Regulatory T Cells Modulate CD4 Proliferation after Severe Trauma via IL-10
Abstract
1. Introduction
2. Experimental Section
2.1. Ethics
2.2. Study Setting and Population
2.3. Study Protocol
2.4. Blood Sampling
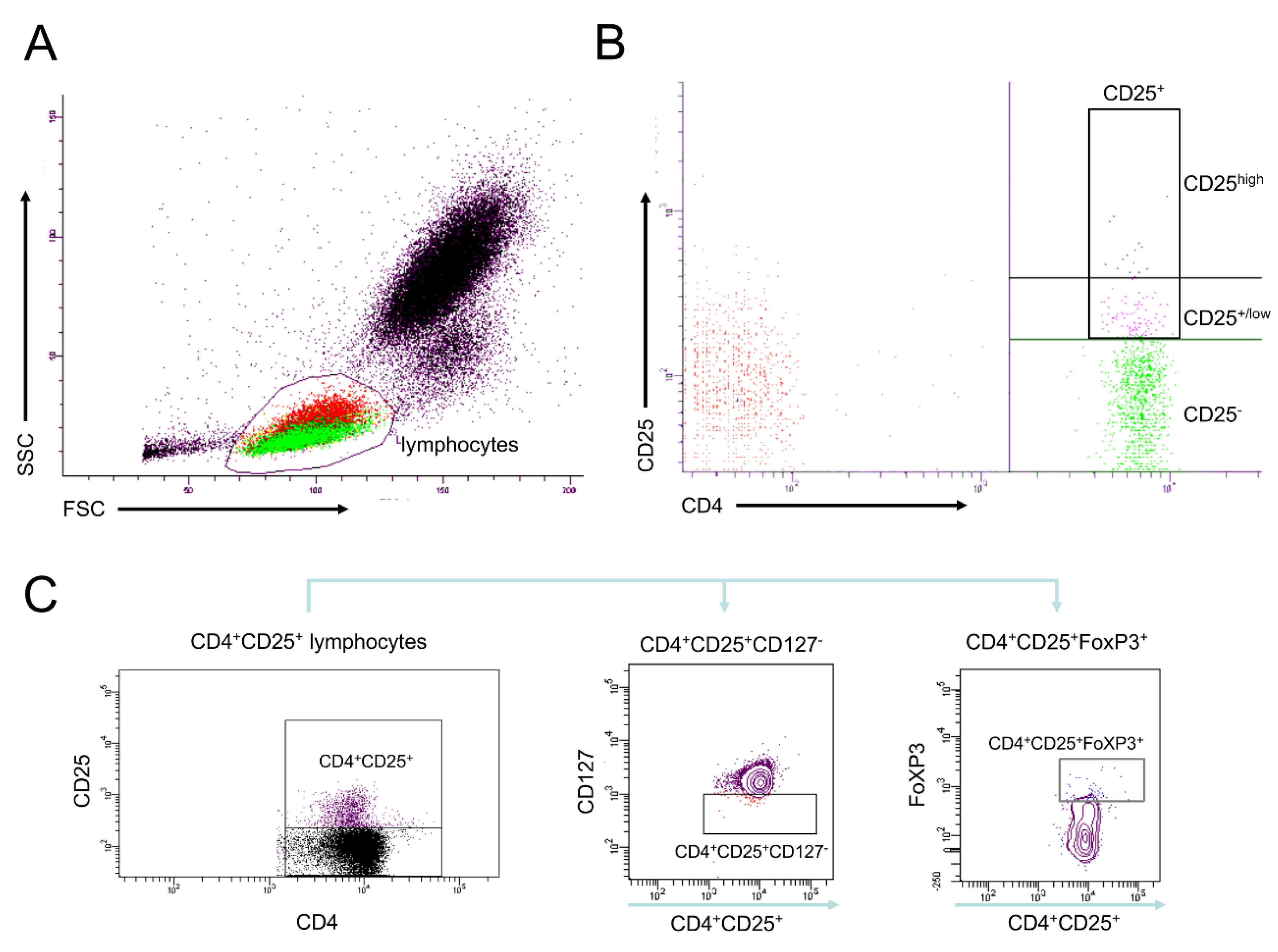
2.5. Analyses of Cell Surface Receptors
2.6. Isolation of CD4+ Cells Including Tregs
2.7. Isolation of CD4+ Cells without Tregs
2.8. Proliferation of Lymphocytes in a CD4+ Culture with and without Tregs
2.9. Statistical Analysis
3. Results
3.1. Main Findings
3.1.1. Study Population
3.1.2. Frequencies of Differentially Defined Tregs after Trauma
3.1.3. Proliferation of CD4+ Cells Depending on Tregs
3.1.4. Tregs-Mediated Proliferation of CD4+ Cells Depends on IL-10
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peden, M.; Hyder, A. Road traffic injuries are a global public health problem. BMJ 2002, 324, 1153. [Google Scholar] [CrossRef]
- World Health Organization. Injuries and Violence: The Facts 2014; World Health Organization: Geneva, Switzerland, 2014; Available online: https://apps.who.int/iris/handle/10665/149798. (accessed on 7 March 2020).
- Acosta, J.A.; Yang, J.C.; Winchell, R.J.; Simons, R.K.; Fortlage, D.A.; Hollingsworth-Fridlund, P.; Hoyt, D.B. Lethal injuries and time to death in a level I trauma center. J. Am. Coll. Surg. 1998, 186, 528–533. [Google Scholar] [CrossRef]
- Pfeifer, R.; Tarkin, I.S.; Rocos, B.; Pape, H.C. Patterns of mortality and causes of death in polytrauma patients--has anything changed? Injury 2009, 40, 907–911. [Google Scholar] [CrossRef]
- Probst, C.; Pape, H.C.; Hildebrand, F.; Regel, G.; Mahlke, L.; Giannoudis, P.; Krettek, C.; Grotz, M.R. 30 years of polytrauma care: An analysis of the change in strategies and results of 4849 cases treated at a single institution. Injury 2009, 40, 77–83. [Google Scholar] [CrossRef]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Coats, T.J.; Duranteau, J.; Fernandez-Mondejar, E.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Nardi, G.; et al. Management of bleeding and coagulopathy following major trauma: An updated European guideline. Crit. Care 2013, 17, R76. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Mallina, R.; Harwood, P.; Perry, S.; Sante, E.D.; Pape, H.C. Pattern of release and relationship between HMGB-1 and IL-6 following blunt trauma. Injury 2010, 41, 1323–1327. [Google Scholar] [CrossRef]
- Seong, S.Y.; Matzinger, P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004, 4, 469–478. [Google Scholar] [CrossRef]
- Wutzler, S.; Lustenberger, T.; Relja, B.; Lehnert, M.; Marzi, I. Pathophysiology of multiple trauma: Intensive care medicine and timing of treatment. Chirurg 2013, 84, 753–758. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef]
- Lenz, A.; Franklin, G.A.; Cheadle, W.G. Systemic inflammation after trauma. Injury 2007, 38, 1336–1345. [Google Scholar] [CrossRef]
- Relja, B.; Land, W.G. Damage-associated molecular patterns in trauma. Eur. J. Trauma Emerg. Surg. 2019, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Relja, B.; Mors, K.; Marzi, I. Danger signals in trauma. Eur. J. Trauma Emerg. Surg. 2018, 44, 301–336. [Google Scholar] [CrossRef] [PubMed]
- Kasten, K.R.; Goetzman, H.S.; Reid, M.R.; Rasper, A.M.; Adediran, S.G.; Robinson, C.T.; Cave, C.M.; Solomkin, J.S.; Lentsch, A.B.; Johannigman, J.A.; et al. Divergent adaptive and innate immunological responses are observed in humans following blunt trauma. BMC Immunol. 2010, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Bond, M.W.; Mosmann, T.R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989, 170, 2081–2095. [Google Scholar] [CrossRef]
- Marik, P.E.; Flemmer, M. The immune response to surgery and trauma: Implications for treatment. J. Trauma Acute Care Surg. 2012, 73, 801–808. [Google Scholar] [CrossRef]
- Venet, F.; Chung, C.S.; Monneret, G.; Huang, X.; Horner, B.; Garber, M.; Ayala, A. Regulatory T cell populations in sepsis and trauma. J. Leukoc. Biol. 2008, 83, 523–535. [Google Scholar] [CrossRef]
- Monneret, G.; Venet, F.; Pachot, A.; Lepape, A. Monitoring immune dysfunctions in the septic patient: A new skin for the old ceremony. Mol. Med. 2008, 14, 64–78. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar]
- Seddiki, N.; Santner-Nanan, B.; Martinson, J.; Zaunders, J.; Sasson, S.; Landay, A.; Solomon, M.; Selby, W.; Alexander, S.I.; Nanan, R.; et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 2006, 203, 1693–1700. [Google Scholar] [CrossRef]
- Liu, W.; Putnam, A.L.; Xu-Yu, Z.; Szot, G.L.; Lee, M.R.; Zhu, S.; Gottlieb, P.A.; Kapranov, P.; Gingeras, T.R.; Fazekas de St Groth, B.; et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006, 203, 1701–1711. [Google Scholar] [CrossRef]
- Khattri, R.; Cox, T.; Yasayko, S.A.; Ramsdell, F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 2003, 4, 337–342. [Google Scholar] [CrossRef] [PubMed]
- MacConmara, M.P.; Maung, A.A.; Fujimi, S.; McKenna, A.M.; Delisle, A.; Lapchak, P.H.; Rogers, S.; Lederer, J.A.; Mannick, J.A. Increased CD4+ CD25+ T regulatory cell activity in trauma patients depresses protective Th1 immunity. Ann. Surg. 2006, 244, 514–523. [Google Scholar] [CrossRef]
- Hein, F.; Massin, F.; Cravoisy-Popovic, A.; Barraud, D.; Levy, B.; Bollaert, P.E.; Gibot, S. The relationship between CD4+CD25+CD127- regulatory T cells and inflammatory response and outcome during shock states. Crit. Care 2010, 14, R19. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.R.; Liberal, R.; Mieli-Vergani, G.; Vergani, D.; Longhi, M.S. Regulatory T-cells in autoimmune diseases: Challenges, controversies and--yet--unanswered questions. Autoimmun. Rev. 2015, 14, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Venet, F.; Pachot, A.; Debard, A.L.; Bohe, J.; Bienvenu, J.; Lepape, A.; Monneret, G. Increased percentage of CD4+CD25+ regulatory T cells during septic shock is due to the decrease of CD4+CD25- lymphocytes. Crit. Care Med. 2004, 32, 2329–2331. [Google Scholar] [CrossRef] [PubMed]
- Murphey, E.D.; Lin, C.Y.; McGuire, R.W.; Toliver-Kinsky, T.; Herndon, D.N.; Sherwood, E.R. Diminished bacterial clearance is associated with decreased IL-12 and interferon-gamma production but a sustained proinflammatory response in a murine model of postseptic immunosuppression. Shock 2004, 21, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Gogos, C.A.; Drosou, E.; Bassaris, H.P.; Skoutelis, A. Proversus anti-inflammatory cytokine profile in patients with severe sepsis: A marker for prognosis and future therapeutic options. J. Infect. Dis. 2000, 181, 176–180. [Google Scholar] [CrossRef]
- Junger, W.G.; Hoyt, D.B.; Liu, F.C.; Loomis, W.H.; Coimbra, R. Immunosuppression after endotoxin shock: The result of multiple anti-inflammatory factors. J. Trauma 1996, 40, 702–709. [Google Scholar] [CrossRef]
- Mokart, D.; Capo, C.; Blache, J.L.; Delpero, J.R.; Houvenaeghel, G.; Martin, C.; Mege, J.L. Early postoperative compensatory anti-inflammatory response syndrome is associated with septic complications after major surgical trauma in patients with cancer. Br. J. Surg. 2002, 89, 1450–1456. [Google Scholar] [CrossRef]
- Menges, T.; Engel, J.; Welters, I.; Wagner, R.M.; Little, S.; Ruwoldt, R.; Wollbrueck, M.; Hempelmann, G. Changes in blood lymphocyte populations after multiple trauma: Association with posttraumatic complications. Crit. Care Med. 1999, 27, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Rivkind, A.I.; Siegel, J.H.; Guadalupi, P.; Littleton, M. Sequential patterns of eicosanoid, platelet, and neutrophil interactions in the evolution of the fulminant post-traumatic adult respiratory distress syndrome. Ann. Surg. 1989, 210, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.P.; O’Neill, B.; Haddon, W., Jr.; Long, W.B. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J. Trauma 1974, 14, 187–196. [Google Scholar] [CrossRef]
- Relja, B.; Lustenberger, T.; Puttkammer, B.; Jakob, H.; Morser, J.; Gabazza, E.C.; Takei, Y.; Marzi, I. Thrombin-activatable fibrinolysis inhibitor (TAFI) is enhanced in major trauma patients without infectious complications. Immunobiology 2013, 218, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Relja, B.; Menke, J.; Wagner, N.; Auner, B.; Voth, M.; Nau, C.; Marzi, I. Effects of positive blood alcohol concentration on outcome and systemic interleukin-6 in major trauma patients. Injury 2016, 47, 640–645. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Brown, J.A.; Freeman, G.J.; Hafler, D.A. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 2001, 167, 1245–1253. [Google Scholar] [CrossRef]
- Groux, H.; O’Garra, A.; Bigler, M.; Rouleau, M.; Antonenko, S.; de Vries, J.E.; Roncarolo, M.G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 1997, 389, 737–742. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Smith, R.M.; Perry, S.L.; Windsor, A.J.; Dickson, R.A.; Bellamy, M.C. Immediate IL-10 expression following major orthopaedic trauma: Relationship to anti-inflammatory response and subsequent development of sepsis. Intensive Care Med. 2000, 26, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Kitani, A.; Chua, K.; Nakamura, K.; Strober, W. Activated self-MHC-reactive T cells have the cytokine phenotype of Th3/T regulatory cell 1 T cells. J. Immunol. 2000, 165, 691–702. [Google Scholar] [CrossRef]
- Levings, M.K.; Sangregorio, R.; Roncarolo, M.G. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 2001, 193, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.N.; Yao, Y.M.; Sheng, Z.Y. The role of regulatory T cells in the pathogenesis of sepsis and its clinical implication. J. Interferon Cytokine Res. 2012, 32, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Monneret, G.; Lepape, A.; Voirin, N.; Bohe, J.; Venet, F.; Debard, A.L.; Thizy, H.; Bienvenu, J.; Gueyffier, F.; Vanhems, P. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006, 32, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.F.; Yao, Y.M.; Dong, N.; Yu, Y.; He, L.X.; Sheng, Z.Y. Association between regulatory T cell activity and sepsis and outcome of severely burned patients: A prospective, observational study. Crit. Care 2010, 14, R3. [Google Scholar] [CrossRef]
- Kramer, T.J.; Hack, N.; Bruhl, T.J.; Menzel, L.; Hummel, R.; Griemert, E.V.; Klein, M.; Thal, S.C.; Bopp, T.; Schafer, M.K.E. Depletion of regulatory T cells increases T cell brain infiltration, reactive astrogliosis, and interferon-gamma gene expression in acute experimental traumatic brain injury. J. Neuroinflamm. 2019, 16, 163. [Google Scholar] [CrossRef]
- Dai, H.; Sun, T.; Liu, Z.; Zhang, J.; Zhou, M. The imbalance between regulatory and IL-17-secreting CD4(+)T cells in multiple-trauma rat. Injury 2013, 44, 1521–1527. [Google Scholar] [CrossRef]
- Wang, H.; Daniel, V.; Sadeghi, M.; Opelz, G. Differences in the induction of induced human CD4(+) CD25(+) FoxP3(+) T-regulatory cells and CD3(+) CD8(+) CD28(-) T-suppressor cells subset phenotypes in vitro: Comparison of phorbol 12-myristate 13-acetate/ionomycin and phytohemagglutinin stimulation. Transpl. Proc. 2013, 45, 1822–1831. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Daniel, V.; Sadeghi, M.; Opelz, G. Plasticity and overlap of in vitro-induced regulatory T-cell markers in healthy humans. Transpl. Proc. 2013, 45, 1816–1821. [Google Scholar] [CrossRef]
- Anderson, S.J.; Coleclough, C. Regulation of CD4 and CD8 expression on mouse T cells. Active removal from the cell surface by two mechanisms. J. Immunol. 1993, 151, 5123–5134. [Google Scholar] [PubMed]
- Petersen, C.M.; Christensen, E.I.; Andresen, B.S.; Moller, B.K. Internalization, lysosomal degradation and new synthesis of surface membrane CD4 in phorbol ester-activated T-lymphocytes and U-937 cells. Exp. Cell Res. 1992, 201, 160–173. [Google Scholar] [CrossRef]
- O’Neil-Andersen, N.J.; Lawrence, D.A. Differential modulation of surface and intracellular protein expression by T cells after stimulation in the presence of monensin or brefeldin A. Clin. Diagn. Lab. Immunol. 2002, 9, 243–250. [Google Scholar] [CrossRef][Green Version]
- Mukherjee, S.; Maiti, P.K.; Nandi, D. Role of CD80, CD86, and CTLA4 on mouse CD4(+) T lymphocytes in enhancing cell-cycle progression and survival after activation with PMA and ionomycin. J. Leukoc. Biol. 2002, 72, 921–931. [Google Scholar] [PubMed]
- Fiorentino, D.F.; Zlotnik, A.; Vieira, P.; Mosmann, T.R.; Howard, M.; Moore, K.W.; O’Garra, A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 1991, 146, 3444–3451. [Google Scholar] [PubMed]
- De Waal Malefyt, R.; Haanen, J.; Spits, H.; Roncarolo, M.G.; te Velde, A.; Figdor, C.; Johnson, K.; Kastelein, R.; Yssel, H.; de Vries, J.E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med. 1991, 174, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.G.; Chen, G.; Feng, H.; Liu, J.; Jiang, Y.J.; Shang, H.; Cao, Y.M. Plasmodium chabaudi AS: Distinct CD4(+)CD25(+)Foxp3(+) regulatory T cell responses during infection in DBA/2 and BALB/c mice. Parasitol. Int. 2013, 62, 24–31. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sturm, R.; Xanthopoulos, L.; Heftrig, D.; Oppermann, E.; Vrdoljak, T.; Dunay, I.R.; Marzi, I.; Relja, B. Regulatory T Cells Modulate CD4 Proliferation after Severe Trauma via IL-10. J. Clin. Med. 2020, 9, 1052. https://doi.org/10.3390/jcm9041052
Sturm R, Xanthopoulos L, Heftrig D, Oppermann E, Vrdoljak T, Dunay IR, Marzi I, Relja B. Regulatory T Cells Modulate CD4 Proliferation after Severe Trauma via IL-10. Journal of Clinical Medicine. 2020; 9(4):1052. https://doi.org/10.3390/jcm9041052
Chicago/Turabian StyleSturm, Ramona, Lara Xanthopoulos, David Heftrig, Elsie Oppermann, Teodora Vrdoljak, Ildiko Rita Dunay, Ingo Marzi, and Borna Relja. 2020. "Regulatory T Cells Modulate CD4 Proliferation after Severe Trauma via IL-10" Journal of Clinical Medicine 9, no. 4: 1052. https://doi.org/10.3390/jcm9041052
APA StyleSturm, R., Xanthopoulos, L., Heftrig, D., Oppermann, E., Vrdoljak, T., Dunay, I. R., Marzi, I., & Relja, B. (2020). Regulatory T Cells Modulate CD4 Proliferation after Severe Trauma via IL-10. Journal of Clinical Medicine, 9(4), 1052. https://doi.org/10.3390/jcm9041052




