Breast Mammographic Density: Stromal Implications on Breast Cancer Detection and Therapy
Abstract
1. Introduction
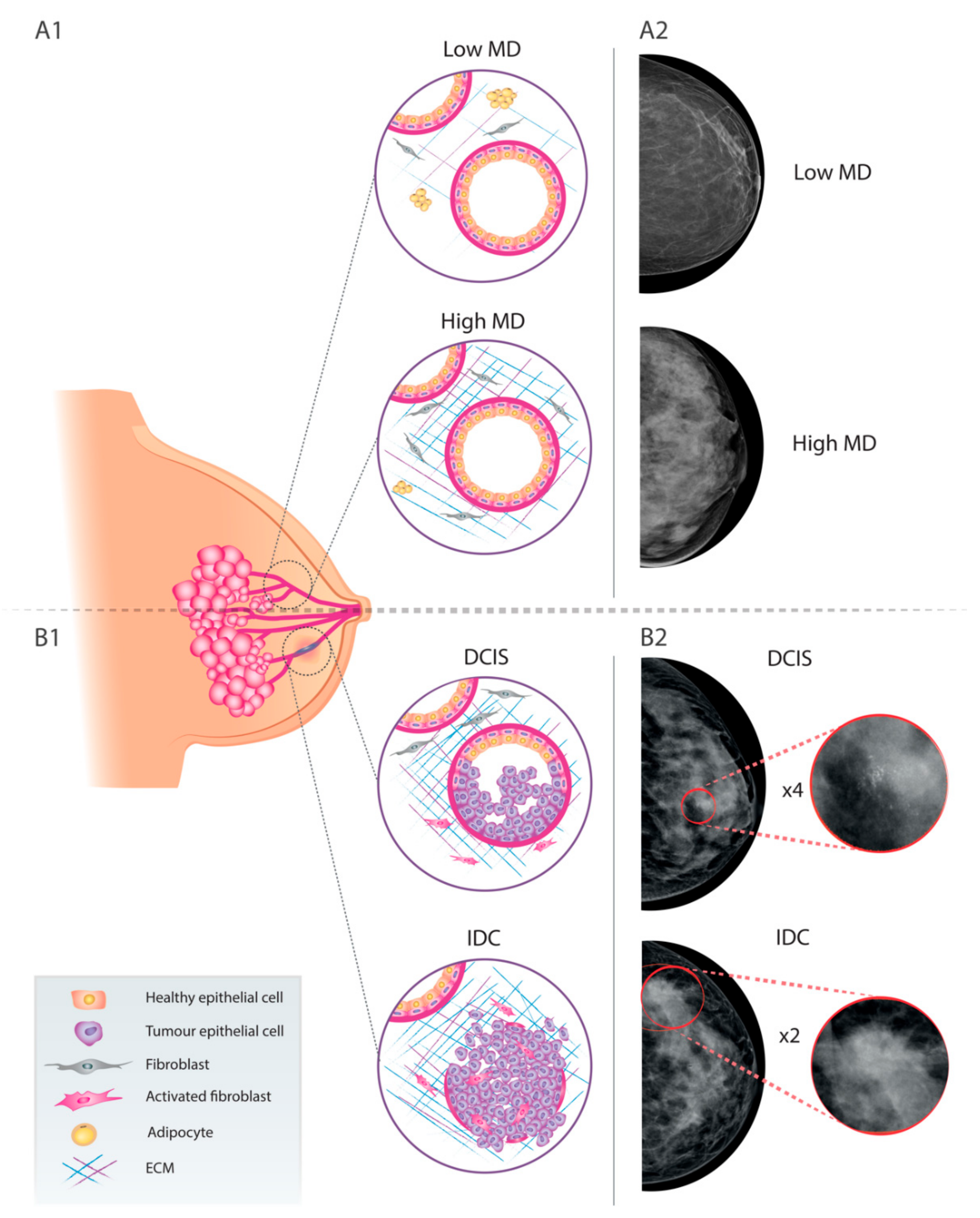
2. Breast Cancer and Mammographic Density
3. Hormonal Therapy and Mammographic Density
4. Relevance of MD in the DCIS-to-ICD Transition
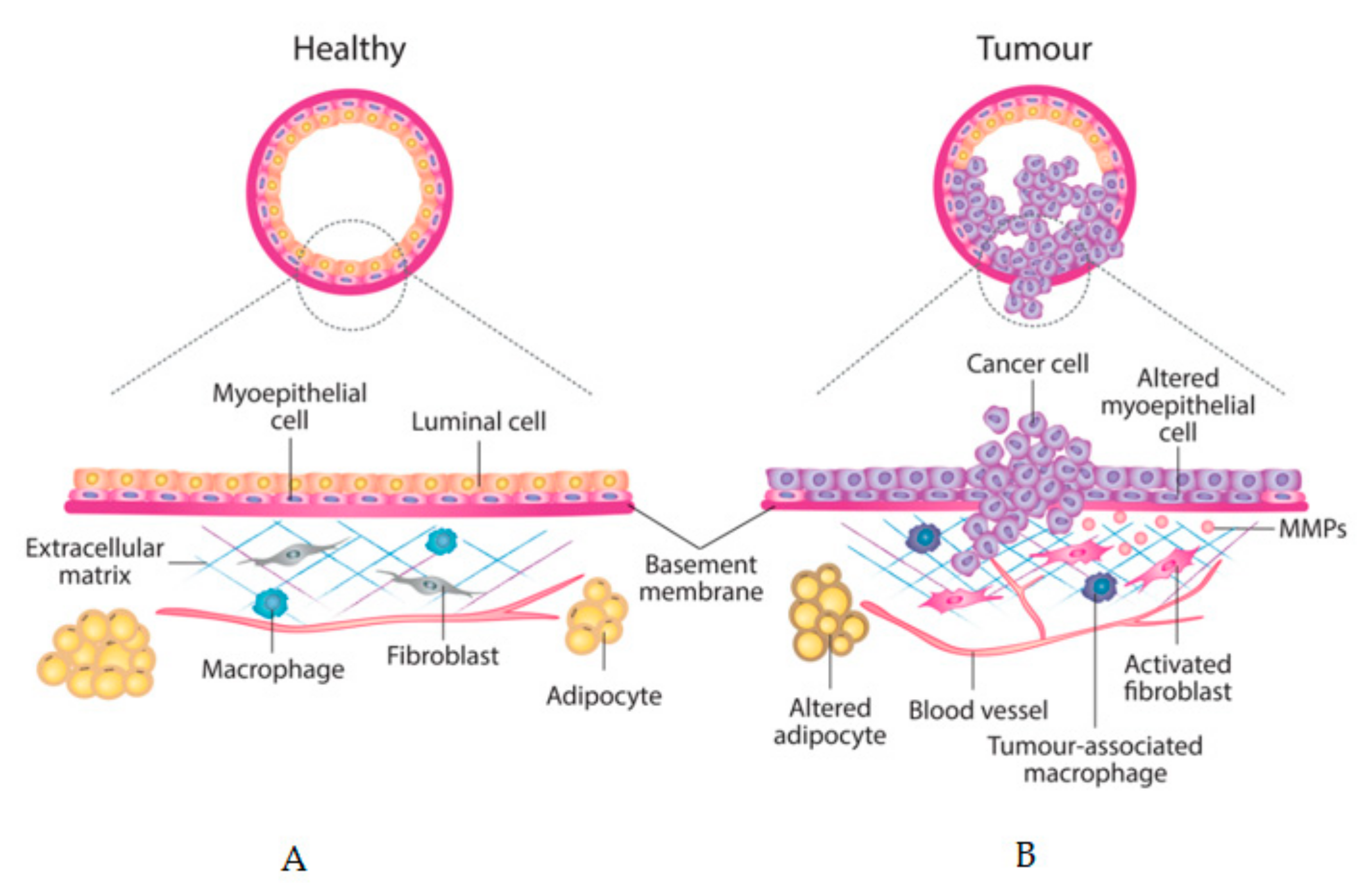
5. Tumour Stroma as a Prognostic Factor
6. CAFs, MD, Cancer Progression and Chemoresistance
7. Future Perspectives: CAFs as Therapeutic Targets and Improved Mammographic Monitoring
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.W.; Morrish, O.W.E.; Allgood, P.C.; Black, R.; Gillan, M.G.C.; Willsher, P.; Cooke, J.; Duncan, K.A.; Michell, M.J.; Dobson, H.M.; et al. Mammographic density and breast cancer risk in breast screening assessment cases and women with a family history of breast cancer. Eur. J. Cancer 2018, 88, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Pellacani, D.; Tan, S.; Lefort, S.; Eaves, C.J. Transcriptional regulation of normal human mammary cell heterogeneity and its perturbation in breast cancer. EMBO J. 2019, 38, e100330. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Hines, W.C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011, 17, 320–329. [Google Scholar] [CrossRef]
- Saftlas, A.F.; Szklo, M. Mammographic parenchymal patterns and breast cancer risk. Epidemiol. Rev. 1987, 9, 146–174. [Google Scholar] [CrossRef]
- Martin, L.J.; Boyd, N.F. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: Hypotheses based on epidemiological evidence. Breast Cancer Res. 2008, 10, 201. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; Del Rio Hernandez, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef]
- Kerlikowske, K.; Zhu, W.; Hubbard, R.A.; Geller, B.; Dittus, K.; Braithwaite, D.; Wernli, K.J.; Miglioretti, D.L.; O’Meara, E.S. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern. Med. 2013, 173, 807–816. [Google Scholar] [CrossRef]
- Euler-Chelpin, M.V.; Lillholm, M.; Napolitano, G.; Vejborg, I.; Nielsen, M.; Lynge, E. Screening mammography: Benefit of double reading by breast density. Breast Cancer Res. Treat. 2019, 171, 767–776. [Google Scholar] [CrossRef]
- Monticciolo, D.L.; Newell, M.S.; Moy, L.; Niell, B.; Monsees, B.; Sickles, E.A. Breast Cancer Screening in Women at Higher-Than-Average Risk: Recommendations From the ACR. J. Am. Coll. Radiol. 2018, 15, 408–414. [Google Scholar] [CrossRef]
- Bakker, M.F.; de Lange, S.V.; Pijnappel, R.M.; Mann, R.M.; Peeters, P.H.M.; Monninkhof, E.M.; Emaus, M.J.; Loo, C.E.; Bisschops, R.H.C.; Lobbes, M.B.I.; et al. Supplemental MRI Screening for Women with Extremely Dense Breast Tissue. N. Engl. J. Med. 2019, 381, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.K.; Strobel, K.; Bieling, H.; Leutner, C.; Schild, H.H.; Schrading, S. Supplemental Breast MR Imaging Screening of Women with Average Risk of Breast Cancer. Radiology 2017, 283, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Green, V.L. Mammographic Breast Density and Breast Cancer Risk: Implications of the Breast Density Legislation for Health Care Practitioners. Clin. Obstet. Gynecol. 2016, 59, 419–438. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.; Lange, T.; Huynh, S.; Aro, A.R.; von Euler-Chelpin, M.; Vejborg, I.; Tjonneland, A.; Lynge, E.; Andersen, Z.J. Hormone replacement therapy, mammographic density, and breast cancer risk: A cohort study. Cancer Causes Control 2018, 29, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Hjerkind, K.V.; Ellingjord-Dale, M.; Johansson, A.L.V.; Aase, H.S.; Hoff, S.R.; Hofvind, S.; Fagerheim, S.; Dos-Santos-Silva, I.; Ursin, G. Volumetric Mammographic Density, Age-Related Decline, and Breast Cancer Risk Factors in a National Breast Cancer Screening Program. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Kerlikowske, K.; Zhu, W.; Tosteson, A.N.; Sprague, B.L.; Tice, J.A.; Lehman, C.D.; Miglioretti, D.L. Identifying women with dense breasts at high risk for interval cancer: A cohort study. Ann. Intern. Med. 2015, 162, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.S.; Mukherjee, P. An overview of mammographic density and its association with breast cancer. Breast Cancer 2018, 25, 259–267. [Google Scholar] [CrossRef]
- Bissell, M.J.; Radisky, D. Putting tumours in context. Nat. Rev. Cancer 2001, 1, 46–54. [Google Scholar] [CrossRef]
- Pike, M.C.; Krailo, M.D.; Henderson, B.E.; Casagrande, J.T.; Hoel, D.G. ‘Hormonal’ risk factors, ‘breast tissue age’ and the age-incidence of breast cancer. Nature 1983, 303, 767–770. [Google Scholar] [CrossRef]
- Greendale, G.A.; Reboussin, B.A.; Slone, S.; Wasilauskas, C.; Pike, M.C.; Ursin, G. Postmenopausal hormone therapy and change in mammographic density. J. Natl. Cancer Inst. 2003, 95, 30–37. [Google Scholar] [CrossRef]
- Bertrand, K.A.; Eliassen, A.H.; Hankinson, S.E.; Rosner, B.A.; Tamimi, R.M. Circulating Hormones and Mammographic Density in Premenopausal Women. Horm. Cancer 2018, 9, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Shawky, M.S.; Martin, H.; Hugo, H.J.; Lloyd, T.; Britt, K.L.; Redfern, A.; Thompson, E.W. Mammographic density: A potential monitoring biomarker for adjuvant and preventative breast cancer endocrine therapies. Oncotarget 2017, 8, 5578–5591. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; He, W.; Eriksson, M.; Humphreys, K.; Bergh, J.; Hall, P.; Czene, K. Adjuvant Therapy and Mammographic Density Changes in Women With Breast Cancer. JNCI Cancer Spectr. 2018, 2, pky071. [Google Scholar] [CrossRef] [PubMed]
- Mullooly, M.; Gierach, G.L. The Potential for Mammographic Breast Density Change as a Biosensor of Adjuvant Tamoxifen Therapy Adherence and Response. JNCI Cancer Spectr. 2018, 2, pky072. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Humphreys, K.; Eriksson, L.; Edgren, G.; Czene, K.; Hall, P. Mammographic density reduction is a prognostic marker of response to adjuvant tamoxifen therapy in postmenopausal patients with breast cancer. J. Clin. Oncol. 2013, 31, 2249–2256. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Warwick, J.; Pinney, E.; Duffy, S.W.; Cawthorn, S.; Howell, A.; Forbes, J.F.; Warren, R.M. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: A nested case-control study. J. Natl. Cancer Inst. 2011, 103, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Cho, N.; Kim, Y.S.; Yi, A. Mammographic density changes following discontinuation of tamoxifen in premenopausal women with oestrogen receptor-positive breast cancer. Eur. Radiol. 2018, 28, 3176–3184. [Google Scholar] [CrossRef]
- Nyante, S.J.; Sherman, M.E.; Pfeiffer, R.M.; Berrington de Gonzalez, A.; Brinton, L.A.; Bowles, E.J.; Hoover, R.N.; Glass, A.; Gierach, G.L. Longitudinal Change in Mammographic Density among ER-Positive Breast Cancer Patients Using Tamoxifen. Cancer Epidemiol. Biomark. Prev. 2016, 25, 212–216. [Google Scholar] [CrossRef]
- Yeong, J.; Thike, A.A.; Tan, P.H.; Iqbal, J. Identifying progression predictors of breast ductal carcinoma in situ. J. Clin. Pathol. 2017, 70, 102–108. [Google Scholar] [CrossRef]
- Polyak, K. Is breast tumor progression really linear? Clin. Cancer Res. 2008, 14, 339–341. [Google Scholar] [CrossRef][Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Groen, E.J.; Elshof, L.E.; Visser, L.L.; Rutgers, E.J.T.; Winter-Warnars, H.A.O.; Lips, E.H.; Wesseling, J. Finding the balance between over- and under-treatment of ductal carcinoma in situ (DCIS). Breast 2017, 31, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Levinsohn, E.; Altman, M.; Chagpar, A.B. Controversies Regarding the Diagnosis and Management of Ductal Carcinoma In Situ. Am. Surg. 2018, 84, 1–6. [Google Scholar] [PubMed]
- Hanna, W.M.; Parra-Herran, C.; Lu, F.I.; Slodkowska, E.; Rakovitch, E.; Nofech-Mozes, S. Ductal carcinoma in situ of the breast: An update for the pathologist in the era of individualized risk assessment and tailored therapies. Mod. Pathol. 2019, 32, 896–915. [Google Scholar] [CrossRef]
- Conklin, M.W.; Keely, P.J. Why the stroma matters in breast cancer: Insights into breast cancer patient outcomes through the examination of stromal biomarkers. Cell Adhes. Migr. 2012, 6, 249–260. [Google Scholar] [CrossRef]
- Ma, X.J.; Dahiya, S.; Richardson, E.; Erlander, M.; Sgroi, D.C. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2009, 11, R7. [Google Scholar] [CrossRef]
- Gill, J.K.; Maskarinec, G.; Pagano, I.; Kolonel, L.N. The association of mammographic density with ductal carcinoma in situ of the breast: The Multiethnic Cohort. Breast Cancer Res. 2006, 8, R30. [Google Scholar] [CrossRef]
- Ursin, G.; Hovanessian-Larsen, L.; Parisky, Y.R.; Pike, M.C.; Wu, A.H. Greatly increased occurrence of breast cancers in areas of mammographically dense tissue. Breast Cancer Res. 2005, 7, R605. [Google Scholar] [CrossRef]
- Habel, L.A.; Capra, A.M.; Achacoso, N.S.; Janga, A.; Acton, L.; Puligandla, B.; Quesenberry, C.P., Jr. Mammographic density and risk of second breast cancer after ductal carcinoma in situ. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2488–2495. [Google Scholar] [CrossRef]
- Boyd, N.F.; Martin, L.J.; Yaffe, M.J.; Minkin, S. Mammographic density and breast cancer risk: Current understanding and future prospects. Breast Cancer Res. 2011, 13, 223. [Google Scholar] [CrossRef]
- Navaratna, R.; Gastounioti, A.; Hsieh, M.-K.; Pantalone, L.; Shelanski, M.; Conant, E.F.; Kontos, D. Associations between mammographic phenotypes and histopathologic features in ductal carcinoma in situ. Proc. SPIE Med Imaging 2019, 10950, 1–6. [Google Scholar]
- Huo, C.W.; Hill, P.; Chew, G.; Neeson, P.J.; Halse, H.; Williams, E.D.; Henderson, M.A.; Thompson, E.W.; Britt, K.L. High mammographic density in women is associated with protumor inflammation. Breast Cancer Res. 2018, 20, 92. [Google Scholar] [CrossRef] [PubMed]
- Werb, Z.; Lu, P. The Role of Stroma in Tumor Development. Cancer J. 2015, 21, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Arendt, L.M.; Rudnick, J.A.; Keller, P.J.; Kuperwasser, C. Stroma in breast development and disease. Semin. Cell Dev. Biol. 2010, 21, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Orimo, A.; Weinberg, R.A. Stromal fibroblasts in cancer: A novel tumor-promoting cell type. Cell Cycle 2006, 5, 1597–1601. [Google Scholar] [CrossRef] [PubMed]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef]
- Pietras, K.; Ostman, A. Hallmarks of cancer: Interactions with the tumor stroma. Exp. Cell Res. 2010, 316, 1324–1331. [Google Scholar] [CrossRef]
- Yoshida, G.J.; Azuma, A.; Miura, Y.; Orimo, A. Activated Fibroblast Program Orchestrates Tumor Initiation and Progression; Molecular Mechanisms and the Associated Therapeutic Strategies. Int. J. Mol. Sci. 2019, 20, 2256. [Google Scholar] [CrossRef]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Knittel, J.G.; Yan, L.; Rueden, C.T.; White, J.G.; Keely, P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008, 6, 11. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, S.X.; Brunner, A.L.; van de Rijn, M.; West, R.B. Next generation sequencing-based expression profiling identifies signatures from benign stromal proliferations that define stromal components of breast cancer. Breast Cancer Res. 2013, 15, R117. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Gierach, G.L.; Sandhu, R.; Williams, T.; Midkiff, B.R.; Lissowska, J.; Wesolowska, E.; Boyd, N.F.; Johnson, N.B.; Figueroa, J.D.; et al. Relationship of mammographic density and gene expression: Analysis of normal breast tissue surrounding breast cancer. Clin. Cancer Res. 2013, 19, 4972–4982. [Google Scholar] [CrossRef] [PubMed]
- Huo, C.W.; Chew, G.; Hill, P.; Huang, D.; Ingman, W.; Hodson, L.; Brown, K.A.; Magenau, A.; Allam, A.H.; McGhee, E.; et al. High mammographic density is associated with an increase in stromal collagen and immune cells within the mammary epithelium. Breast Cancer Res. 2015, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, M.R.; Avagliano, A.; Granato, G.; Imparato, V.; Masone, S.; Masullo, M.; Nasso, R.; Montagnani, S.; Arcucci, A. Involvement of Breast Cancer-Associated Fibroblasts in Tumor Development, Therapy Resistance and Evaluation of Potential Therapeutic Strategies. Curr. Med. Chem. 2018, 25, 3414–3434. [Google Scholar] [CrossRef]
- Ironside, A.J.; Jones, J.L. Stromal characteristics may hold the key to mammographic density: The evidence to date. Oncotarget 2016, 7, 31550–31562. [Google Scholar] [CrossRef][Green Version]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.L.; Gabbiani, G. The myofibroblast: One function, multiple origins. Am. J. Pathol. 2007, 170, 1807–1816. [Google Scholar] [CrossRef]
- Pein, M.; Oskarsson, T. Microenvironment in metastasis: Roadblocks and supportive niches. Am. J. Physiol. Cell Physiol. 2015, 309, C627–C638. [Google Scholar] [CrossRef]
- DeFilippis, R.A.; Chang, H.; Dumont, N.; Rabban, J.T.; Chen, Y.Y.; Fontenay, G.V.; Berman, H.K.; Gauthier, M.L.; Zhao, J.; Hu, D.; et al. CD36 repression activates a multicellular stromal program shared by high mammographic density and tumor tissues. Cancer Discov. 2012, 2, 826–839. [Google Scholar] [CrossRef]
- Nienhuis, H.H.; Gaykema, S.B.; Timmer-Bosscha, H.; Jalving, M.; Brouwers, A.H.; Lub-de Hooge, M.N.; van der Vegt, B.; Overmoyer, B.; de Vries, E.G.; Schroder, C.P. Targeting breast cancer through its microenvironment: Current status of preclinical and clinical research in finding relevant targets. Pharmacol. Ther. 2015, 147, 63–79. [Google Scholar] [CrossRef]
- Lafkas, D.; Trimis, G.; Papavassiliou, A.G.; Kiaris, H. P53 mutations in stromal fibroblasts sensitize tumors against chemotherapy. Int. J. Cancer 2008, 123, 967–971. [Google Scholar] [CrossRef]
- Qiao, A.; Gu, F.; Guo, X.; Zhang, X.; Fu, L. Breast cancer-associated fibroblasts: Their roles in tumor initiation, progression and clinical applications. Front. Med. 2016, 10, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Slany, A.; Bileck, A.; Muqaku, B.; Gerner, C. Targeting breast cancer-associated fibroblasts to improve anti-cancer therapy. Breast 2015, 24, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Amornsupak, K.; Insawang, T.; Thuwajit, P.; Pornchai, O.; Eccles, S.A.; Thuwajit, C. Cancer-associated fibroblasts induce high mobility group box 1 and contribute to resistance to doxorubicin in breast cancer cells. BMC Cancer 2014, 14, 955. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.L.; Madden, J.M.; Zoratti, G.L.; Kuperwasser, C.; List, K.; Boerner, J.L. Fibroblast-secreted hepatocyte growth factor mediates epidermal growth factor receptor tyrosine kinase inhibitor resistance in triple-negative breast cancers through paracrine activation of Met. Breast Cancer Res. 2012, 14, R104. [Google Scholar] [CrossRef] [PubMed]
- Crawford, Y.; Ferrara, N. Tumor and stromal pathways mediating refractoriness/resistance to anti-angiogenic therapies. Trends Pharmacol. Sci. 2009, 30, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Nogueira, P.; Mancino, M.; Fuster, G.; Lopez-Plana, A.; Jauregui, P.; Almendro, V.; Enreig, E.; Menendez, S.; Rojo, F.; Noguera-Castells, A.; et al. Tumor Associated Fibroblasts Promote HER2-Targeted Therapy Resistance through FGFR2 Activation. Clin. Cancer Res. 2019. Online ahead of print. [Google Scholar] [CrossRef]
- Marusyk, A.; Tabassum, D.P.; Janiszewska, M.; Place, A.E.; Trinh, A.; Rozhok, A.I.; Pyne, S.; Guerriero, J.L.; Shu, S.; Ekram, M.; et al. Spatial Proximity to Fibroblasts Impacts Molecular Features and Therapeutic Sensitivity of Breast Cancer Cells Influencing Clinical Outcomes. Cancer Res. 2016, 76, 6495–6506. [Google Scholar] [CrossRef]
- Saito, S.; Morishima, K.; Ui, T.; Hoshino, H.; Matsubara, D.; Ishikawa, S.; Aburatani, H.; Fukayama, M.; Hosoya, Y.; Sata, N.; et al. The role of HGF/MET and FGF/FGFR in fibroblast-derived growth stimulation and lapatinib-resistance of esophageal squamous cell carcinoma. BMC Cancer 2015, 15, 82. [Google Scholar] [CrossRef]
- Brechbuhl, H.M.; Finlay-Schultz, J.; Yamamoto, T.M.; Gillen, A.E.; Cittelly, D.M.; Tan, A.C.; Sams, S.B.; Pillai, M.M.; Elias, A.D.; Robinson, W.A.; et al. Fibroblast Subtypes Regulate Responsiveness of Luminal Breast Cancer to Estrogen. Clin. Cancer Res. 2017, 23, 1710–1721. [Google Scholar] [CrossRef]
- Catalano, S.; Giordano, C.; Panza, S.; Chemi, F.; Bonofiglio, D.; Lanzino, M.; Rizza, P.; Romeo, F.; Fuqua, S.A.; Maggiolini, M.; et al. Tamoxifen through GPER upregulates aromatase expression: A novel mechanism sustaining tamoxifen-resistant breast cancer cell growth. Breast Cancer Res. Treat. 2014, 146, 273–285. [Google Scholar] [CrossRef]
- Tchou, J.; Conejo-Garcia, J. Targeting the tumor stroma as a novel treatment strategy for breast cancer: Shifting from the neoplastic cell-centric to a stroma-centric paradigm. Adv. Pharmacol. 2012, 65, 45–61. [Google Scholar] [PubMed]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Takeyama, H. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers 2015, 7, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Gonda, T.A.; Varro, A.; Wang, T.C.; Tycko, B. Molecular biology of cancer-associated fibroblasts: Can these cells be targeted in anti-cancer therapy? Semin. Cell Dev. Biol. 2010, 21, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Calone, I.; Souchelnytskyi, S. Inhibition of TGFbeta signaling and its implications in anticancer treatments. Exp. Oncol. 2012, 34, 9–16. [Google Scholar] [PubMed]
- Liu, R.; Li, H.; Liu, L.; Yu, J.; Ren, X. Fibroblast activation protein: A potential therapeutic target in cancer. Cancer Biol. Ther. 2012, 13, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, E.; Garin-Chesa, P.; Heider, K.H.; Kalat, M.; Lamche, H.; Puri, C.; Kerjaschki, D.; Rettig, W.J.; Adolf, G.R. Effective immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts. Clin. Cancer Res. 2008, 14, 4584–4592. [Google Scholar] [CrossRef]
- Howell, A.; Landberg, G.; Bergh, J. Breast tumour stroma is a prognostic indicator and target for therapy. Breast Cancer Res. 2009, 11, S16. [Google Scholar] [CrossRef]
- Del Valle, P.R.; Milani, C.; Brentani, M.M.; Katayama, M.L.; de Lyra, E.C.; Carraro, D.M.; Brentani, H.; Puga, R.; Lima, L.A.; Rozenchan, P.B.; et al. Transcriptional profile of fibroblasts obtained from the primary site, lymph node and bone marrow of breast cancer patients. Genet. Mol. Biol. 2014, 37, 480–489. [Google Scholar] [CrossRef]
- Gonzalez, L.; Eiro, N.; Fernandez-Garcia, B.; Gonzalez, L.O.; Dominguez, F.; Vizoso, F.J. Gene expression profile of normal and cancer-associated fibroblasts according to intratumoral inflammatory cells phenotype from breast cancer tissue. Mol. Carcinog. 2016, 55, 1489–1502. [Google Scholar] [CrossRef]
- Herrera, M.; Islam, A.B.; Herrera, A.; Martin, P.; Garcia, V.; Silva, J.; Garcia, J.M.; Salas, C.; Casal, I.; de Herreros, A.G.; et al. Functional heterogeneity of cancer-associated fibroblasts from human colon tumors shows specific prognostic gene expression signature. Clin. Cancer Res. 2013, 19, 5914–5926. [Google Scholar] [CrossRef]
- Huliak, I.; Bodai, L.; Czepan, M.; Kovacs, D.; Szabo, A.; Tiszlavicz, L.; Lazar, G.; Rakonczay, Z., Jr.; Hegyi, P.; Boros, I.M.; et al. Genetic, epigenetic and transcriptional comparison of esophagus tumorassociated and adjacent normal myofibroblasts. Oncol. Rep. 2019, 41, 839–852. [Google Scholar] [PubMed]
- O’Day, S.; Pavlick, A.; Loquai, C.; Lawson, D.; Gutzmer, R.; Richards, J.; Schadendorf, D.; Thompson, J.A.; Gonzalez, R.; Trefzer, U.; et al. A randomised, phase II study of intetumumab, an anti-alphav-integrin mAb, alone and with dacarbazine in stage IV melanoma. Br. J. Cancer 2011, 105, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Hersey, P.; Sosman, J.; O’Day, S.; Richards, J.; Bedikian, A.; Gonzalez, R.; Sharfman, W.; Weber, R.; Logan, T.; Buzoianu, M.; et al. A randomized phase 2 study of etaracizumab, a monoclonal antibody against integrin alpha(v)beta(3), + or—Dacarbazine in patients with stage IV metastatic melanoma. Cancer 2010, 116, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Wiseman, G.; Welt, S.; Adjei, A.; Lee, F.T.; Hopkins, W.; Divgi, C.R.; Hanson, L.H.; Mitchell, P.; Gansen, D.N.; et al. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin. Cancer Res. 2003, 9, 1639–1647. [Google Scholar] [PubMed]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef]
- Trust, O.U.H.N. A Study to Correlate Ultrasound Elastography with Histopathology to Monitor the Response of Locally Advanced Breast Cancer to Neoadjuvant Chemotherapy; Clinical Trials.gov: Bethesda, MD, USA, 2012. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Nogueira, P.; Mancino, M.; Fuster, G.; Bragado, P.; Prats de Puig, M.; Gascón, P.; Casado, F.J.; Carbó, N. Breast Mammographic Density: Stromal Implications on Breast Cancer Detection and Therapy. J. Clin. Med. 2020, 9, 776. https://doi.org/10.3390/jcm9030776
Fernández-Nogueira P, Mancino M, Fuster G, Bragado P, Prats de Puig M, Gascón P, Casado FJ, Carbó N. Breast Mammographic Density: Stromal Implications on Breast Cancer Detection and Therapy. Journal of Clinical Medicine. 2020; 9(3):776. https://doi.org/10.3390/jcm9030776
Chicago/Turabian StyleFernández-Nogueira, Patricia, Mario Mancino, Gemma Fuster, Paloma Bragado, Miquel Prats de Puig, Pere Gascón, Francisco Javier Casado, and Neus Carbó. 2020. "Breast Mammographic Density: Stromal Implications on Breast Cancer Detection and Therapy" Journal of Clinical Medicine 9, no. 3: 776. https://doi.org/10.3390/jcm9030776
APA StyleFernández-Nogueira, P., Mancino, M., Fuster, G., Bragado, P., Prats de Puig, M., Gascón, P., Casado, F. J., & Carbó, N. (2020). Breast Mammographic Density: Stromal Implications on Breast Cancer Detection and Therapy. Journal of Clinical Medicine, 9(3), 776. https://doi.org/10.3390/jcm9030776




