Velocity Vector Imaging Assessment of Functional Change in the Right Ventricle during Transcatheter Closure of Atrial Septal Defect by Intracardiac Echocardiography
Abstract
:1. Introduction
2. Methods
2.1. Patients
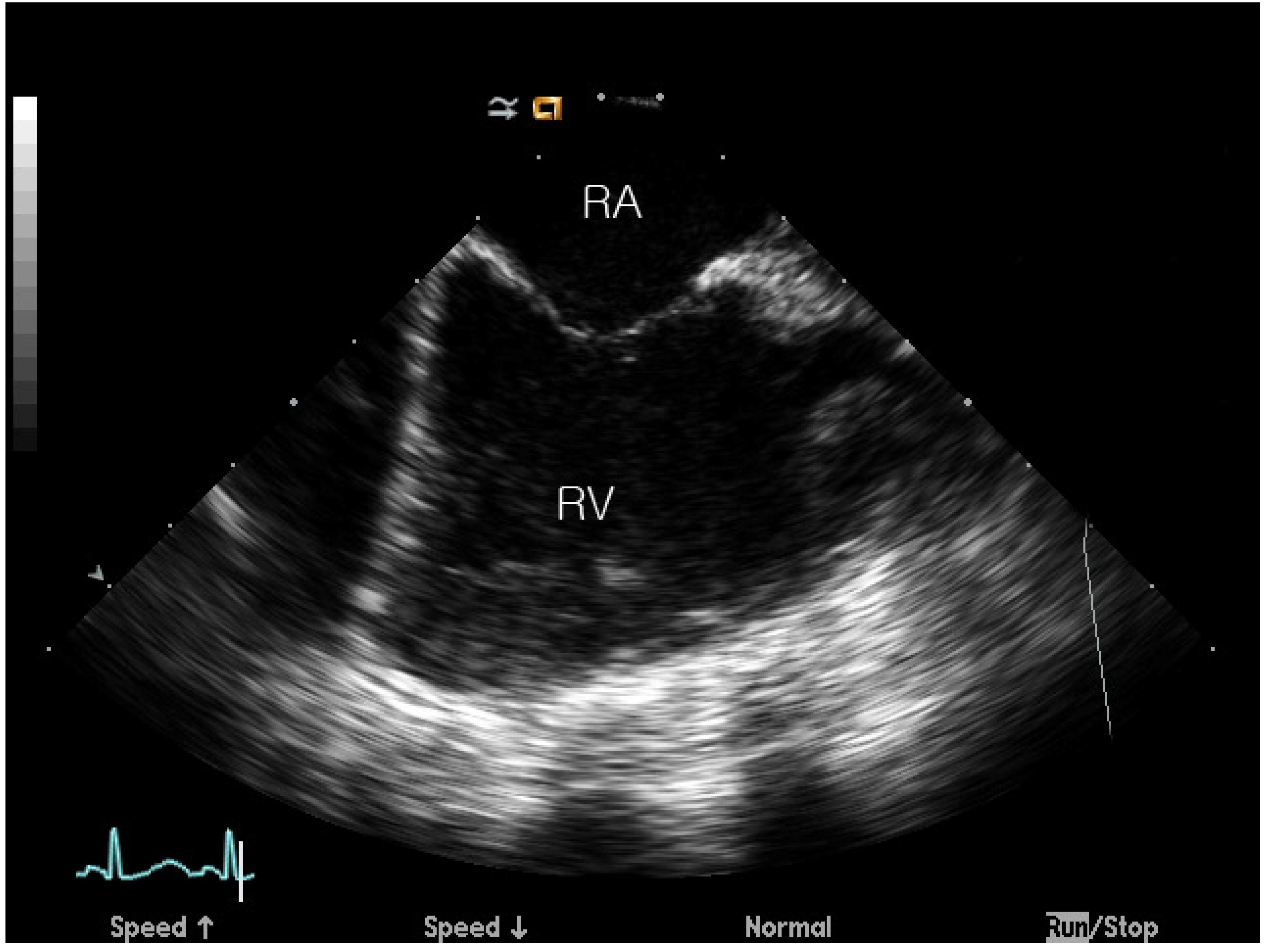
2.2. ASD Device Closure and ICE Imaging Protocol
2.3. Image Capture and Analysis
2.4. Statistical Analysis
2.5. Ethics Statement
3. Results
3.1. Baseline Characteristics of the Enrolled Patients
3.2. Velocity in Each RV Segment on VVI
3.3. Strain in Each RV Segment on VVI
3.4. SR in Each RV Segment on VVI
3.5. Longitudinal Displacement in Each RV Segment on VVI
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kort, S. Intracardiac echocardiography: Evolution, recent advances, and current applications. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2006, 19, 1192–1201. [Google Scholar] [CrossRef]
- Hijazi, Z.M.; Shivkumar, K.; Sahn, D.J. Intracardiac echocardiography during interventional and electrophysiological cardiac catheterization. Circulation 2009, 119, 587–596. [Google Scholar] [CrossRef] [Green Version]
- Okumura, Y.; Watanabe, I.; Ashino, S.; Kofune, M.; Ohkubo, K.; Takagi, Y.; Kawauchi, K.; Yamada, T.; Hashimoto, K.; Shindo, A.; et al. Electrophysiologic and anatomical characteristics of the right atrial posterior wall in patients with and without atrial flutter: Analysis by intracardiac echocardiography. Circ. J. Off. J. Jpn. Circ. Soc. 2007, 71, 636–642. [Google Scholar]
- Kim, N.K.; Park, S.; Shin, J.I.; Choi, J.Y. Eight-french intracardiac echocardiography. Circ. J. 2012, 76, 2119–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Uijl, D.W.; Tops, L.F.; Tolosana, J.M.; Schuijf, J.D.; Trines, S.A.; Zeppenfeld, K.; Bax, J.J.; Schalij, M.J. Real-time integration of intracardiac echocardiography and multislice computed tomography to guide radiofrequency catheter ablation for atrial fibrillation. Heart Rhythm 2008, 5, 1403–1410. [Google Scholar] [CrossRef]
- Calkins, H. Preventing complications following catheter ablation of atrial fibrillation: Is intracardiac echocardiography the answer we are seeking? Europace 2013, 15, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, T.; Muller, S.; Biviano, A.; Hahn, R.T. Why is intracardiac echocardiography helpful? Benefits, costs, and how to learn. Eur. Heart J. 2014, 35, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Kutty, S.; Deatsman, S.L.; Nugent, M.L.; Russell, D.; Frommelt, P.C. Assessment of regional right ventricular velocities, strain, and displacement in normal children using velocity vector imaging. Echocardiography 2008, 25, 294–307. [Google Scholar] [CrossRef]
- Deng, Y.; Pandit, A.; Heilman, R.L.; Chakkera, H.A.; Mazur, M.J.; Mookadam, F. Left ventricular torsion changes post kidney transplantation. J. Cardiovasc. Ultrasound 2013, 21, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Williams, L.K.; Urbano-Moral, J.A.; Rowin, E.J.; Jamorski, M.; Bruchal-Garbicz, B.; Carasso, S.; Pandian, N.G.; Maron, M.S.; Rakowski, H. Velocity vector imaging in the measurement of left ventricular myocardial mechanics on cardiac magnetic resonance imaging: Correlations with echocardiographically derived strain values. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2013, 26, 1153–1162. [Google Scholar] [CrossRef]
- Hayabuchi, Y.; Ono, A.; Kagami, S. Pulmonary annular motion velocity assessed using doppler tissue imaging- novel echocardiographic evaluation of right ventricular outflow tract function. Circ. J. Off. J. Jpn. Circ. Soc. 2015, 80, 168–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, Y.; Watanabe, I.; Ashino, S.; Kofune, M.; Yamada, T.; Takagi, Y.; Kawauchi, K.; Okubo, K.; Hashimoto, K.; Shindo, A.; et al. Anatomical characteristics of the cavotricuspid isthmus in patients with and without typical atrial flutter: Analysis with two- and three-dimensional intracardiac echocardiography. J. Interv. Card. Electrophysiol. Int. J. Arrhythm. Pacing 2006, 17, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Yu, W. Intracardiac echocardiography-assisted transseptal puncture. J. Chin. Med. Assoc. 2010, 73, 509–510. [Google Scholar] [CrossRef] [Green Version]
- Hollender, P.J.; Wolf, P.D.; Goswami, R.; Trahey, G.E. Intracardiac echocardiography measurement of dynamic myocardial stiffness with shear wave velocimetry. Ultrasound Med. Biol. 2012, 38, 1271–1283. [Google Scholar] [CrossRef] [Green Version]
- Haber, I.; Metaxas, D.N.; Geva, T.; Axel, L. Three-dimensional systolic kinematics of the right ventricle. American journal of physiology. Heart Circ. Physiol. 2005, 289, H1826–H1833. [Google Scholar] [CrossRef] [Green Version]
- Kukulski, T.; Hubbert, L.; Arnold, M.; Wranne, B.; Hatle, L.; Sutherland, G.R. Normal regional right ventricular function and its change with age: A doppler myocardial imaging study. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2000, 13, 194–204. [Google Scholar] [CrossRef]
- Eyskens, B.; Ganame, J.; Claus, P.; Boshoff, D.; Gewillig, M.; Mertens, L. Ultrasonic strain rate and strain imaging of the right ventricle in children before and after percutaneous closure of an atrial septal defect. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2006, 19, 994–1000. [Google Scholar] [CrossRef]
- Vitarelli, A.; Sardella, G.; Roma, A.D.; Capotosto, L.; De Curtis, G.; D’Orazio, S.; Cicconetti, P.; Battaglia, D.; Caranci, F.; De Maio, M.; et al. Assessment of right ventricular function by three-dimensional echocardiography and myocardial strain imaging in adult atrial septal defect before and after percutaneous closure. Int. J. Cardiovasc. Imaging 2012, 28, 1905–1916. [Google Scholar] [CrossRef]
- Pascotto, M.; Caso, P.; Santoro, G.; Caso, I.; Cerrato, F.; Pisacane, C.; D’Andrea, A.; Severino, S.; Russo, M.G.; Calabro, R. Analysis of right ventricular doppler tissue imaging and load dependence in patients undergoing percutaneous closure of atrial septal defect. Am. J. Cardiol. 2004, 94, 1202–1205. [Google Scholar] [CrossRef]
- Du, Z.D.; Cao, Q.L.; Koenig, P.; Heitschmidt, M.; Hijazi, Z.M. Speed of normalization of right ventricular volume overload after transcatheter closure of atrial septal defect in children and adults. Am. J. Cardiol. 2001, 88, 1450–1453. [Google Scholar] [CrossRef]
- Veldtman, G.R.; Razack, V.; Siu, S.; El-Hajj, H.; Walker, F.; Webb, G.D.; Benson, L.N.; McLaughlin, P.R. Right ventricular form and function after percutaneous atrial septal defect device closure. J. Am. Coll. Cardiol. 2001, 37, 2108–2113. [Google Scholar] [CrossRef] [Green Version]
- Kijima, Y.; Akagi, T.; Takaya, Y.; Akagi, S.; Nakagawa, K.; Kusano, K.; Sano, S.; Ito, H. Treat and repair strategy in patients with atrial septal defect and significant pulmonary arterial hypertension. Circ. J. Off. J. Jpn. Circ. Soc. 2015, 80, 227–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Total Patients | N = 74 |
|---|---|
| Age (years) | 21.7 (range, 3–59) |
| Male/Female | 20/54 |
| Weight (Kg) | 35.9 ± 23.3 |
| Height (cm) | 125.9 ± 34.4 |
| BSA (m2) | 1.1 ± 0.5 |
| Systolic pulmonary artery pressure (mmHg) | 37± 11 |
| LVEF (%) | 67.0 ± 5.2 |
| Qp/Qs | 2.2 ± 0.7 |
| Device Size (mm) | 20 (10–36) |
| Major complication | none |
| Residual shunt | none |
| Location | Preprocedure | Postprocedure | p-Value | Mixed-Model * |
|---|---|---|---|---|
| RV Inlet | 5.05 ± 2.53 | 4.16 ± 2.34 | 0.0077 | 0.01 |
| Midinlet | 4.11 ± 2.15 | 4.03 ± 2.69 | 0.8358 | 0.8 |
| Apex-inlet | 3.18 ± 2.03 | 3.33 ± 2.27 | 0.6264 | 0.618 |
| Apex-outlet | 1.89 ± 1.31 | 1.70 ± 1.53 | 0.2402 | 0.222 |
| Midoutlet | 4.12 ± 2.00 | 3.49 ± 1.63 | 0.1545 | 0.04 |
| RV Outlet | 5.42 ± 2.09 | 4.67 ± 1.69 | 0.0015 | 0.001 |
| Average | 3.97 ± 1.48 | 3.56 ± 1.40 | 0.0248 | 0.022 |
| Location | Preprocedure | Postprocedure | p-Value | Mixed-Model * |
|---|---|---|---|---|
| RV Inlet | −15.73 ± 10.11 | −15.33 ± 12.11 | 0.7687 | 0.817 |
| Midinlet | −15.26 ± 9.26 | −12.05 ± 9.40 | 0.015 | 0.02 |
| Apex-inlet | −16.72 ± 10.84 | −14.85 ± 10.21 | 0.2733 | 0.22 |
| Apex-outlet | −24.13 ± 9.56 | −22.61 ± 11.24 | 0.3526 | 0.323 |
| Midoutlet | −25.02 ± 10.41 | −21.89 ± 10.59 | 0.0333 | 0.028 |
| RV Outlet | −18.42 ± 12.04 | −14.51 ± 10.21 | 0.0312 | 0.027 |
| Average | −19.21 ± 5.79 | −16.87 ± 5.03 | 0.0021 | 0.002 |
| Location | Preprocedure | Postprocedure | p-Value | Mixed-Model * |
|---|---|---|---|---|
| RV Inlet | −2.21 ± 1.54 | −1.90 ± 1.47 | 0.1388 | 0.197 |
| Midinlet | −2.02 ± 1.14 | −1.78 ± 1.06 | 0.0819 | 0.139 |
| Apex-inlet | −2.07 ± 1.03 | −1.93 ± 1.10 | 0.605 | 0.458 |
| Apex-outlet | −2.78 ± 1.14 | −2.46 ± 1.37 | 0.0651 | 0.062 |
| Midoutlet | −2.63 ± 1.31 | −2.47 ± 1.01 | 0.3489 | 0.338 |
| RV Outlet | −1.96 ± 1.15 | −1.79 ± 1.51 | 0.3187 | 0.438 |
| Average | −2.28 ± 0.64 | −2.03 ± 0.61 | 0.006 | 0.008 |
| Location | Preprocedure | Postprocedure | p-Value | Mixed-Model * |
|---|---|---|---|---|
| RV Inlet | 5.37 ± 3.45 | 4.22 ± 3.11 | 0.0045 | 0.005 |
| Midinlet | 4.25 ± 2.81 | 3.75 ± 2.88 | 0.221 | 0.951 |
| Apex-inlet | 3.34 ± 2.59 | 3.25 ± 2.91 | 0.822 | 0.816 |
| Apex-outlet | 2.77 ± 2.43 | 2.33 ± 2.13 | 0.1499 | 0.155 |
| Midoutlet | 5.50 ± 3.19 | 4.91 ± 2.62 | 0.1261 | 0.104 |
| RV Outlet | 7.73 ± 3.44 | 6.87 ± 3.07 | 0.025 | 0.02 |
| Average | 4.83 ± 2.34 | 4.22 ± 1.99 | 0.3161 | 0.321 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.Y.; Shin, J.I.; Choi, J.Y.; Park, S.-J.; Kim, N.K. Velocity Vector Imaging Assessment of Functional Change in the Right Ventricle during Transcatheter Closure of Atrial Septal Defect by Intracardiac Echocardiography. J. Clin. Med. 2020, 9, 1132. https://doi.org/10.3390/jcm9041132
Jung SY, Shin JI, Choi JY, Park S-J, Kim NK. Velocity Vector Imaging Assessment of Functional Change in the Right Ventricle during Transcatheter Closure of Atrial Septal Defect by Intracardiac Echocardiography. Journal of Clinical Medicine. 2020; 9(4):1132. https://doi.org/10.3390/jcm9041132
Chicago/Turabian StyleJung, Se Yong, Jae Il Shin, Jae Young Choi, Su-Jin Park, and Nam Kyun Kim. 2020. "Velocity Vector Imaging Assessment of Functional Change in the Right Ventricle during Transcatheter Closure of Atrial Septal Defect by Intracardiac Echocardiography" Journal of Clinical Medicine 9, no. 4: 1132. https://doi.org/10.3390/jcm9041132
APA StyleJung, S. Y., Shin, J. I., Choi, J. Y., Park, S.-J., & Kim, N. K. (2020). Velocity Vector Imaging Assessment of Functional Change in the Right Ventricle during Transcatheter Closure of Atrial Septal Defect by Intracardiac Echocardiography. Journal of Clinical Medicine, 9(4), 1132. https://doi.org/10.3390/jcm9041132







