Combining Novel Biomarkers for Risk Stratification of Two-Year Cardiovascular Mortality in Patients with ST-Elevation Myocardial Infarction
Abstract
:1. Introduction
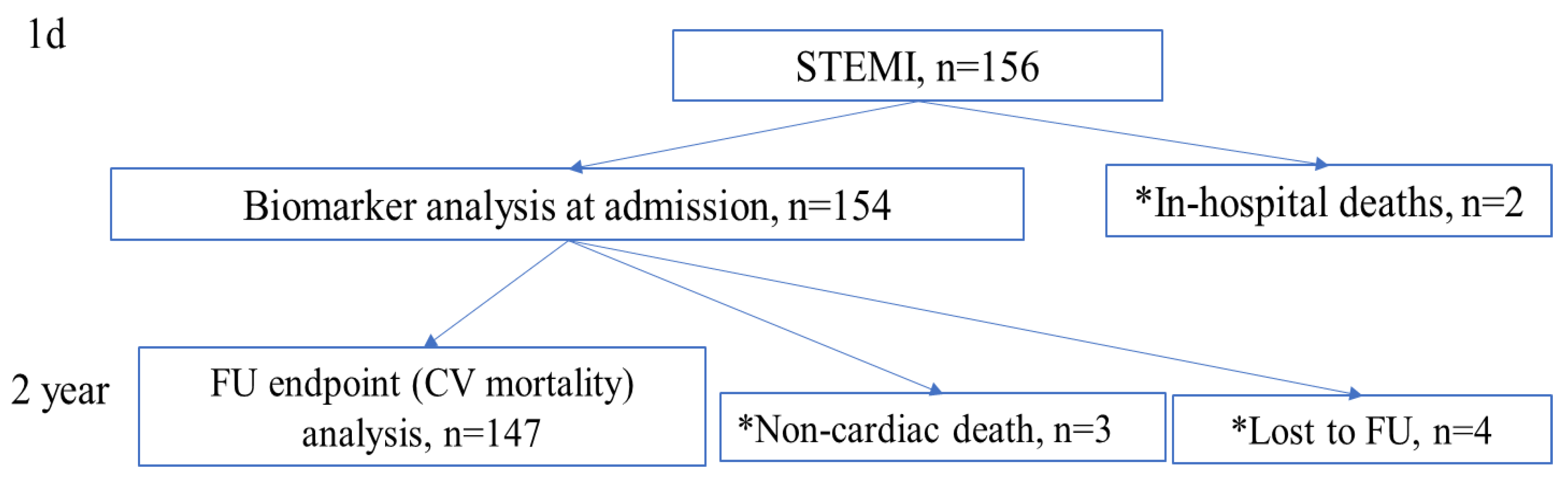
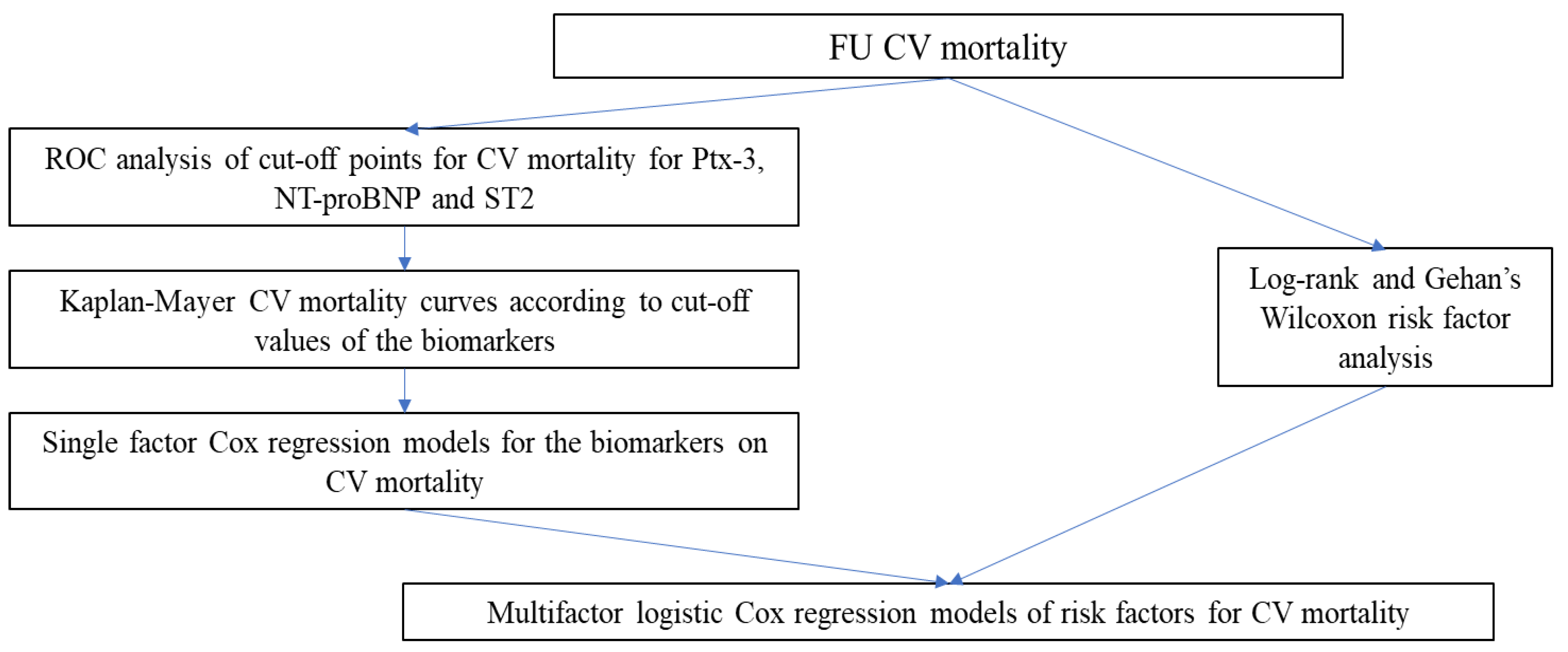
2. Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Ibanez, B.; Lames, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed] [Green Version]
- Magnussen, C.; Blankenberg, S. Biomarkers for heart failure: Small molecules with high clinical relevance. J. Intern. Med. 2018, 283, 530–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarko, J.; Pollack, C.V. Cardiac troponins. J. Emerg. Med. 2002, 23, 57–65. [Google Scholar] [CrossRef]
- Margit, M.B.; Klaus, H.; Schröder, A.; Ebert, C.; Borgya, A.; Gerhardt, W.; Remppis, A.; Zehelein, J.; Katus, H.A. Improved troponin T ELISA specific for cardiac troponin T isoform: Assay development and analytical and clinical validation. Clin. Chem. 1997, 43, 458–466. [Google Scholar]
- Garg, P.; Morris, P.; Fazlanie, A.L.; Vijayan, S.; Dancso, B.; Dastidar, A.G.; Plein, S.; Mueller, C.; Haaf, P. Cardiac biomarkers of acute coronary syndrome: From history to high-sensitivity cardiac troponin. Intern. Emerg. Med. 2017, 12, 147–155. [Google Scholar] [CrossRef] [Green Version]
- James, S.; Lindback, J.; Tilly, J.; Siegbahn, A.; Venge, P.; Armstrong, P.; Califf, R.; Simoons, M.L.; Wallentin, L.; Lindahl, B. Troponin-T and N-terminal pro-B-type natriuretic peptide predict mortality benefit from coronary revascularization in acute coronary syndromes: A GUSTO-IV substudy. J. Am. Coll. Cardiol. 2006, 48, 1146–1154. [Google Scholar] [CrossRef] [Green Version]
- Casula, M.; Montecucco, F.; Bonaventura, A.; Liberale, L.; Vecchié, A.; Dallegri, F.; Carbone, F. Update on the role of Pentraxin 3 in atherosclerosis and cardiovascular diseases. Vasc. Pharm. 2017, 99, 1–12. [Google Scholar] [CrossRef]
- Ristagno, G.; Fumagalli, F.; Bottazzi, B.; Mantovani, A.; Olivari, D.; Novelli, D.; Latini, R. Pentraxin 3 in Cardiovascular Disease. Front. Immunol. 2019, 10, 823. [Google Scholar] [CrossRef]
- Fornai, F.; Carrizzo, A.; Forte, M.; Ambrosio, M.; Damato, A.; Ferrucci, M.; Biagioni, F.; Busceti, C.; Puca, A.A.; Vecchione, C. The inflammatory protein Pentraxin 3 in cardiovascular disease. Immun. Ageing 2016, 13, 25. [Google Scholar] [CrossRef] [Green Version]
- Ciccone, M.M.; Cortese, F.; Gesualdo, M.; Riccardi, R.; Di Nunzio, D.; Moncelli, M.; Iacoviello, M.; Scicchitano, P. A novel cardiac bio-marker: ST2: A review. Molecules 2013, 18, 15314–15328. [Google Scholar] [CrossRef]
- Aydin, S.; Ugur, K.; Aydin, S.; Sahin, İ.; Yardim, M. Biomarkers in acute myocardial infarction: Current perspectives. Vasc. Health Risk Manag. 2019, 17, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helseth, R.; Solheim, S.; Opstad, T.; Hoffmann, P.; Arnesen, H.; Seljeflot, I. The time profile of Pentraxin 3 in patients with acute ST-elevation myocardial infarction and stable angina pectoris undergoing percutaneous coronary intervention. Mediat. Inflamm. 2014, 2014, 608414. [Google Scholar] [CrossRef] [PubMed]
- Morishita, T.; Uzui, H.; Nakano, A.; Fukuoka, Y.; Ikeda, H.; Amaya, N.; Kaseno, K.; Ishida, K.; Lee, J.D.; Tada, H. Association of Plasma pentraxin-3 Levels With Coronary Risk Factors and the Lipid Profile: A Cross-Sectional Study in Japanese Patients With Stable Angina Pectoris. Heart Vessel. 2018, 33, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Akgul, O.; Baycan, O.F.; Nakano, A.; Fukuoka, Y.; Ikeda, H.; Amaya, N.; Kaseno, K.; Ishida, K.; Lee, J.D.; Tada, H. Long-term prognostic value of elevated pentraxin 3 in patients undergoing primary angioplasty for ST-elevation myocardial infarction. Coron. Artery Dis. 2015, 26, 592–597. [Google Scholar] [CrossRef]
- Mjelva, O.R.; Ponitz, V.; Brügger-Andersen, T.; Grundt, H.; Staines, H.; Nilsen, D.W. Long-term prognostic utility of pentraxin 3 and D-dimer as compared to high-sensitivity C-reactive protein and B-type natriuretic peptide in suspected acute coronary syndrome. Eur. J. Prev. Cardiol. 2016, 23, 1130–1140. [Google Scholar] [CrossRef]
- Altay, S.; Cakmak, H.A.; Kemaloğlu Öz, T.; Özpamuk Karadeniz, F.; Türer, A.; Erer, H.B.; Kılıç, G.F.; Keleş, İ.; Can, G.; Eren, M. Long-term prognostic significance of pentraxin-3 in patients with acute myocardial infarction: 5-year prospective cohort study. Anatol. J. Cardiol. 2017, 17, 202–209. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Januzzi, J.L. The biology of ST2: The International ST2 Consensus Panel. Am. J. Cardiol. 2015, 115, 3B–7B. [Google Scholar] [CrossRef]
- Miñana, G.; Núñez, J.; Bayés-Genís, A.; Revuelta-López, E.; Ríos-Navarro, C.; Núñez, E.; Chorro, F.J.; López-Lereu, M.P.; Monmeneu, J.V.; Lupón, J.; et al. ST2 and Left Ventricular Remodeling After ST-segment Elevation Myocardial Infarction: A Cardiac Magnetic Resonance Study. Int. J. Cardiol. 2018, 270, 336–342. [Google Scholar] [CrossRef]
- Weir, R.A.; Miller, A.M.; Murphy, G.E.; Clements, S.; Steedman, T.; Connell, J.M.; McInnes, I.B.; Dargie, H.J.; McMurray, J.J. Serum soluble ST2: A potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J. Am. Coll. Cardiol. 2010, 55, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Lupón, J.; de Antonio, M.; Galán, A.; Vila, J.; Zamora, E.; Urrutia, A.; Bayes-Genis, A. Combined use of the novel biomarkers high-sensitivity troponin T and ST2 for heart failure risk stratification vs conventional assessment. Mayo Clin. Proc. 2013, 88, 234–243. [Google Scholar] [CrossRef] [Green Version]
- Bayes-Genis, A.; Richards, A.M.; Maisel, A.S.; Mueller, C.; Ky, B. Multimarker testing with ST2 in chronic heart failure. Am. J. Cardiol. 2015, 115, 76B–80B. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.M.; Somma, S. Di.; Mueller, T. ST2 in stable and unstable ischemic heart diseases. Am. J. Cardiol. 2015, 115, 48B–58B. [Google Scholar] [CrossRef] [Green Version]
- Maisel, A.S.; Richards, A.M.; Pascual-Figal, D.; Mueller, C. Serial ST2 testing in hospitalized patients with acute heart failure. Am. J. Cardiol. 2015, 115, 32B–37B. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [PubMed]
- Lichtenauer, M.; Jirak, P.; Wernly, B.; Paar, V.; Rohm, I.; Jung, C.; Schernthaner, C.; Kraus, J.; Motloch, L.J.; Yilmaz, A.; et al. A comparative analysis of novel cardiovascular biomarkers in patients with chronic heart failure. Eur. J. Intern. Med. 2017, 44, 31–38. [Google Scholar] [CrossRef]
- Jirak, P.; Fejzic, D.; Paar, V.; Wernly, B.; Pistulli, R.; Rohm, I.; Jung, C.; Hoppe, U.C.; Schulze, P.C.; Lichtenauer, M.; et al. Influences of Ivabradine treatment on serum levels of cardiac biomarkers sST2, GDF-15, suPAR and H-FABP in patients with chronic heart failure. Acta Pharm. Sin. 2018, 39, 1189–1196. [Google Scholar] [CrossRef]
- Oremus, M.; McKelvie, R.; Don-Wauchope, A.; Santaguida, P.L.; Ali, U.; Balion, C.; Hill, S.; Booth, R.; Brown, J.A.; Bustamam, A.; et al. A systematic review of BNP and NT-proBNP in the management of heart failure: Overview and methods. Heart Fail. Rev. 2014, 19, 413–419. [Google Scholar] [CrossRef]
- Fan, J.; Ma, J.; Xia, N.; Sun, L.; Li, B.; Liu, H. Clinical Value of Combined Detection of CK-MB, MYO, cTnI and Plasma NT-proBNP in Diagnosis of Acute Myocardial Infarction. Clin. Lab. 2017, 63, 427–433. [Google Scholar] [CrossRef]
- Reinstadler, S.J.; Feistritzer, H.J.; Reindl, M.; Klug, G.; Metzler, B. Utility of NT-proBNP in predicting infarct scar and left ventricular dysfunction at a chronic stage after myocardial infarction. Eur. J. Intern. Med. 2016, 29, 16–18. [Google Scholar] [CrossRef]
- Kopec, M.; Duma, A.; Helwani, M.A.; Brown, J.; Brown, F.; Gage, B.F.; Gibson, D.W.; Miller, J.P.; Novak, E.; Jaffe, A.S.; et al. Improving Prediction of Postoperative Myocardial Infarction With High-Sensitivity Cardiac Troponin T and NT-proBNP. Anesth. Analg. 2017, 124, 398–405. [Google Scholar] [CrossRef] [Green Version]
- Drewniak, W.; Szybka, W.; Bielecki, D.; Malinowski, M.; Kotlarska, J.; Krol-Jaskulska, A.; Popielarz-Grygalewicz, A.; Konwicka, A.; Dąbrowski, M. Prognostic Significance of NT-proBNP Levels in Patients Over 65 Presenting Acute Myocardial Infarction Treated Invasively or Conservatively. Biomed Res. Int. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenny, N.S.; Arnold, A.M.; Kuller, L.H.; Tracy, R.P.; Psaty, B.M. Associations of pentraxin 3 with cardiovascular disease and all-cause death: The Cardiovascular Health Study. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Jenny, N.S.; Blumenthal, R.S.; Kuller, L.H.; Tracy, R.P.; Psaty, B.M. Associations of pentraxin 3 with cardiovascular disease: The multiethnic study of atherosclerosis. J. Thromb. Haemost. 2014, 12, 999–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.R.; McKenzie, A.N.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Investig. 2007, 117, 1538–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggers, K.M.; Armstrong, P.W.; Califf, R.M.; Simoons, M.L.; Venge, P.; Wallentin, L.; James, S.K. ST2 and mortality in non-ST-segment elevation acute coronary syndrome. Am. Heart J. 2010, 159, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, W.S.; Roger, V.L.; Jaffe, A.S.; Weston, S.A.; AbouEzzeddine, O.F.; Jiang, R.; Manemann, S.M.; Enriquez-Sarano, M. Prognostic Value of Soluble ST2 After Myocardial Infarction: A Community Perspective. Am. J. Med. 2017, 130, 1112. [Google Scholar] [CrossRef] [Green Version]
- Sabatine, M.S.; Morrow, D.A.; Higgins, L.J.; MacGillivray, C.; Guo, W.; Bode, C.; Rifai, N.; Cannon, C.P.; Gerszten, R.E.; Lee, R.T. Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone B-type natriuretic peptide in patients with ST-elevation myocardial infarction. Circulation. 2008, 117, 1936–1944. [Google Scholar] [CrossRef] [Green Version]
- Jirak, P.; Mirna, M.; Wernly, B.; Paar, V.; Thieme, M.; Betge, S.; Franz, M.; Hoppe, U.; Lauten, A.; Kammler, J.; et al. Analysis of novel cardiovascular biomarkers in patients with peripheral artery disease (PAD). Minerva Med. 2018, 109, 443–450. [Google Scholar] [CrossRef]
- Gierlotka, M.; Zdrojewski, T.; Wojtyniak, B.; Poloński, L.; Stokwiszewski, J.; Gąsior, M.; Kozierkiewicz, A.; Kalarus, Z.; Wierucki, Ł.; Chlebus, K.; et al. Incidence, treatment, in-hospital mortality and one-year outcomes of acute myocardial infarction in Poland in 2009–2012—Nationwide AMI-PL database. Kardiol. Pol. 2015, 73, 142–158. [Google Scholar] [CrossRef]
- Nowbar, A.N.; Howard, J.P.; Finegold, J.A.; Asaria, P.; Francis, D.P. 2014 global geographic analysis of mortality from ischaemic heart disease by country, age and income: Statistics from World Health Organisation and United Nations. Int. J. Cardiol. 2014, 174, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Sidorenkov, O.; Nilssen, O.; Grjibovski, A.M. Metabolic syndrome in Russian adults: Associated factors and mortality from cardiovascular diseases and all causes. BMC Public Health 2010, 10, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Parameter | Value |
|---|---|
| n | 147 |
| Gender (male) | 118 (80.3 %) |
| Age | 60.9 ± 12.1 |
| LVEF (%) | 52.8 ± 7.2 |
| Hx stroke (%) | 5 (3.4) |
| Hx MI (%) | 34 (23.1) |
| Smoker (%) | 86(58.5) |
| Arterial hypertension (%) | 138 (93.9) |
| Dyslipidemia (%) | 111 (75.5) |
| DMT2 (%) | 37 (25.2) |
| Revascularization strategy | |
| Acute thrombolytic therapy (%) | 35 (23.8) |
| Successful thrombolytic therapy (%) | 17 (48.6) |
| Acute thrombolytic therapy followed by rescue PCI (%) | 18 (51.4) |
| Acute PCI only (%) | 112 (76.2) |
| Successful PCI (%) | 126 (96.9) |
| Target vessel in acute/rescue PCA: LCA (%) LAD (%) CX (%) RCA (%) Multivessel approach (%) | 1 (0.7) 51 (38.1) 12 (8.9) 48 (35.8) 12 (8.9) |
| Discharge medication | |
| ACE inhibitors/Angiotensin receptor blockers n (%) | 143 (97.3) |
| Beta-blockers (%) | 139 (94.6) |
| Diuretics (%) | 51 (34.7) |
| Aldosterone antagonists (%) | 37 (25.2) |
| Ivabradine (%) | 12 (8.1) |
| Statins (%) | 139 (94.6) |
| Acetylsalicylic acid (%) | 142 (96.0) |
| Thienopyridines (%) | 138 (93.8) |
| Warfarin | 1 (0.7) |
| NOAK (%) | 7 (4.8) |
| Parameter | Median (Q1, Q3) |
|---|---|
| n | 147 |
| CK-MB, mmol/L | 100.8; (38, 175) |
| hs-Troponin I, ng/mL | 688.4; (41, 2270) |
| NT-proBNP, pg/mL | 518.5; (54, 2130) |
| ST2, ng/mL | 43.8; (24.8, 56.5) |
| Pentraxin-3, ng/mL | 131.5; (110.8, 164.3) |
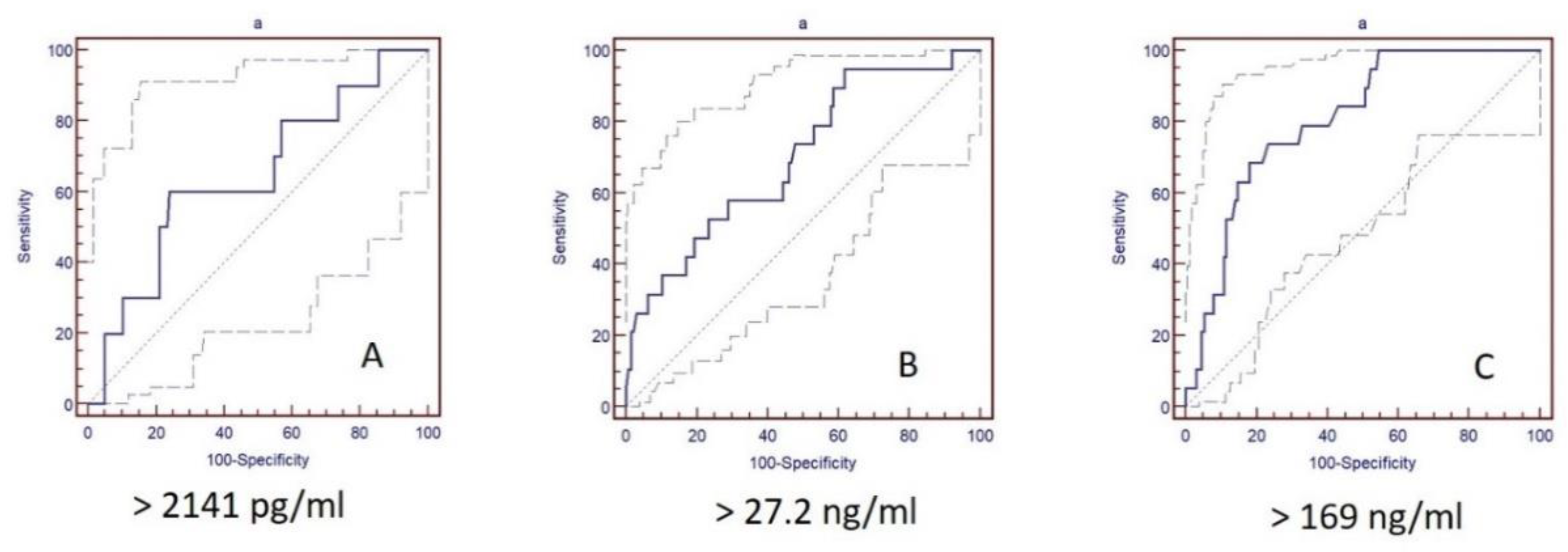
| Biomarker | CV Mortality | ||||
|---|---|---|---|---|---|
| Cut-Off | Sens. % | Spec. % | AUC | p-Value | |
| Ptx-3, ng/mL | >169 | 68.4 | 82.0 | 0.804 | 0.063 |
| NT-pro-BNP, pg/mL | >2141 | 73.7 | 80.5 | 0.801 | 0.063 |
| ST2, ng/mL | >27.2 | 94.7 | 38.3 | 0.698 | 0.071 |
| NT-proBNP, pg/mL | ST2, ng/mL | Ptx-3, ng/mL | ||||
|---|---|---|---|---|---|---|
| >2141 | ≤2141 | >27.2 | ≤27.2 | >169.0 | ≤169.0 | |
| n | 39 | 108 | 97 | 50 | 36 | 111 |
| CV mortality, n (%) | 14(35.9) | 5 (4.6) | 18(18.6) | 1(2.0) | 13(36.1) | 6(5.4) |
| Non-CV mortality, n (%) | 25(64.1) | 103 (95.4) | 79(81.4) | 49 (98.0) | 23(63.8) | 105(94.6) |
| n | NT-proBNP, pg/mL | ST2, ng/mL | Ptx-3, ng/mL | |
|---|---|---|---|---|
| CV mortality | 33 | 3019.0 ± 2270.5 | 93.7 ± 97.1 | 236.8 ± 158.5 |
| Non-CV mortality | 114 | 1015.8 ± 972.2 | 51.3 ± 47.3 | 158.2 ± 103.6 |
| Biomarker | Coefficient ± SE | Hazard Ratio | AUC | CI | p-Value |
|---|---|---|---|---|---|
| Log (NT-proBNP) | 0.49 ± 0.15 | 1.64 | 0.777 | 1.21–2.21 | 0.001 |
| ST2 | 0.000013 ± 0.000006 | 1.000022 | 0.800 | 1.00–1.001 | <0.001 |
| Log (Ptx-3) | 1.12±0.32 | 3.1 | 0.738 | 1.63–5.39 | 0.005 |
| Biomarker and Cut-Off Value | Coefficient ± SD | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|---|
| ST2 (AIC = 220, BIC = 226, p = 0.002, MER = 0.49, MEV = 0.39) | ||||
| ST2 > 27.2 ng/mL | 1.36 ± 0.57 | 3.88 | 1.27–11.84 | 0.017 |
| Age > 65 years | 1.32 ± 0.48 | 3.75 | 1.45–9.73 | 0.006 |
| Male gender | 0.52 ± 0.42 | 1.68 | 0.70–4.05 | 0.242 |
| hs-Troponin I | 0.43 ± 0.20 | 1.54 | 1.25–1.88 | 0.088 |
| LVEF < 60% | −0.37 ± 0.51 | 0.69 | 0.25–1.86 | 0.460 |
| Ptx-3 (AIC = 211, BIC = 217, p < 0.001, MER = 0.69, MEV = 0.56) | ||||
| Ptx-3 > 169 ng/mL | 1.66 ± 0.44 | 5.26 | 2.23–12.36 | 0.0001 |
| Age > 65 years | 1.04 ± 0.467 | 2.83 | 1.13–7.09 | 0.026 |
| Male gender | 1.02 ± 0.45 | 2.77 | 1.15–6.66 | 0.022 |
| hs-Troponin I | 0.63 ± 0.29 | 1.88 | 1.41–2.51 | 0.021 |
| LVEF < 60% | −0.28 ± 0.45 | 0.75 | 0.31–1.84 | 0.534 |
| NT-proBNP (AIC = 213, BIC = 219, p < 0.001, MER = 0.66, MEV = 0.54) | ||||
| NT-proBNP > 2141 pg/mL | 1.74 ± 0.52 | 5.67 | 2.05–15.61 | 0.0008 |
| Age > 65 years | 0.53 ± 0.54 | 1.70 | 0.59–4.88 | 0.322 |
| Male gender | 0.36 ± 0.45 | 1.43 | 0.59–3.44 | 0.427 |
| hs-Troponin I | 0.29 ± 0.22 | 1.34 | 1.07–1.66 | 0.208 |
| LVEF < 60% | −0.31 ± 0.46 | 0,74 | 0.30–1.82 | 0.507 |
| NT-proBNP + Ptx-3 combination (AIC = 209, BIC = 214, p = 0.001, MER = 0.75, MEV = 0.64) | ||||
| NT-proBNP > 2141 pg/mL | 1.67 ± 0.51 | 5.32 | 1.95–14.46 | 0.001 |
| Ptx-3 >169 ng/mL | 1.19 ± 0.44 | 3.28 | 1.39–7.73 | 0.007 |
| Age > 65 years | 0.51 ± 0.51 | 1.67 | 0.60–4.62 | 0.326 |
| Male gender | 0.12 ± 0.21 | 1.13 | 0.91–1.39 | 0.591 |
| hs-Troponin I | 0.44 ± 0.22 | 1.54 | 1.23–1.92 | 0.065 |
| LVEF < 60% | 0.08 ± 0.12 | 1.08 | 0.96–1.22 | 0.692 |
| NT-proBNP + ST2 combination (AIC = 212, BIC = 217, p < 0.001, MER = 0.68, MEV = 0.57) | ||||
| NT-proBNP > 2141 pg/mL | 1.79 ± 0.49 | 5.98 | 2.29–15.60 | 0.0003 |
| ST2 > 27.2 ng/mL | 1.25 ± 0.58 | 3.48 | 1.10–10.99 | 0.03 |
| age > 65 years | 0.81 ± 0.51 | 2.25 | 0.83–6.10 | 0.111 |
| Male gender | 0.18 ± 0.22 | 1.20 | 0.96–1.49 | 0.281 |
| hs-Troponin I | 0.44 ± 0.23 | 1.54 | 1.23–1.96 | 0.058 |
| LVEF < 60% | 0.08 ± 0.12 | 1.08 | 0.96–1.22 | 0.696 |
| ST2 + Ptx-3 combination (AIC = 217, BIC = 222, p < 0.001, MER = 0.52, MEV = 0.40) | ||||
| ST2 > 27.2 ng/mL | 1.05 ± 0.59 | 2.88 | 0.91–9.08 | 0.071 |
| Ptx-3 > 169 ng/mL | 1.32 ± 0.44 | 3.74 | 1.58–8.86 | 0.003 |
| Age > 65 years | 1.26 ± 0.463 | 3.53 | 1.43–8.75 | 0.006 |
| Male gender | 0.14 ± 0.22 | 1.15 | 0.92–1.43 | 0.428 |
| hs-Troponin I | 0.44 ± 0.21 | 1.54 | 1.25–1.88 | 0.071 |
| LVEF < 60% | 0.09 ± 0.11 | 1.09 | 0.98–1.23 | 0.641 |
| NT-proBNP + ST2 + Ptx-3 combination (AIC = 208, BIC = 214, p < 0.001, MER = 0.77, MEV = 0.66) | ||||
| NT-proBNP > 2141 pg/mL | 1.60 ± 0.49 | 4.95 | 1.87–13.17 | 0.001 |
| ST2 > 27.2 ng/mL | 0.99 ± 0.59 | 2.70 | 0.84–8.69 | 0.095 |
| Ptx-3 > 169 ng/mL | 1.08 ± 0.44 | 2.94 | 1.24–6.99 | 0.055 |
| Age > 65 years | 0.73 ± 0.52 | 2.08 | 0.76–5.73 | 0.155 |
| Male gender | 0.11 ± 0.21 | 1.12 | 0.90–1.38 | 0.612 |
| hs-Troponin I | 0.43 ± 0.22 | 1.537 | 1.23–1.93 | 0.073 |
| LVEF < 60% | 0.07 ± 0.12 | 1.07 | 0.95–1.21 | 0.702 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagidullin, N.; Motloch, L.J.; Gareeva, D.; Hamitova, A.; Lakman, I.; Krioni, I.; Popov, D.; Zulkarneev, R.; Paar, V.; Kopp, K.; et al. Combining Novel Biomarkers for Risk Stratification of Two-Year Cardiovascular Mortality in Patients with ST-Elevation Myocardial Infarction. J. Clin. Med. 2020, 9, 550. https://doi.org/10.3390/jcm9020550
Zagidullin N, Motloch LJ, Gareeva D, Hamitova A, Lakman I, Krioni I, Popov D, Zulkarneev R, Paar V, Kopp K, et al. Combining Novel Biomarkers for Risk Stratification of Two-Year Cardiovascular Mortality in Patients with ST-Elevation Myocardial Infarction. Journal of Clinical Medicine. 2020; 9(2):550. https://doi.org/10.3390/jcm9020550
Chicago/Turabian StyleZagidullin, Naufal, Lukas J. Motloch, Diana Gareeva, Aysilu Hamitova, Irina Lakman, Ilja Krioni, Denis Popov, Rustem Zulkarneev, Vera Paar, Kristen Kopp, and et al. 2020. "Combining Novel Biomarkers for Risk Stratification of Two-Year Cardiovascular Mortality in Patients with ST-Elevation Myocardial Infarction" Journal of Clinical Medicine 9, no. 2: 550. https://doi.org/10.3390/jcm9020550





