Decreased Glucose Utilization Contributes to Memory Impairment in Patients with Glufosinate Ammonium Intoxication
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. MRI and FDG-PET Acquisition
2.3. FDG-PET Image Preprocessing and Voxel-Based Single-Subject Analysis
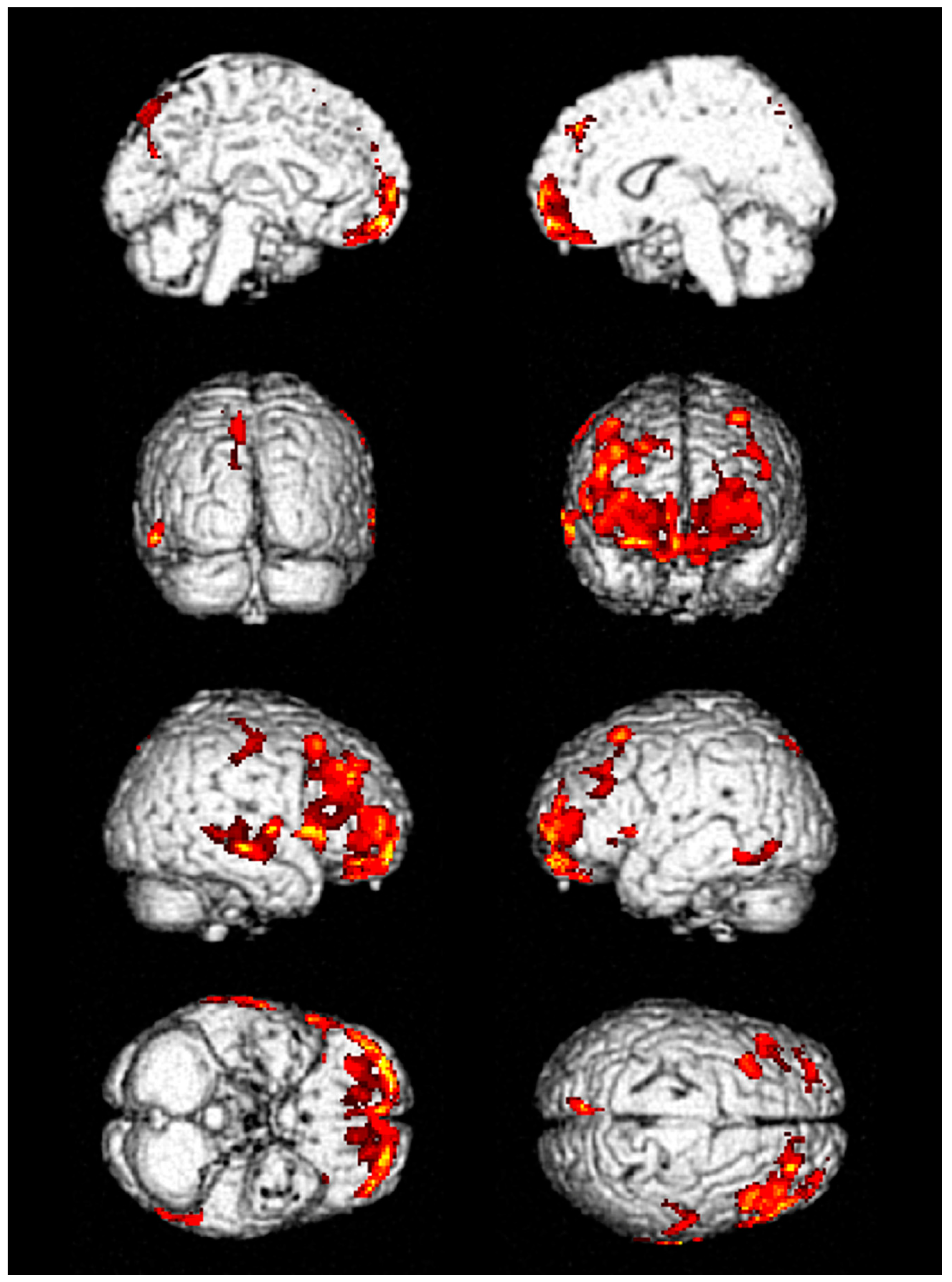
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoerlein, G. Glufosinate (phosphinothricin), a natural amino acid with unexpected herbicidal properties. Rev. Environ. Contam. Toxicol. 1994, 138, 73–145. [Google Scholar] [PubMed]
- Lee, J.W.; Hwang, I.W.; Kim, J.W.; Moon, H.J.; Kim, K.H.; Park, S.; Gil, H.W.; Hong, S.Y. Common pesticides used in suicide attempts following the 2012 paraquat ban in Korea. J. Korean Med. Sci. 2015, 30, 1517–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebert, E.; Leist, K.H.; Mayer, D. Summary of safety evaluation toxicity studies of glufosinate ammonium. Food. Chem. Toxicol. 1990, 28, 339–349. [Google Scholar] [CrossRef]
- Watanabe, T.; Sano, T. Neurological effects of glufosinate poisoning with a brief review. Hum. Exp. Toxicol. 1998, 17, 35–39. [Google Scholar] [CrossRef]
- Mao, Y.C.; Hung, D.Z.; Wu, M.L.; Tsai, W.J.; Wang, L.M.; Ger, J.; Deng, J.F.; Yang, C.C. Acute human glufosinate-containing herbicide poisoning. Clin. Toxicol. (Phila.) 2012, 50, 396–402. [Google Scholar] [CrossRef]
- Park, J.S.; Kwak, S.J.; Gil, H.W.; Kim, S.Y.; Hong, S.Y. Glufosinate herbicide intoxication causing unconsciousness, convulsion, and 6th cranial nerve palsy. J. Korean Med. Sci. 2013, 28, 1687–1689. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Kim, D.E.; Park, S.Y.; Gil, H.W.; Hong, S.Y. Seizures in patients with acute pesticide intoxication, with a focus on glufosinate ammonium. Hum. Exp. Toxicol. 2018, 37, 331–337. [Google Scholar] [CrossRef]
- Lantz, S.R.; Mack, C.M.; Wallace, K.; Key, E.F.; Shafer, T.J.; Casida, J.E. Glufosinate binds N-methyl-D-aspartate receptors and increases neuronal network activity in vitro. Neurotoxicology 2014, 45, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, N.; Takeuchi, C.; Hishikawa, K.; Fujii, T.; Nakaki, T. Glufosinate ammonium induces convulsion through N-methyl-D-aspartate receptors in mice. Neurosci. Lett. 2001, 304, 123–125. [Google Scholar] [CrossRef]
- Li, F.; Tsien, J.Z. Memory and the NMDA receptors. N. Engl. J. Med. 2009, 361, 302–303. [Google Scholar] [CrossRef] [Green Version]
- Park, H.Y.; Lee, P.H.; Shin, D.H.; Kim, G.W. Anterograde amnesia with hippocampal lesions following glufosinate intoxication. Neurology 2006, 67, 914–915. [Google Scholar] [CrossRef] [PubMed]
- Calas, A.G.; Richard, O.; Meme, S.; Beloeil, J.C.; Doan, B.T.; Gefflaut, T.; Meme, W.; Crusio, W.E.; Pichon, J.; Montecot, C. Chronic exposure to glufosinate-ammonium induces spatial memory impairments, hippocampal MRI modifications and glutamine synthetase activation in mice. Neurotoxicology 2008, 29, 740–747. [Google Scholar] [CrossRef] [Green Version]
- Cunnane, S.; Nugent, S.; Roy, M.; Courchesne-Loyer, A.; Croteau, E.; Tremblay, S.; Castellano, A.; Pifferi, F.; Bocti, C.; Paquet, N.; et al. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition 2011, 27, 3–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverman, D.H.; Mosconi, L.; Ercoli, L.; Chen, W.; Small, G.W. Positron emission tomography scans obtained for the evaluation of cognitive dysfunction. Semin. Nucl. Med. 2008, 38, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Lange, C.; Suppa, P.; Frings, L.; Brenner, W.; Spies, L.; Buchert, R. Optimization of statistical single subject analysis of brain FDG PET for the prognosis of mild cognitive impairment-to-alzheimer’s disease conversion. J. Alzheimers Dis. 2016, 49, 945–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Morgan, P.S.; Ashburner, J.; Smith, J.; Rorden, C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J. Neurosci. Methods 2016, 264, 47–56. [Google Scholar] [CrossRef]
- Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Kiebel, S.; Friston, K.J. Anatomically informed basis functions in multisubject studies. Hum. Brain Mapp. 2002, 16, 36–46. [Google Scholar] [CrossRef] [PubMed]
- De Blasi, B.; Barnes, A.; Galazzo, I.B.; Hua, C.H.; Shulkin, B.; Koepp, M.; Tisdall, M. Age-Specific (18)F-FDG image processing pipelines and analysis are essential for individual mapping of seizure foci in pediatric patients with intractable epilepsy. J. Nucl. Med. 2018, 59, 1590–1596. [Google Scholar] [CrossRef] [Green Version]
- Baldi, I.; Filleul, L.; Mohammed-Brahim, B.; Fabrigoule, C.; Dartigues, J.F.; Schwall, S.; Drevet, J.P.; Salamon, R.; Brochard, P. Neuropsychologic effects of long-term exposure to pesticides: Results from the French Phytoner study. Environ. Health Perspect. 2001, 109, 839–844. [Google Scholar] [CrossRef]
- Petrovitch, H.; Ross, G.W.; Abbott, R.D.; Sanderson, W.T.; Sharp, D.S.; Tanner, C.M.; Masaki, K.H.; Blanchette, P.L.; Popper, J.S.; Foley, D.; et al. Plantation work and risk of Parkinson disease in a population-based longitudinal study. Arch. Neurol. 2002, 59, 1787–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldi, I.; Lebailly, P.; Mohammed-Brahim, B.; Letenneur, L.; Dartigues, J.F.; Brochard, P. Neurodegenerative diseases and exposure to pesticides in the elderly. Am. J. Epidemiol. 2003, 157, 409–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayden, K.M.; Norton, M.C.; Darcey, D.; Ostbye, T.; Zandi, P.P.; Breitner, J.C.; Welsh-Bohmer, K.A.; Cache County Study, I. Occupational exposure to pesticides increases the risk of incident AD: The Cache County study. Neurology 2010, 74, 1524–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldi, I.; Gruber, A.; Rondeau, V.; Lebailly, P.; Brochard, P.; Fabrigoule, C. Neurobehavioral effects of long-term exposure to pesticides: Results from the 4-year follow-up of the PHYTONER study. Occup. Environ. Med. 2011, 68, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.Y.; Song, S.Y.; Lee, S.H.; Lee, S.Y.; Kim, S.H.; Ryu, S.W. Vasogenic edema in striatum following ingestion of glufosinate-containing herbicide. J. Clin. Neurosci. 2009, 16, 1372–1373. [Google Scholar] [CrossRef] [PubMed]
- Jeong, T.O.; Yoon, J.C.; Lee, J.B.; Jin, Y.H.; Hwang, S.B. Reversible splenial lesion syndrome (RESLES) following glufosinate ammonium poisoning. J. Neuroimaging 2015, 25, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L.; Tsui, W.H.; Herholz, K.; Pupi, A.; Drzezga, A.; Lucignani, G.; Reiman, E.M.; Holthoff, V.; Kalbe, E.; Sorbi, S.; et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J. Nucl. Med. 2008, 49, 390–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohnen, N.I.; Djang, D.S.; Herholz, K.; Anzai, Y.; Minoshima, S. Effectiveness and safety of 18F-FDG PET in the evaluation of dementia: A review of the recent literature. J. Nucl. Med. 2012, 53, 59–71. [Google Scholar] [CrossRef] [Green Version]
- Gallucci, M.; Limbucci, N.; Catalucci, A.; Caulo, M. Neurodegenerative diseases. Radiol. Clin. N. Am. 2008, 46, 799–817. [Google Scholar] [CrossRef]
- Brown, R.K.; Bohnen, N.I.; Wong, K.K.; Minoshima, S.; Frey, K.A. Brain PET in suspected dementia: Patterns of altered FDG metabolism. Radiographics 2014, 34, 684–701. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, D.L.; Rice, H.J.; Cooper, J.J.; Cabeza, R.; Rubin, D.C.; Labar, K.S. Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia 2005, 43, 659–674. [Google Scholar] [CrossRef] [Green Version]
- Newcomer, J.W.; Farber, N.B.; Jevtovic-Todorovic, V.; Selke, G.; Melson, A.K.; Hershey, T.; Craft, S.; Olney, J.W. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology 1999, 20, 106–118. [Google Scholar] [CrossRef]
- Ghasemi, M.; Schachter, S.C. The NMDA receptor complex as a therapeutic target in epilepsy: A review. Epilepsy Behav. 2011, 22, 617–640. [Google Scholar] [CrossRef]
- Hirose, S.; Umetani, Y.; Amitani, M.; Hosoi, R.; Momosaki, S.; Hatazawa, J.; Gee, A.; Inoue, O. Role of NMDA receptors in the increase of glucose metabolism in the rat brain induced by fluorocitrate. Neurosci. Lett. 2007, 415, 259–263. [Google Scholar] [CrossRef]
- Riedel, G.; Platt, B.; Micheau, J. Glutamate receptor function in learning and memory. Behav. Brain Res. 2003, 140, 1–47. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef] [Green Version]
- Zimmer, E.R.; Parent, M.J.; Souza, D.G.; Leuzy, A.; Lecrux, C.; Kim, H.I.; Gauthier, S.; Pellerin, L.; Hamel, E.; Rosa-Neto, P. [(18)F]FDG PET signal is driven by astroglial glutamate transport. Nat. Neurosci. 2017, 20, 393–395. [Google Scholar] [CrossRef] [Green Version]
- Morris, R.G. NMDA receptors and memory encoding. Neuropharmacology 2013, 74, 32–40. [Google Scholar] [CrossRef]
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Walker, R.H.; Magistretti, P.J.; Alberini, C.M. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 2011, 144, 810–823. [Google Scholar] [CrossRef] [Green Version]
- Won, H.J.; Chang, K.H.; Cheon, J.E.; Kim, H.D.; Lee, D.S.; Han, M.H.; Kim, I.O.; Lee, S.K.; Chung, C.K. Comparison of MR imaging with PET and ictal SPECT in 118 patients with intractable epilepsy. AJNR Am. J. Neuroradiol. 1999, 20, 593–599. [Google Scholar]
- Goffin, K.; Dedeurwaerdere, S.; Van Laere, K.; Van Paesschen, W. Neuronuclear assessment of patients with epilepsy. Semin. Nucl. Med. 2008, 38, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, I. PET studies in epilepsy. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 416–430. [Google Scholar] [PubMed]


| Variables | Values |
|---|---|
| Male sex, n (%) | 5 (55.6%) |
| Age, year | 57 (38–83) |
| Hypertension, n (%) | 3 (33.3%) |
| Diabetes mellitus, n (%) | 1 (11.1%) |
| Depression, n (%) | 2 (22.2%) |
| Current smoker, n (%) | 4 (44.4%) |
| Alcohol history, bottles/week | 1 (0–14) |
| † Ingested volume, mL | 300 (100–500) |
| Seizure, n (%) | 7 (77.8%) |
| † Initial GCS, score | 9 (3–15) |
| † Initial ammonia, mg/dL | 130.6 (69.1–230.0) |
| † MMSE (n = 6) | 24.5 (14–28) |
| †* Orientation (10) | 8.5 (6–10) |
| †* Registration (3) | 3 (0–3) |
| †* Attention and Calculation (5) | 3 (0–4) |
| †* Recall (3) | 2.5 (0–3) |
| †* Language (9) | 9 (6–9) |
| † CDR (n = 6) | 0.5 (0–0.5) |
| APACHE II score | 20 (6–35) |
| Systemic hypotension, n (%) | 6 (66.7%) |
| Acute kidney injury, n (%) | 1 (11.1%) |
| Mechanical ventilation, n (%) | 7 (77.8%) |
| † Hospital day | 12 (6–53) |
| † ICU stay day | 6 (3–41) |
| No. | FDG-PET Finding | Hospital Stay, Days | ICU Stay, Days | Seizure Duration, Days | F/U Duration, Months | ||
|---|---|---|---|---|---|---|---|
| Initial | Follow up (I) | Follow up (II) | |||||
| 1 | Decreased metabolic activity in frontal and temporal lobes | Improved but remained | Improved but remained | 53 | 41 | 6 | 12 |
| 2 | Decreased metabolic activity in frontal and temporal lobes | Improved | Improved | 6 | 3 | No | 3 |
| 3 | Decreased metabolic activity in frontal and temporal lobes | Not performed | Not performed | 14 | 7 | 2 | 6 |
| 4 | Decreased metabolic activity in inferior frontal lobe | Not performed | Not performed | 10 | 6 | 1 | 1 |
| 5 | Decreased metabolic activity in frontal lobe | Not performed | Not performed | 12 | 4 | 1 | 1 |
| 6 | Decreased metabolic activity in frontal and temporal lobes | Not performed | Not performed | 24 | 8 | 3 | 2 |
| 7 | Decreased metabolic activity in frontal and temporal lobes | Not performed | Not performed | 19 | 13 | 3 | 1 |
| 8 | Decreased metabolic activity in frontal and temporal lobes | Improved | Not performed | 9 | 3 | No | 2 |
| 9 | Decreased metabolic activity in frontal and temporal lobes | Not performed | Not performed | 11 | 5 | 2 | Loss |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Kim, J.I.; Cho, N.-j.; Oh, S.W.; Park, J.; Yoo, I.D.; Gil, H.-W.; Lee, S.M. Decreased Glucose Utilization Contributes to Memory Impairment in Patients with Glufosinate Ammonium Intoxication. J. Clin. Med. 2020, 9, 1213. https://doi.org/10.3390/jcm9041213
Park S, Kim JI, Cho N-j, Oh SW, Park J, Yoo ID, Gil H-W, Lee SM. Decreased Glucose Utilization Contributes to Memory Impairment in Patients with Glufosinate Ammonium Intoxication. Journal of Clinical Medicine. 2020; 9(4):1213. https://doi.org/10.3390/jcm9041213
Chicago/Turabian StylePark, Samel, Joong Il Kim, Nam-jun Cho, Se Won Oh, Jongkyu Park, Ik Dong Yoo, Hyo-Wook Gil, and Sang Mi Lee. 2020. "Decreased Glucose Utilization Contributes to Memory Impairment in Patients with Glufosinate Ammonium Intoxication" Journal of Clinical Medicine 9, no. 4: 1213. https://doi.org/10.3390/jcm9041213






