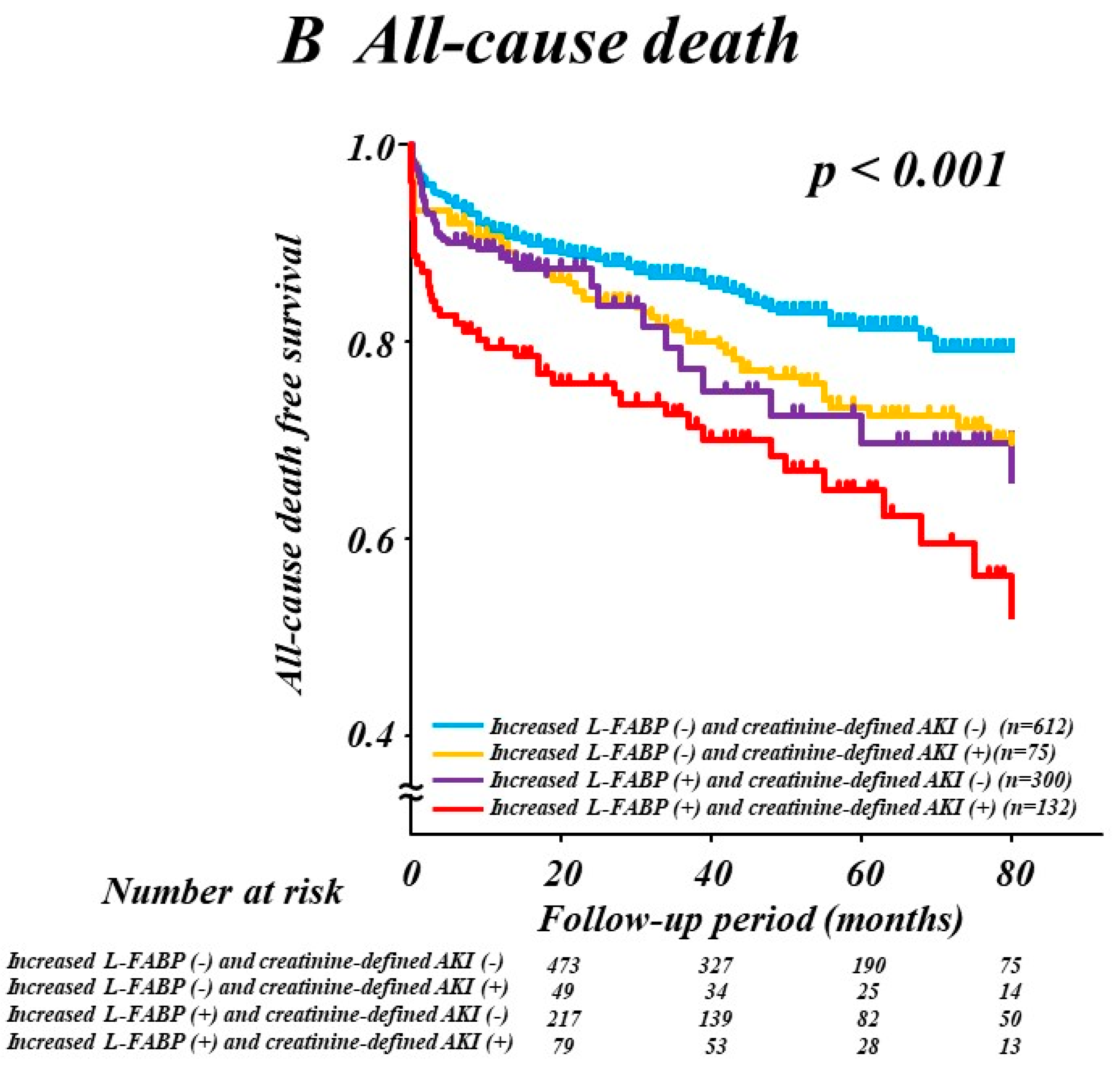
3.4. Combination of L-FABP and Creatinine-Defined AKI
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Portilla, D. Energy metabolism and cytotoxicity. Semin. Nephrol. 2003, 23, 432–438. [Google Scholar] [CrossRef]
- Yamamoto, T.; Noiri, E.; Ono, Y.; Doi, K.; Negishi, K.; Kamijo, A.; Kimura, K.; Fujita, T.; Kinukawa, T.; Taniguchi, H.; et al. Renal L-type fatty acid-binding protein in acute ischemic injury. J. Am. Soc. Nephrol. 2007, 18, 2894–2902. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xie, Y.; Shao, X.; Ni, Z.; Mou, S. L-FABP: a novel biomarker of kidney disease. Clin. Chim. Acta. 2015, 445, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Ito, K.; Kato, Y.; Sugaya, T.; Kubo, Y.; Tsuji, A. L-type fatty acid binding protein transgenic mouse as a novel tool to explore cytotoxicity to renal proximal tubules. Drug Metab. Pharmacokinet. 2008, 23, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Naruse, H.; Ishii, J.; Takahashi, H.; Kitagawa, F.; Nishimura, H.; Kawai, H.; Muramatsu, T.; Harada, M.; Yamada, A.; Motoyama, S.; et al. Predicting acute kidney injury using urinary liver-type fatty-acid binding protein and serum N-terminal pro-B-type natriuretic peptide levels in patients treated at medical cardiac intensive care units. Crit. Care. 2018, 22, 197. [Google Scholar] [CrossRef]
- Naruse, H.; Takahashi, H.; Ishii, J. Authors’ response to letter “Prediction of acute kidney injury in intensive care unit patients”. Crit. Care. 2019, 23, 58. [Google Scholar] [CrossRef]
- De Oliveira, B.D.; Xu, K.; Shen, T.H.; Callahan, M.; Kiryluk, K.; D’Agati, V.D.; Tatonetti, N.P.; Barasch, J.; Devarajan, P. Molecular nephrology: types of acute tubular injury. Nat. Rev. Nephrol. 2019, 15, 599–612. [Google Scholar]
- Doi, K.; Negishi, K.; Ishizu, T.; Katagiri, D.; Fujita, T.; Matsubara, T.; Yahagi, N.; Sugaya, T.; Noiri, E. Evaluation of new acute kidney injury biomarkers in a mixed intensive care unit. Crit. Care. Med. 2011, 39, 2464–2469. [Google Scholar] [CrossRef]
- McIlroy, D.R.; Farkas, D.; Matto, M.; Lee, H.T. Neutrophil gelatinase-associated lipocalin combined with delta serum creatinine provides early risk stratification for adverse outcomes after cardiac surgery: A prospective observational study. Crit. Care. Med. 2015, 43, 1043–1052. [Google Scholar] [CrossRef]
- Haase, M.; Devarajan, P.; Haase-Fielitz, A.; Bellomo, R.; Cruz, D.N.; Wagener, G.; Krawczeski, C.D.; Koyner, J.L.; Murray, P.; Zappitelli, M.; et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: A multicenter pooled analysis of prospective studies. J. Am. Coll. Cardiol. 2011, 57, 1752–1761. [Google Scholar] [CrossRef]
- Coca, S.G.; Garg, A.X.; Thiessen-Philbrook, H.; Koyner, J.L.; Patel, U.D.; Krumholz, H.M.; Shlipak, M.G.; Parikh, C.R. Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J. Am. Soc. Nephrol. 2014, 25, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.; Albert, A.; Kube, J.; Bellomo, R.; Wettersten, N.; Kuppe, H.; Westphal, S.; Haase, M.; Haase-Fielitz, A. Urinary biomarkers may provide prognostic information for subclinical acute kidney injury after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2018, 155, 2441–2452. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving global outcomes (KDIGO) acute kidney injury work group (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138.
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1998, 44, 837–845. [Google Scholar] [CrossRef]
- Pencina, M.J.; D’Agostino, R.B., Sr.; D’Agostino, R.B., Jr.; Vasan, R.S. Evaluating the added predictive ability of a new marker from area under the ROC curve to reclassification and beyond. Stat Med. 2008, 27, 157–172. [Google Scholar] [CrossRef]
- Cook, N.R. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007, 115, 928–935. [Google Scholar] [CrossRef]
- Holland, E.M.; Moss, T.J. Acute Noncardiovascular Illness in the Cardiac Intensive Care Unit. J. Am. Coll. Cardiol. 2017, 69, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Iwagami, M.; Yasunaga, H.; Noiri, E.; Horiguchi, H.; Fushimi, K.; Matsubara, T.; Yahagi, N.; Nangaku, M.; Doi, K. Choice of renal replacement therapy modality in intensive care units: data from a Japanese Nationwide Administrative Claim Database. J. Crit. Care 2015, 30, 381–385. [Google Scholar] [CrossRef]
- Katagiri, D.; Doi, K.; Honda, K.; Negishi, K.; Fujita, T.; Hisagi, M.; Ono, M.; Matsubara, T.; Yahagi, N.; Iwagami, M.; et al. Combination of two urinary biomarkers predicts acute kidney injury after adult cardiac surgery. Ann. Thorac. Surg. 2012, 93, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Noiri, E.; Maeda-Mamiya, R.; Ishii, T.; Negishi, K.; Hamasaki, Y.; Fujita, T.; Yahagi, N.; Koide, H.; Sugaya, T.; et al. Urinary L-type fatty acid-binding protein as a new biomarker of sepsis complicated with acute kidney injury. Crit. Care Med. 2010, 38, 2037–2042. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Yang, H.N.; Jo, S.K.; Cho, W.Y.; Kim, H.K. The role of urinary liver-type fatty acid-binding protein in critically ill patients. J. Korean Med. Sci. 2013, 28, 100–105. [Google Scholar] [CrossRef]
- Parr, S.K.; Clark, A.J.; Bian, A.; Shintani, A.K.; Wickersham, N.E.; Ware, L.B.; Ikizler, T.A.; Siew, E.D. Urinary L-FABP predicts poor outcomes in critically ill patients with early acute kidney injury. Kidney Int. 2015, 87, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Parikh, C.R.; Puthumana, J.; Shlipak, M.G.; Koyner, J.L.; Thiessen-Philbrook, H.; McArthur, E.; Kerr, K.; Kavsak, P.; Whitlock, R.P.; Garg, A.X.; et al. Relationship of Kidney Injury Biomarkers with Long-Term Cardiovascular Outcomes after Cardiac Surgery. J. Am. Soc. Nephrol. 2017, 28, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Kamijo-Ikemori, A.; Sugaya, T.; Yoshida, M.; Hoshino, S.; Akatsu, S.; Yamazaki, S.; Kimura, K.; Shibagaki, Y. Clinical utility of urinary liver-type fatty acid binding protein measured by latex-enhanced turbidimetric immunoassay in chronic kidney disease. Clin. Chem. Lab. Med. 2016, 54, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Waikar, S.S.; Sabbisetti, V.S.; Bonventre, J.V. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010, 78, 486–494. [Google Scholar] [CrossRef]

| Acute coronary syndrome, n (%) | 529 (47) |
| STEMI, n | 217 |
| NSTEM, n | 264 |
| Unstable angina, n | 48 |
| Acute decompensated heart failure, n (%) | 424 (38) |
| With reduced ejection fraction (LVEF < 40%), n | 217 |
| With mid-range ejection fraction (40% ≤ LVEF < 50%), n | 67 |
| With preserved ejection fraction (LVEF ≥ 50%), n | 140 |
| Arrhythmia, n (%) | 51 (5) |
| Supraventricular tachycardia, n | 6 |
| Ventricular tachycardia, n | 14 |
| Sick sinus syndrome, n | 13 |
| Second- or third-degree atrioventricular block, n | 18 |
| Primary pulmonary hypertension, n (%) | 32 (3) |
| Acute aortic syndrome, n (%) | 24 (2) |
| Infective endocarditis, n (%) | 14 (1) |
| Takotsubo cardiomyopathy, n (%) | 11 (1) |
| Others, n (%) | 34 (3) |
| All Patients | Primary Endpoint (+) | Primary Endpoint (-) | p Value | |
|---|---|---|---|---|
| Number | 1119 | 242 | 877 | |
| Age (year) | 68 ± 12 | 73 ± 9 | 67 ± 13 | <0.001 |
| Male, n (%) | 732 (65) | 157 (65) | 575 (66) | 0.84 |
| Hypertension, n (%) | 724 (65) | 158 (65) | 566 (65) | 0.83 |
| Dyslipidemia, n (%) | 520 (47) | 97 (40) | 423 (48) | 0.02 |
| Diabetes, n (%) | 420 (38) | 88 (36) | 332 (38) | 0.67 |
| Current or ex-smoker, n (%) | 324 (29) | 70 (29) | 254 (29) | 0.99 |
| Previous myocardial infarction, n (%) | 214 (19) | 61 (25) | 153 (17) | 0.007 |
| Prior hospitalization for worsening heart failure, n (%) | 215 (19) | 53 (22) | 162 (19) | 0.23 |
| Previous coronary revascularization, n (%) | 213 (19) | 59 (24) | 154 (18) | 0.02 |
| Paroxysmal or persistent AF, n (%) | 248 (22) | 77 (32) | 171 (20) | <0.001 |
| Acute decompensated heart failure, n (%) | 424 (38) | 143 (59) | 281 (32) | <0.001 |
| SOFA score | 2 (1–4) | 4 (2–5) | 2 (1–4) | <0.001 |
| Systolic blood pressure, mmHg | 141 ± 31 | 135 ± 32 | 143 ± 31 | <0.001 |
| Heart rate, beats per minutes | 86 ± 25 | 90 ± 24 | 85 ± 26 | 0.001 |
| Emergent CAG or PCI before admission, n (%) | 405 (36) | 69 (29) | 336 (38) | 0.005 |
| Mechanical ventilation before admission, n (%) | 20 (1.8) | 6 (2.5) | 14 (1.6) | 0.36 |
| IABP before admission, n (%) | 96 (8.6) | 20 (8.3) | 76 (8.7) | 0.84 |
| White blood cell count, ×103/μL | 8.7 ± 3.6 | 8.4 ± 3.9 | 8.7 ± 3.4 | 0.19 |
| Hemoglobin, g/dL | 12.7 ± 2.3 | 11.7 ± 2.3 | 13.0 ± 2.2 | <0.001 |
| eGFR, mL/min/1.73 m2 | 66.6 ± 26.6 | 54.2 ± 25.2 | 70.0 ± 26.0 | <0.001 |
| Glucose, mg/dL | 159 ± 70 | 170 ± 75 | 156 ± 68 | 0.006 |
| hs-CRP, mg/L | 2.32 (0.75–10.3) | 4.50 (1.09–24.3) | 1.99 (0.69–8.18) | <0.001 |
| BNP, pg/mL | 186 (53–631) | 581 (158–1210) | 133 (43–479) | <0.001 |
| hs-TnT, pg/mL | 59 (17–445) | 56 (24–290) | 62 (15–51) | 0.43 |
| Urinary L-FABP, ng/mL | 5.8 (2.4–16.9) | 9.2 (3.1–27.0) | 5.2 (2.2–14.5) | <0.001 |
| LVEF, % | 47.3 ± 13.8 | 42.4 ± 14.4 | 48.7 ± 13.3 | <0.001 |
| Treatment at enrollment, n (%) | ||||
| Antiplatelet drugs | 387 (35) | 111 (46) | 276 (32) | <0.001 |
| Statins | 355 (32) | 70 (29) | 285 (33) | 0.29 |
| RAAS inhibitors | 469 (42) | 110 (46) | 359 (41) | 0.21 |
| Beta-blockers | 301 (27) | 84 (35) | 217 (25) | 0.002 |
| Diuretics | 305 (27) | 103 (43) | 202 (23) | <0.001 |
| Anticoagulant drugs | 163 (15) | 52 (22) | 111 (13) | <0.001 |
| Creatinine-defined AKI, n (%) | 207 (18.5) | 68 (28.1) | 139 (15.8) | <0.001 |
| (A) Primary Endpoint | Model 1 | Model 2 | ||
| Variables | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Age (per 10 years increment) | 1.54 (1.32–1.81) | <0.001 | 1.54 (1.32–1.80) | <0.001 |
| Previous myocardial infarction | 0.81 (0.56–1.18) | 0.27 | 0.86 (0.59–1.25) | 0.43 |
| Paroxysmal or persistent AF | 1.16 (0.87–1.55) | 0.32 | 1.16 (0.87–1.56) | 0.31 |
| Previous coronary revascularization | 1.09 (0.75–1.59) | 0.66 | 1.06 (0.73–1.55) | 0.76 |
| Acute decompensated heart failure | 1.03 (0.74–1.42) | 0.87 | 1.06 (0.77–1.47) | 0.72 |
| Systolic blood pressure (per 10 mmHg increment) | 0.94 (0.90–0.98) | 0.004 | 0.93 (0.89–0.97) | 0.002 |
| Heart rate (per 10 beats per minutes increment) | 1.03 (0.98–1.08) | 0.26 | 1.03 (0.98–1.09) | 0.26 |
| Hemoglobin (per 1 g/dL increment) | 0.88 (0.83–0.94) | <0.001 | 0.88 (0.83–0.94) | <0.001 |
| CKD | 1.14 (0.85–1.54) | 0.38 | 1.18 (0.88–1.58) | 0.28 |
| hs-CRP (per 10-fold increment) | 1.08 (0.91–1.27) | 0.40 | 1.09 (0.93–1.29) | 0.29 |
| BNP (per 10-fold increment) | 1.84 (1.37–2.49) | <0.001 | 1.80 (1.34–2.44) | <0.001 |
| Urinary L-FABP (per 10-fold increment) | 1.47 (1.22–1.76) | <0.001 | ||
| Urinary L-FABP (ng/mL) | ||||
| < 9.0 (1st + 2nd + 3rd quintile) | Reference | |||
| ≥ 9.0 (4th + 5th quintile) | 1.63 (1.25–2.12) | <0.001 | ||
| LVEF (per 10% increment) | 0.86 (0.78–0.96) | 0.005 | 0.87 (0.79–0.97) | 0.01 |
| (B) All-cause Mortality | Model 1 | Model 2 | ||
| Variables | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Age (per 10 years increment) | 1.66 (1.41–1.96) | <0.001 | 1.66 (1.40–1.96) | <0.001 |
| Previous myocardial infarction | 0.85 (0.58–1.24) | 0.40 | 0.89 (0.61–1.31) | 0.56 |
| Paroxysmal or persistent AF | 1.20 (0.89–1.61) | 0.24 | 1.20 (0.89–1.62) | 0.24 |
| Previous coronary revascularization | 1.11 (0.75–1.63) | 0.60 | 1.08 (0.73–1.59) | 0.70 |
| Acute decompensated heart failure | 1.00 (0.71–1.39) | 0.99 | 1.02 (0.73–1.43) | 0.90 |
| Systolic blood pressure (per 10 mmHg increment) | 0.93 (0.89–0.97) | 0.002 | 0.92 (0.88–0.97) | <0.001 |
| Heart rate (per 10 beats per minutes increment) | 1.03 (0.97–1.08) | 0.35 | 1.02 (0.97–1.08) | 0.37 |
| Hemoglobin (per 1 g/dL increment) | 0.90 (0.85–0.96) | 0.002 | 0.91 (0.85–0.97) | 0.004 |
| CKD | 1.02 (0.75–1.37) | 0.92 | 1.05 (0.78–1.42) | 0.74 |
| hs-CRP (per 10-fold increment) | 1.13 (0.95–1.35) | 0.17 | 1.15 (0.97–1.37) | 0.10 |
| BNP (per 10-fold increment) | 1.89 (1.39–2.57) | <0.001 | 1.86 (1.36–2.53) | <0.001 |
| Urinary L-FABP (per 10-fold increment) | 1.43 (1.18–1.72) | <0.001 | ||
| Urinary L-FABP (ng/mL) | ||||
| < 9.0 (1st + 2nd + 3rd quintile) | Reference | |||
| ≥ 9.0 (4th + 5th quintile) | 1.50 (1.14–1.97) | 0.003 | ||
| LVEF (per 10% increment) | 0.87 (0.78–0.97) | 0.009 | 0.88 (0.79–0.98) | 0.02 |
| (A) Primary Endpoint | ||||||
| C-index | p Value | NRI | p Value | IDI | p Value | |
| Established risk factor model | 0.756 | Reference | Reference | Reference | ||
| Established risk factor model + L-FABP | 0.763 | 0.76 | 0.252 | <0.001 | 0.013 | 0.002 |
| (B) All-cause Mortality | ||||||
| C-index | p Value | NRI | p Value | IDI | p Value | |
| Established risk factor model | 0.760 | Reference | Reference | Reference | ||
| Established risk factor model + L-FABP | 0.766 | 0.80 | 0.222 | 0.001 | 0.012 | 0.004 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naruse, H.; Ishii, J.; Takahashi, H.; Kitagawa, F.; Nishimura, H.; Kawai, H.; Muramatsu, T.; Harada, M.; Yamada, A.; Fujiwara, W.; et al. Urinary Liver-Type Fatty-Acid-Binding Protein Predicts Long-Term Adverse Outcomes in Medical Cardiac Intensive Care Units. J. Clin. Med. 2020, 9, 482. https://doi.org/10.3390/jcm9020482
Naruse H, Ishii J, Takahashi H, Kitagawa F, Nishimura H, Kawai H, Muramatsu T, Harada M, Yamada A, Fujiwara W, et al. Urinary Liver-Type Fatty-Acid-Binding Protein Predicts Long-Term Adverse Outcomes in Medical Cardiac Intensive Care Units. Journal of Clinical Medicine. 2020; 9(2):482. https://doi.org/10.3390/jcm9020482
Chicago/Turabian StyleNaruse, Hiroyuki, Junnichi Ishii, Hiroshi Takahashi, Fumihiko Kitagawa, Hideto Nishimura, Hideki Kawai, Takashi Muramatsu, Masahide Harada, Akira Yamada, Wakaya Fujiwara, and et al. 2020. "Urinary Liver-Type Fatty-Acid-Binding Protein Predicts Long-Term Adverse Outcomes in Medical Cardiac Intensive Care Units" Journal of Clinical Medicine 9, no. 2: 482. https://doi.org/10.3390/jcm9020482
APA StyleNaruse, H., Ishii, J., Takahashi, H., Kitagawa, F., Nishimura, H., Kawai, H., Muramatsu, T., Harada, M., Yamada, A., Fujiwara, W., Hayashi, M., Motoyama, S., Sarai, M., Watanabe, E., Izawa, H., & Ozaki, Y. (2020). Urinary Liver-Type Fatty-Acid-Binding Protein Predicts Long-Term Adverse Outcomes in Medical Cardiac Intensive Care Units. Journal of Clinical Medicine, 9(2), 482. https://doi.org/10.3390/jcm9020482





