Update on Weaning from Veno-Arterial Extracorporeal Membrane Oxygenation
Abstract
:1. Introduction
2. Short-Term Outcome of Patients Receiving Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO)
3. Weaning Does Not Equal Survival
4. Factors Associated with Poor Outcome of Weaned Patients
4.1. Cardiogenic Shock
4.2. Postcardiotomy Shock
4.3. Extracorporeal Cardiopulmonary Resuscitation
5. Predictors of Successful Weaning from VA-ECMO
5.1. Etiology
5.2. Pulse
5.3. Echocardiography
5.4. Inotropic Support
5.5. Biomarkers
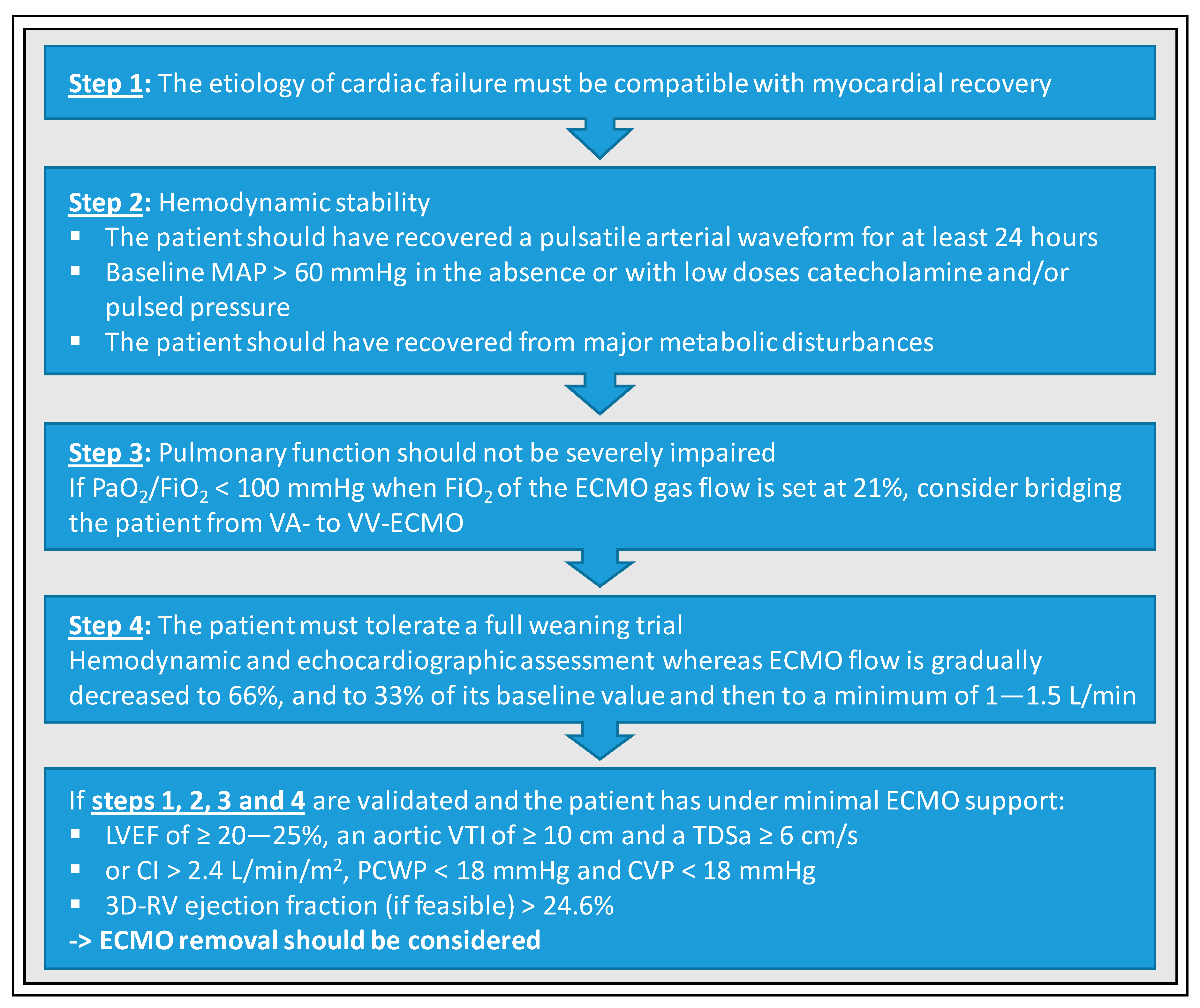
6. Basic Requirements for Promising Weaning Attempts
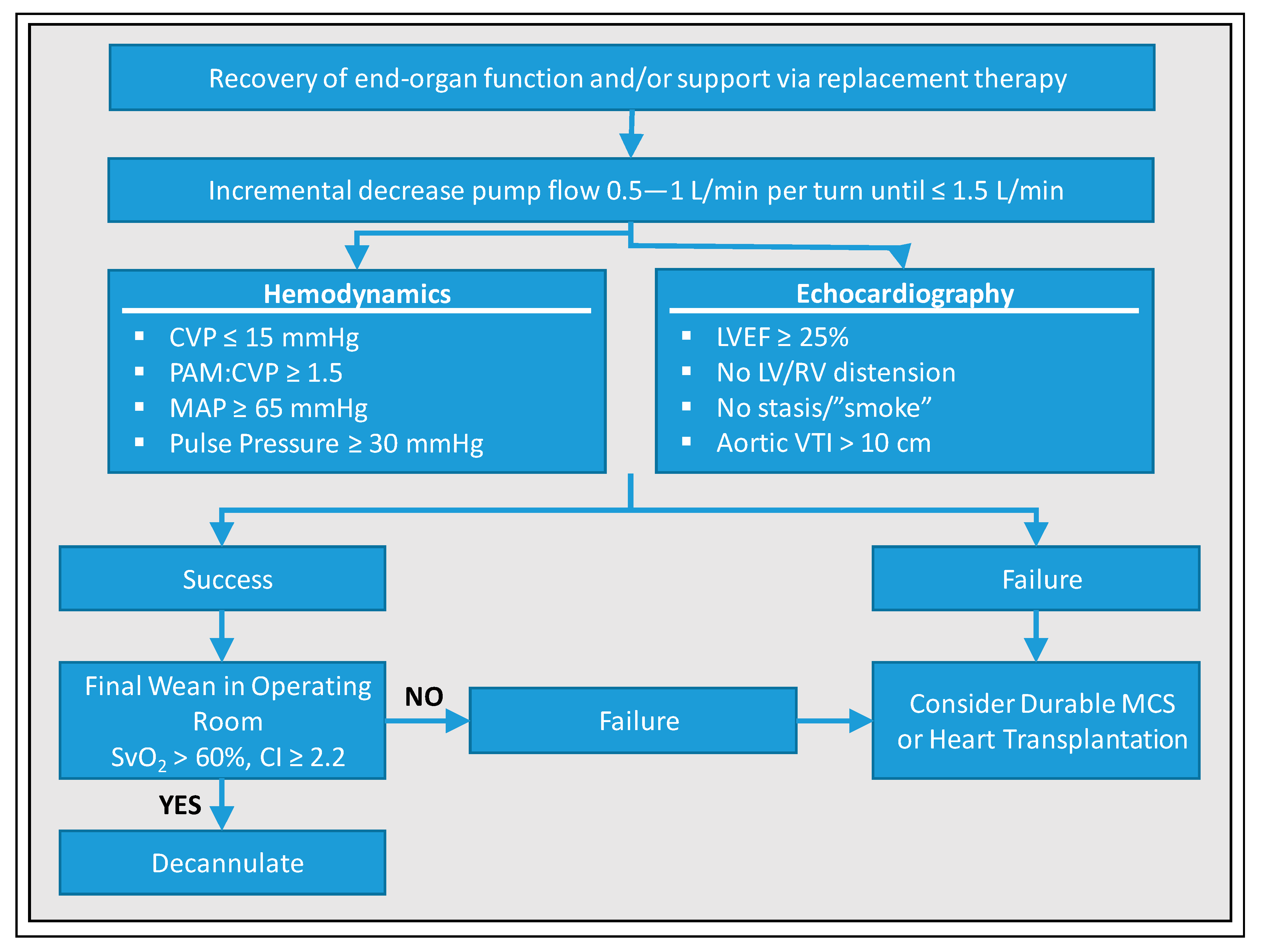
7. Weaning Strategies from VA-ECMO
8. VA-ECMO Weaning—University of Munich, Cardiologic ICU, Weaning Protocol
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xie, A.; Phan, K.; Tsai MY, C.; Yan, T.D.; Forrest, P. Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock and Cardiac Arrest. Circ. Heart Fail. 2018, 11, e004905. [Google Scholar] [CrossRef]
- Rajan, S.; Wissenberg, M.; Folke, F.; Hansen, S.M.; Gerds, T.A.; Kragholm, K.; Hansen, C.M.; Karlsson, L.; Lippert, F.K.; Kber, L. Association of bystander cardiopulmonary resuscitation and survival according to ambulance response times after out-of-hospital cardiac arrest. Circulation 2016, 134, 2095–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guglin, M.; Zucker, M.J.; Bazan, V.M.; Bozkurt, B.; El Banayosy, A.; Estep, J.D.; Gurley, J.; Nelson, K.; Malyala, R.; Panjrath, G.S. Venoarterial ECMO for Adults: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 698–716. [Google Scholar] [CrossRef] [PubMed]
- Napp, L.C.; Kühn, C.; Bauersachs, J. ECMO in cardiac arrest and cardiogenic shock. Herz 2017, 42, 27–44. [Google Scholar] [CrossRef] [Green Version]
- Cavarocchi, N.C.; Pitcher, H.T.; Yang, Q.; Karbowski, P.; Miessau, J.; Hastings, H.M.; Hirose, H. Weaning of extracorporeal membrane oxygenation using continuous hemodynamic transesophageal echocardiography. J. Thorac. Cardiovasc. Surg. 2013, 146, 1474–1479. [Google Scholar] [CrossRef] [Green Version]
- Pappalardo, F.; Pieri, M.; Corada, B.A.; Ajello, S.; Melisurgo, G.; De Bonis, M.; Zangrillo, A. Timing and Strategy for Weaning From Venoarterial ECMO are Complex Issues. J. Cardiothorac. Vasc. Anesth. 2015, 29, 906–911. [Google Scholar] [CrossRef]
- Aissaoui, N.; Luyt, C.E.; Leprince, P.; Trouillet, J.L.; Léger, P.; Pavie, A.; Combes, A. Predictors of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensive Care Med. 2012, 37, 1738–1745. [Google Scholar] [CrossRef]
- Aissaoui, N.; El-Banayosy, A.; Combes, A. How to wean a patient from veno-arterial extracorporeal membrane oxygenation. Intensive Care Med. 2015, 41, 902–905. [Google Scholar] [CrossRef]
- Thiagarajan, R.R.; Barbaro, R.P.; Rycus, P.T. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017, 63, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Phan, K.; Tsai MY, C.; Yan, T.D.; Forrest, P. Veno-Arterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock: An Introduction for the Busy Clinician. Circulation 2019, 140, 2019–2037. [Google Scholar] [CrossRef]
- Corsi, F.; Lebreton, G.; Bréchot, N.; Hekimian, G.; Nieszkowska, A.; Trouillet, J.L.; Schmidt, M. Life-threatening massive pulmonary embolism rescued by venoarterial-extracorporeal membrane oxygenation. Crit. Care 2017, 21, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, B.; Parazino, M.; Omar, H.R.; Davis, G.; Guglin, M.; Gurley, J.; Smyth, S. A retrospective comparison of survivors and non-survivors of massive pulmonary embolism receiving veno-arterial extracorporeal membrane oxygenation support. Resuscitation 2018, 122, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Burrell, A.; Roberts, L.; Bailey, M.; Sheldrake, J.; Rycus, P.T.; Brodie, D. Predicting survival after ECMO for refractory cardiogenic shock: The survival after veno-arterial-ECMO (SAVE)-score. Eur. Heart J. 2015, 36, 2246–2256. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.J.; Tsai, T.H.; Lee, F.Y.; Fang, H.Y.; Sun, C.K.; Leu, S.; Chen, C.J. Early extracorporeal membrane oxygenator-assisted primary percutaneous coronary intervention improved 30-day clinical outcomes in patients with ST-segment elevation myocardial infarction complicated with profound cardiogenic shock. Crit. Care Med. 2010, 38, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Tsao, N.W.; Shih, C.M.; Yeh, J.S.; Kao, Y.T.; Hsieh, M.H.; Ou, K.-L.; Chen, J.-W.; Shyu, K.-G.; Weng, Z.-C.; Chang, N.-C. Extracorporeal membrane oxygenation-assisted primary percutaneous coronary intervention may improve survival of patients with acute myocardial infarction complicated by profound cardiogenic shock. J. Crit. Care 2012, 27, 530. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Lin, J.W.; Yu, H.Y.; Ko, W.J.; Jerng, J.S.; Chang, W.T.; Chen, W.-J.; Huang, S.-C.; Chi, N.-H.; Wang, C.-H. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: An observational study and propensity analysis. Lancet 2008, 372, 554–561. [Google Scholar] [CrossRef]
- Ortega-Deballon, I.; Hornby, L.; Shemie, S.D.; Bhanji, F.; Guadagno, E. Extracorporeal resuscitation for refractory out-of-hospital cardiac arrest in adults: A systematic review of international practices and outcomes. Resuscitation 2016, 101, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Bakhtiary, F.; Keller, H.; Dogan, S.; Dzemali, O.; Oezaslan, F.; Meininger, D.; Ackermann, H.; Zwissler, B.; Kleine, P.; Moritz, A. Venoarterial Extracorporeal Membrane Oxygenation in Cardiogenic Shock. JACC Heart Fail. 2018, 6, 503–516. [Google Scholar] [CrossRef]
- Muller, G.; Flecher, E.; Lebreton, G.; Luyt, C.E.; Trouillet, J.L.; Bréchot, N.; Schmidt, M.; Mastroianni, C.; Chastre, J.; Leprince, P. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med. 2016, 42, 370–378. [Google Scholar] [CrossRef]
- Smedira, N.G.; Moazami, N.; Golding, C.M.; McCarthy, P.M.; Apperson-Hansen, C.; Blackstone, E.H.; Cosgrove, D.M., III. Clinical experience with 202 adults receiving extracorporeal membrane oxygenation for cardiac failure: Survival at five years. J. Thorac. Cardiovasc. Surg. 2001, 122, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Aissaoui, N.; Brehm, C.; El-Banayosy, A.; Combes, A. Chapter 15 Weaning strategy from veno-arterial extracorporeal membrane oxygenation (ECMO). In Extracorporeal Membrane Oxygenation: Advances in Therapy; Firstenberg, M.S., Ed.; InTech Online Publishers: Cambridge, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Ortuno, S.; Delmas, C.; Diehl, J.L.; Bailleul, C.; Lancelot, A.; Naili, M.; Cholley, B.; Pirracchio, R.; Aissaoui, N. Weaning from veno-arterial extra-corporeal membrane oxygenation: Which strategy to use? Ann. Cardiothorac. Surg. 2019, 8, E1–E8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.S.; Chao, A.; Yu, H.Y.; Ko, W.J.; Wu, I.H.; Chen RJ, C.; Wang, S.S. Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. J. Am. Coll. Cardiol. 2003, 41, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Aso, S.; Matsui, H.; Fushimi, K.; Yasunaga, H. In-hospital mortality and successful weaning from venoarterial extracorporeal membrane oxygenation: Analysis of 5,263 patients using a national inpatient database in Japan. Crit. Care 2016, 20, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastan, A.J.; Dege, A.; Mohr, M.; Doll, N.; Falk, V.; Walther, T.; Mohr, F.W. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J. Thorac. Cardiovasc. Surg. 2010, 139, 302–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.J.; Wang, W.; Hu, S.S.; Sun, H.S.; Gao, H.W.; Long, C.; Xu, J.P. Extracorporeal membrane oxygenation for treatment of cardiac failure in adult patients. Interact Cardiovasc. Thorac. Surg. 2009, 9, 296–300. [Google Scholar] [CrossRef] [Green Version]
- Combes, A.; Leprince, P.; Luyt, C.E.; Bonnet, N.; Trouillet, J.L.; Léger, P.; Chastre, J. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit. Care Med. 2008, 36, 1404–1411. [Google Scholar] [CrossRef]
- Sertic, F.; Chavez, L.; Diagne, D.; Richards, T.; Wald, J.; Acker, M.; Birati, E.; Rame, E.; Bermudez, C. Predictors of in-hospital mortality and midterm outcomes of patients successfully weaned from venoarterial extracorporeal membrane oxygenation. J. Thorac. Cardiovasc. Surg. 2019. [Google Scholar] [CrossRef]
- Chang, W.W.; Tsai, F.C.; Tsai, T.Y.; Chang, C.H.; Jenq, C.C.; Chang, M.Y.; Tian, Y.-C.; Hung, C.-C.; Fang, J.-T.; Yang, C.-W. Predictors of mortality in patients successfully weaned from extracorporeal membrane oxygenation. PLoS ONE 2012, 7, e42687. [Google Scholar] [CrossRef]
- Chommeloux, J.; Montero, S.; Franchineau, G.; Bréchot, N.; Hékimian, G.; Lebreton, G.; Le Guennec, L.; Bourcier, S.; Nieszkowska, A.; Leprince, P. Microcirculation Evolution in Patients on Venoarterial Extracorporeal Membrane Oxygenation for Refractory Cardiogenic Shock. Crit. Care Med. 2020, 48, e9–e17. [Google Scholar] [CrossRef]
- Distelmaier, K.; Winter, M.P.; Rützler, K.; Heinz, G.; Lang, I.M.; Maurer, G.; Koinig, H.; Steinlechner, B.; Niessner, A.; Goliasch, G. Serum butyrylcholinesterase predicts survival after extracorporeal membrane oxygenation after cardiovascular surgery. Crit. Care 2014, 18, R24. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.Y.; Lin, P.J.; Lee, M.Y.; Tsai, F.C.; Chu, J.J.; Chang, Y.S.; Haung, Y.-K.; Liu, K.-S. Using extracorporeal life support to resuscitate adult postcardiotomy cardiogenic shock: Treatment strategies and predictors of short-term and midterm survival. Resuscitation 2010, 81, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Wang, H.; Jia, M.; Ma, N.; Meng, X.; Hou, X.T. The early dynamic behavior of lactate is linked to mortality in postcardiotomy patients with extracorporeal membrane oxygenation support: A retrospective observational study. J. Thorac. Cardiovasc. Surg. 2015, 149, 1445–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leick, J.; Liebetrau, C.; Szardien, S.; Fischer-Rasokat, U.; Willmer, M.; van Linden, A.; Blumenstein, J.; Nef, H.; Rolf, A.; Arlt, M. Door-to-implantation time of extracorporeal life support systems predicts mortality in patients with out-of-hospital cardiac arrest. Clin. Res. Cardiol. 2013, 102, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Tsai, F.C.; Chang, C.H.; Lin, C.Y.; Jenq, C.C.; Juan, K.C.; Hsu, H.-H.; Chang, M.-Y.; Tian, Y.-C.; Hung, C.-C. Prognosis of patients on extracorporeal membrane oxygenation: The impact of acute kidney injury on mortality. Ann. Thorac. Surg. 2011, 91, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Asaumi, Y.; Yasuda, S.; Morii, I.; Kakuchi, H.; Otsuka, Y.; Kawamura, A.; Sasako, Y.; Nakatani, T.; Nonogi, H.; Miyazaki, S. Favourable clinical outcome in patients with cardiogenic shock due to fulminant myocarditis supported by percutaneous extracorporeal membrane oxygenation. Eur. Heart J. 2005, 26, 2185–2192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marasco, S.F.; Vale, M.; Pellegrino, V.; Preovolos, A.; Leet, A.; Kras, A.; Schulberg, E.; Bergin, P.; Esmore, D.S. Extracorporeal membrane oxygenation in primary graft failure after heart transplantation. Ann. Thorac. Surg. 2010, 90, 1541–1546. [Google Scholar] [CrossRef]
- Kim, S.J.; Jung, J.S.; Park, J.H.; Park, J.S.; Hong, Y.S.; Lee, S.W. An optimal transition time to extracorporeal cardiopulmonary resuscitation for predicting good neurological outcome in patients with out-of-hospital cardiac arrest: A propensity-matched study. Crit. Care 2014, 18, 535. [Google Scholar] [CrossRef] [Green Version]
- Park, B.W.; Seo, D.C.; Moon, I.K.; Chung, J.W.; Bang, D.W.; Hyon, M.S.; Kim, S.-K.; Chang, W.; Youm, W. Pulse pressure as a prognostic marker in patients receiving extracorporeal life support. Resuscitation 2013, 84, 1404–1408. [Google Scholar] [CrossRef]
- Huang, K.C.; Lin, L.Y.; Chen, Y.S.; Lai, C.H.; Hwang, J.J.; Lin, L.C. Three-Dimensional Echocardiography-Derived Right Ventricular Ejection Fraction Correlates with Success of Decannulation and Prognosis in Patients Stabilized by Venoarterial Extracorporeal Life Support. J. Am. Soc. Echocardiogr. 2018, 31, 169–179. [Google Scholar] [CrossRef]
- Luyt, C.E.; Landivier, A.; Leprince, P.; Bernard, M.; Pavie, A.; Chastre, J.; Combes, A. Usefulness of cardiac biomarkers to predict cardiac recovery in patients on extracorporeal membrane oxygenation support for refractory cardiogenic shock. J. Crit. Care 2012, 27, 524. [Google Scholar] [CrossRef]
- ELSO. Guidelines for Cardiopulmonary Extracorporeal Life Support Extracorporeal Life Support Organization, Version 1.4; ELSO: Ann Arbor, MI, USA, 2017; Available online: http:// www.elsonet.org (accessed on 2 February 2019).
- Carroll, B.J.; Shah, R.V.; Murthy, V.; McCullough, S.A.; Reza, N.; Thomas, S.S.; Song, T.H.; Newton-Cheh, C.H.; Camuso, J.M.; MacGillivray, T. Clinical Features and outcomes in adults with cardiogenic shock supported by extracorporeal membrane oxygenation. Am. J. Cardiol. 2015, 116, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Durinka, J.B.; Bogar, L.J.; Hirose, H.; Brehm, C.; Koerner, M.M.; Pae, W.E.; El-Banayosy, A.; Stephenson, E.R.; Cavarocchi, N.C. End-organ recovery is key to success for extracorporeal membrane oxygenation as a bridge to implantable left ventricular assist device. ASAIO J. 2014, 60, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.; Garan, A.R.; Abdelbary, A.; Bacchetta, M.; Bartlett, R.H.; Beck, J.; Belohlavek, J.; Chen, Y.-S.; Fan, E.; Ferguson, N.D. Position paper for the organization of ECMO programs for cardiac failure in adults. Intensive Care Med. 2018, 44, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Zwischenberger, J.B.; Pitcher, H.T. Extracorporeal Membrane Oxygenation Management: Techniques to Liberate from Extracorporeal Membrane Oxygenation and Manage Post-Intensive Care Unit Issues. Crit. Care Clin. 2017, 33, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Schrutka, L.; Binder, C.; Kriechbaumer, L.; Heinz, G.; Lang, I.M.; Maurer, G.; Koinig, H.; Steinlechner, B.; Niessner, A. Liver function predicts survival in patients undergoing extracorporeal membrane oxygenation following cardiovascular surgery. Crit. Care 2016, 20, 57. [Google Scholar] [CrossRef] [Green Version]
- Khot, U.N.; Mishra, M.; Yamani, M.H.; Smedira, N.G.; Paganini, E.; Yeager, M.; Buda, T.; McCarthy, P.M.; Young, J.B.; Starling, R.C. Severe renal dysfunction complicating cardiogenic shock is not a contraindication to mechanical support as a bridge to cardiac transplantation. J. Am. Coll. Cardiol. 2003, 41, 381–385. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Pellegrino, V.; Combes, A.; Scheinkestel, C.; Cooper, D.J.; Hodgson, C. Mechanical ventilation during extracorporeal membrane oxygenation. Crit. Care 2014, 18, 203. [Google Scholar] [CrossRef] [Green Version]
- Aissaoui, N.; Guerot, E.; Combes, A.; Delouche, A.; Chastre, J.; Leprince, P.; Leger, P.; Diehl, J.L.; Fagon, J.Y.; Diebold, B. Two-dimensional strain rate and Doppler tissue myocardial velocities: Analysis by echocardiography of hemodynamic and functional changes of the failed left ventricle during different degrees of extracorporeal life support. J. Am. Soc. Echocardiogr. 2012, 25, 632–640. [Google Scholar] [CrossRef]
- Ling, L.; Chan, K.M. Weaning adult patients with cardiogenic shock on veno-arterial extracorporeal membrane oxygenation by pump-controlled retrograde trial off. Perfusion 2018, 33, 339–345. [Google Scholar] [CrossRef]
- Westrope, C.; Harvey, C.; Robinson, S.; Speggiorin, S.; Faulkner, G.; Peek, G.J. Pump controlled retrograde trial off from VA-ECMO. ASAIO J. 2013, 59, 517–519. [Google Scholar] [CrossRef]
- Santise, G.; Panarello, G.; Ruperto, C. Extracorporeal membrane oxygenation for graft failure after heart transplantation: A multidisciplinary approach to maximize weaning rate. Int. J. Artif. Organs 2014, 37, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Affronti, A.; di Bella, I.; Carino, D. Levosimendan may improve weaning outcomes in venoarterial ECMO patients. ASAIO J. 2013, 59, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Distelmaier, K.; Roth, C.; Schrutka, L.; Binder, C.; Steinlechner, B.; Heinz, G.; Koinig, H.; Niessner, A. Beneficial effects of levosimendan on survival in patients undergoing extracorporeal membrane oxygenation after cardiovascular surgery. Br. J. Anaesth. 2016, 117, 52–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lüsebrink, E.; Stremmel, C.; Stark, K.; Petzold, T.; Hein-Rothweiler, R.; Scherer, C.; Schttler, D.; Massberg, S.; Orban, M. Percutaneous Decannulation Instead of Surgical Removal for Weaning After Venoarterial Extracorporeal Membrane Oxygenation—A Crossed Perclose ProGlide Closure Device Technique Using a Hemostasis Valve Y Connector. Crit. Care Explor. 2019, 6, e0018. [Google Scholar] [CrossRef] [PubMed]
- Merkle, J.; Azizov, F.; Fatullayev, J.; Weber, C.; Maier, J.; Eghbalzadeh, K.; Sabashnikov, A.; Pfister, R.; Wahlers, T.; Michels, G. Monitoring of adult patient on venoarterial extracorporeal membrane oxygenation in intensive care medicine. J. Thorac. Dis. 2019, 11, S946–S956. [Google Scholar] [CrossRef]
- Bhatia, M.; Katz, J.N. Contemporary Comprehensive Monitoring of Veno-arterial Extracorporeal Membrane Oxygenation Patients. Can. J. Cardiol. 2020, 36, 291–299. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lüsebrink, E.; Stremmel, C.; Stark, K.; Joskowiak, D.; Czermak, T.; Born, F.; Kupka, D.; Scherer, C.; Orban, M.; Petzold, T.; et al. Update on Weaning from Veno-Arterial Extracorporeal Membrane Oxygenation. J. Clin. Med. 2020, 9, 992. https://doi.org/10.3390/jcm9040992
Lüsebrink E, Stremmel C, Stark K, Joskowiak D, Czermak T, Born F, Kupka D, Scherer C, Orban M, Petzold T, et al. Update on Weaning from Veno-Arterial Extracorporeal Membrane Oxygenation. Journal of Clinical Medicine. 2020; 9(4):992. https://doi.org/10.3390/jcm9040992
Chicago/Turabian StyleLüsebrink, Enzo, Christopher Stremmel, Konstantin Stark, Dominik Joskowiak, Thomas Czermak, Frank Born, Danny Kupka, Clemens Scherer, Mathias Orban, Tobias Petzold, and et al. 2020. "Update on Weaning from Veno-Arterial Extracorporeal Membrane Oxygenation" Journal of Clinical Medicine 9, no. 4: 992. https://doi.org/10.3390/jcm9040992
APA StyleLüsebrink, E., Stremmel, C., Stark, K., Joskowiak, D., Czermak, T., Born, F., Kupka, D., Scherer, C., Orban, M., Petzold, T., von Samson-Himmelstjerna, P., Kääb, S., Hagl, C., Massberg, S., Peterss, S., & Orban, M. (2020). Update on Weaning from Veno-Arterial Extracorporeal Membrane Oxygenation. Journal of Clinical Medicine, 9(4), 992. https://doi.org/10.3390/jcm9040992





