Outcome Comparison between Low-Dose Rabbit Anti-Thymocyte Globulin and Basiliximab in Low-Risk Living Donor Kidney Transplantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Groups with Induction Agents
2.3. Baseline Characteristics
2.4. Maintenance Agent
2.5. Graft Failure and Overall Survival
2.6. PRA Screening and HLA Single Identification
2.7. CMV (cytomegalovirus) Infection Screening and Management
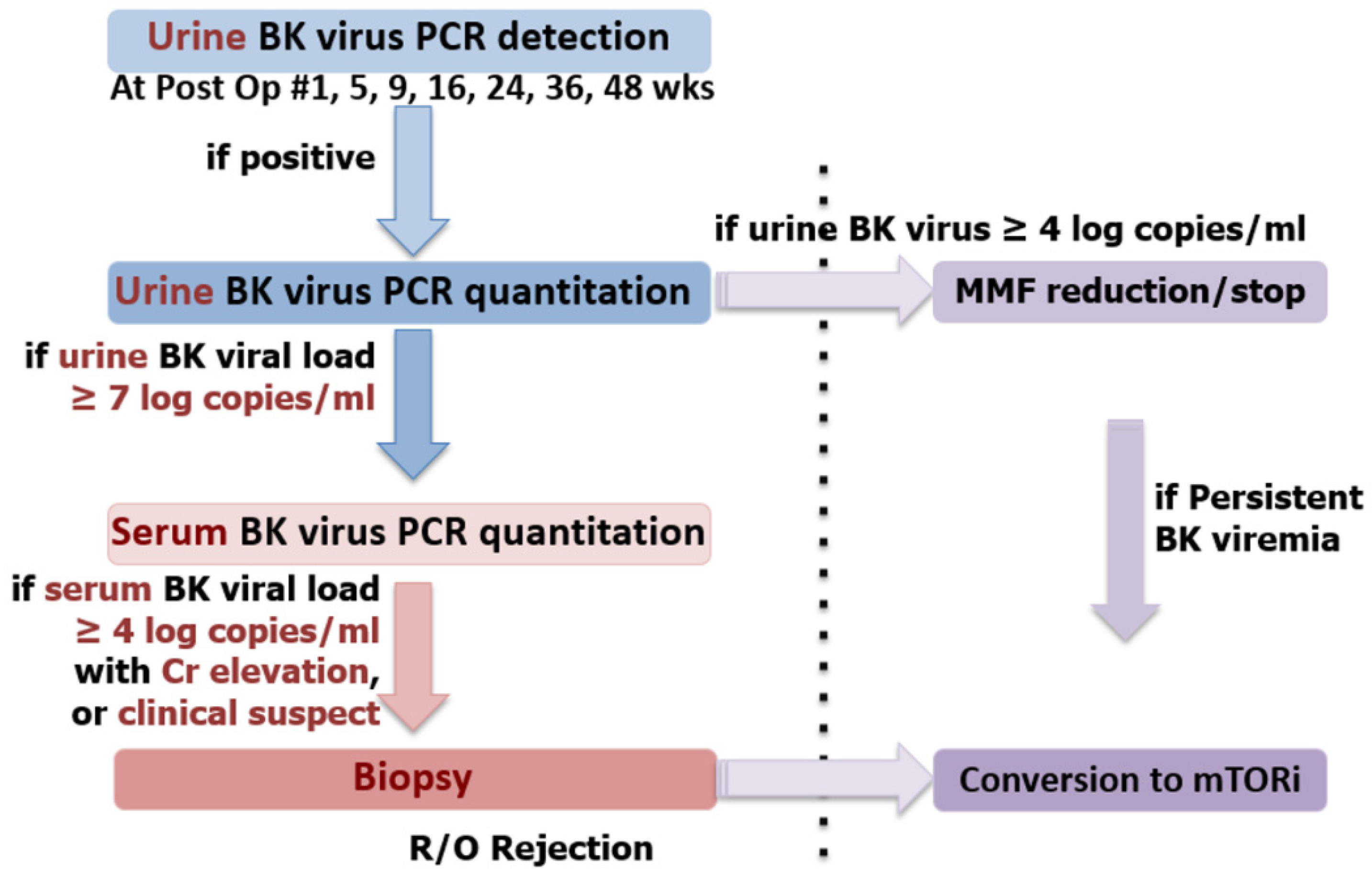
2.8. BK Polyomavirus Infection Screening and Management
2.9. Other Infections
2.10. Biopsy Proven Acute Rejection
2.11. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Graft and Patient Survival
3.3. Biopsy Proven Acute Rejection (BPAR) and De Novo DSA
3.4. Renal Function after KT
3.5. CMV, BK Virus, and Other Infections
3.6. Comparison of de novo DSA in Case of CMV, BK Viral Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hariharan, S. Long-term kidney transplant survival. Am. J. Kidney Dis. 2001, 38, S44–S50. [Google Scholar] [CrossRef] [PubMed]
- Hardinger, K.L.; Brennan, D.C.; Klein, C.L. Selection of induction therapy in kidney transplantation. Transpl. Int. 2013, 26, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.C.; Daller, J.A.; Lake, K.D.; Cibrik, D.; Del, C.D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N. Engl. J. Med. 2006, 355, 1967–1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siedlecki, A.; Irish, W.; Brennan, D.C. Delayed graft function in the kidney transplant. Am. J. Transplant. 2011, 11, 2279–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, H.H.; Kim, T.S.; Lee, S.; Song, S.; Shin, M.; Park, J.B.; Kim, J.M.; Kwon, C.H.; Joh, J.W.; Lee, S.K.; et al. Monitoring and treatment for BK virus after kidney transplantation. Transplant. Proc. 2013, 45, 2980–2983. [Google Scholar] [CrossRef]
- Azuero, A. A note on the magnitude of hazard ratios. Cancer 2016, 122, 1298–1299. [Google Scholar] [CrossRef]
- Nafar, M.; Dalili, N.; Poor-Reza-Gholi, F.; Ahmadpoor, P.; Samadian, F.; Samavat, S. The appropriate dose of thymoglobulin induction therapy in kidney transplantation. Clin. Transplant. 2017, 31, e12977. [Google Scholar] [CrossRef]
- Laftavi, M.R.; Alnimri, M.; Weber-Shrikant, E.; Kohli, R.; Said, M.; Patel, S.; Pankewycz, O. Low-dose rabbit antithymocyte globulin versus basiliximab induction therapy in low-risk renal transplant recipients: 8-year follow-up. Transplant. Proc. 2011, 43, 458–461. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.; Jeon, J.S.; Kwon, S.H.; Noh, H.; Han, D.C.; Yun, S.; Song, D. Thymoglobulin Versus Basiliximab Induction Therapy in Low-Risk Kidney Transplant Recipients: A Single-Center Experience. Transplant. Proc. 2018, 50, 1285–1288. [Google Scholar] [CrossRef]
- Lee, K.W.; Park, J.B.; Cho, C.W.; Lee, N.; Yoo, H.; Kim, K.; Park, H.; Kang, E.S.; Huh, W.; Kim, S. The Impact of Donor-Specific Anti-Human Leukocyte Antigen (HLA) Antibody Rebound on the Risk of Antibody Mediated Rejection in Sensitized Kidney Transplant Recipients. Ann. Transplant. 2017, 22, 166–176. [Google Scholar] [CrossRef]
- Zhang, R. Donor-Specific Antibodies in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2018, 13, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, C.; Gibson, I.W.; Blydt-Hansen, T.D.; Karpinski, M.; Ho, J.; Storsley, L.J.; Goldberg, A.; Birk, P.E.; Rush, D.N.; Nickerson, P.W. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am. J. Transplant. 2012, 12, 1157–1167. [Google Scholar] [CrossRef]
- Pascual, J.; Zuckermann, A.; Djamali, A.; Hertigc, A.; Naesens, M. Rabbit antithymocyte globulin and donor-specific antibodies in kidney transplantation—A review. Transplant. Rev. (Orlando) 2016, 30, 85–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, D.C.; Schnitzler, M.A. Long-Term Results of Rabbit Antithymocyte Globulin and Basiliximab Induction. N. Engl. J. Med. 2008, 359, 1736–1738. [Google Scholar] [CrossRef] [PubMed]
- Gaber, A.O.; Matas, A.J.; Henry, M.L.; Brennan, D.C.; Stevens, R.B.; Kapur, S.; Ilsley, J.N.; Kistler, K.D.; Cosimi, A.B. Antithymocyte globulin induction in living donor renal transplant recipients: Final report of the TAILOR registry. Transplantation 2012, 94, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Kotton, C.; Kumar, D.; Caliendo, A.; Asberg, A.; Chou, S.; Snydman, D.R.; Allen, U.; Humar, A. International Consensus Guidelines on the Management of Cytomegalovirus in Solid-Organ Transplantation. Transplantation 2010, 89, 779–795. [Google Scholar] [CrossRef]
- Franco, R.F.; Montenegro, R.M.; Machado, A.B.; Paris, F.; Menezes, D.S.; Manfro, R.C. Evaluation of diagnostic tests for cytomegalovirus active infection in renal transplant recipients. J. Braz. Nefrol. 2017, 39, 46–54. [Google Scholar] [CrossRef]
- Kim, D.J.; Kim, S.J.; Park, J.; Choi, G.S.; Lee, S.; Kwon, C.D.; Ki, C.; Joh, J. Real-time PCR assay compared with antigenemia assay for detecting cytomegalovirus infection in kidney transplant recipients. Transplant. Proc. 2007, 39, 1458–1460. [Google Scholar] [CrossRef]
- Rhee, J.Y.; Peck, K.R.; Lee, N.Y.; Song, J.H. Clinical usefulness of plasma quantitative polymerase chain reaction assay: Diagnosis of cytomegalovirus infection in kidney transplant recipients. Transplant. Proc. 2011, 43, 2624–2629. [Google Scholar] [CrossRef]
- Kim, C.K.; Song, J.H.; Kim, S.M.; Peck, K.R.; Oh, W.; Huh, W.; Kim, Y.G.; Kim, S.J.; Joh, J.W.; Lee, N.Y.; et al. Clinical usefulness of human cytomegalovirus antigenemia assay after kidney transplantation. Transplantation 2003, 75, 2151–2155. [Google Scholar] [CrossRef] [Green Version]
- Jung, G.O.; Kim, S.J.; Choi, G.S.; Moon, J.I.; Kim, J.M.; Sin, M.J.; Kim, E.Y.; Kwon, C.H.; Joh, J.W.; Lee, S.K. The effect of cytomegalovirus antigenemia titer on the efficacy of preemptive therapy for the prevention of cytomegalovirus disease after kidney transplantation. Transplant. Proc. 2010, 42, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Humar, A.; Lebranchu, Y.; Vincenti, F.; Blumberg, E.A.; Punch, J.D.; Limaye, A.P.; Abramowicz, D.; Jardine, A.G.; Voulgari, A.T.; Ives, J.; et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am. J. Transplant. 2010, 10, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Sawinski, D.; Goral, S. BK virus infection: An update on diagnosis and treatment. Nephrol. Dial. Transplant. 2015, 30, 209–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dharnidharka, V.R.; Cherikh, W.S.; Abbott, K.C. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation 2009, 87, 1019–1026. [Google Scholar] [CrossRef]
- Scurt, F.G.; Ewert, L.; Mertens, P.R.; Haller, H.; Schmidt, B.M.W.; Chatzikyrkou, C. Clinical outcomes after ABO-incompatible renal transplantation: A systematic review and meta-analysis. Lancet 2019, 393, 2059–2072. [Google Scholar] [CrossRef]
- Rivera-Sanchez, R.; Delgado-Ochoa, D.; Flores-Paz, R.R.; García-Jiménez, E.E.; Espinosa-Hernández, R.; Bazan-Borges, A.A.; Arriaga-Alba, M. Prospective study of urinary tract infection surveillance after kidney transplantation. BMC Infect. Dis. 2010, 10, 245. [Google Scholar] [CrossRef] [Green Version]
- Chuang, P.; Parikh, C.R.; Langone, A. Urinary tract infections after renal transplantation: A retrospective review at two US transplant centers. Clin. Transplant. 2005, 19, 230–235. [Google Scholar] [CrossRef]
- Patlolla, V.; Zhong, X.; Reed, G.W.; Mandelbrot, D.A. Efficacy of anti-IL-2 receptor antibodies compared to no induction and to antilymphocyte antibodies in renal transplantation. Am. J. Transplant. 2007, 7, 1832–1842. [Google Scholar] [CrossRef]
- Sheashaa, H.A.; Bakr, M.A.; Ismail, A.M.; Gheith, O.E.; El-dahshan, K.F.; Sobh, M.A.; Ghoneim, M.A. Long-term evaluation of basiliximab induction therapy in live donor kidney transplantation: A five-year prospective randomized study. Am. J. Nephrol. 2005, 25, 221–225. [Google Scholar] [CrossRef]
- Hellemans, R.; Hazzan, M.; Durand, D.; Mourad, G.; Lang, P.; Kessler, M.; Charpentier, B.; Touchard, G.; Berthoux, F.; Merville, P.; et al. Daclizumab Versus Rabbit Antithymocyte Globulin in High-Risk Renal Transplants: Five-Year Follow-up of a Randomized Study. Am. J. Transplant. 2015, 15, 1923–1932. [Google Scholar] [CrossRef] [Green Version]
- Ciancio, G.; Burke, G.W.; Gaynor, J.J.; Carreno, M.R.; Cirocco, R.E.; Mathew, J.M.; Mattiazzi, A.; Cordovilla, T.; Roth, D.; Kupin, W.; et al. A randomized trial of three renal transplant induction antibodies: Early comparison of tacrolimus, mycophenolate mofetil, and steroid dosing, and newer immune-monitoring. Transplantation 2005, 80, 457–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willoughby, L.M.; Schnitzler, M.A.; Brennan, D.C.; Pinsky, B.W.; Dzebisashvili, N.; Buchanan, P.M.; Neri, L.; Rocca-Rey, L.A.; Abbott, K.C.; Lentine, K.L. Early outcomes of thymoglobulin and basiliximab induction in kidney transplantation: Application of statistical approaches to reduce bias in observational comparisons. Transplantation 2009, 87, 1520–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodle, E.S.; First, M.R.; Pirsch, J.; Shihab, F.; Gaber, A.O.; Van Veldhuisen, P. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann. Surg. 2008, 248, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Grafals, M.; Smith, B.; Murakami, N.; Trabucco, A.; Hamill, K.; Marangos, E.; Gilligan, H.; Pomfret, E.A.; Pomposelli, J.J.; Simpson, M.A.; et al. Immunophenotyping and efficacy of low dose ATG in non-sensitized kidney recipients undergoing early steroid withdrawal: A randomized pilot study. PLoS ONE 2014, 9, e104408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomusch, O.; Wiesener, M.; Opgenoorth, M.; Pascher, A.; Woitas, R.P.; Witzke, O.; Jaenigen, B.; Rentsch, M.; Wolters, H.; Rath, T.; et al. Rabbit-ATG or basiliximab induction for rapid steroid withdrawal after renal transplantation (Harmony): An open-label, multicentre, randomised controlled trial. Lancet 2016, 388, 3006–3016. [Google Scholar] [CrossRef]

| Time (years) | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|---|
| Basiliximab | survival rate | 1 | 0.996 | 0.987 | 0.987 | 0.977 | 0.964 | 0.938 | 0.92 |
| # at risk | 231 | 229 | 223 | 207 | 179 | 140 | 92 | 43 | |
| r-ATG | survival rate | 1 | 1 | 1 | 1 | 0.971 | 0.971 | 0.971 | 0.971 |
| # at risk | 37 | 36 | 36 | 34 | 30 | 16 | 5 | 2 |


| Total (n = 268) | Low Dose r-ATG (n = 37) | Basiliximab (n = 231) | p-Value | |
|---|---|---|---|---|
| Recipient | ||||
| Age (yr), median (range) | 47 (19–72) | 46 (19–68) | 47 (20–72) | 0.435 |
| Sex (M/F) | 172/96 | 23/14 | 149/82 | 0.783 |
| BMI (kg/m2) | 22.5 | 22.7 | 22.4 | 0.620 |
| DM (%) | 69 (25.8) | 9 (24.3) | 60 (22.4) | 0.831 |
| HLA Class I MM, median (range) | 2 (0–4) | 2 (0–4) | 2 (0–4) | 0.176 |
| HLA Class II MM, median (range) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.593 |
| CMV status (%) | ||||
| Donor +/Recipient + | 254 (94.8) | 35 (94.6) | 219 (94.8) | 0.974 |
| Donor +/Recipient - | 6 (2.2) | 1 (2.7) | 5 (2.2) | |
| Donor -/Recipient + | 8 (3.0) | 1 (2.7) | 7 (3.0) | |
| Dialysis duration (months), median (range) | 7.5 (0–637) | 7.6 (0–637) | 7.3 (0–547) | 0.981 |
| Cause of ESRD | 0.760 | |||
| DM (%) | 61 (22.3) | 9 (24.3) | 52 (22.5) | |
| GN (%) | 88 (32.8) | 14 (37.8) | 74 (32.0) | |
| PCKD (%) | 9 (3.4) | 1 (2.7) | 8 (3.5) | |
| HTN (%) | 39 (14.6) | 5 (13.5) | 34 (14.7) | |
| Other (%) | 14 (5.2) | 3 (8.1) | 11 (4.8) | |
| Unknown (%) | 57 (21.3) | 5 (13.5) | 52 (22.5) | |
| PRA > 50% | 6 (2.3) | 2 (5.7) | 4 (1.8) | 0.182 |
| Donor | ||||
| Age (yr), median (range) | 44 (18–80) | 47 (19–66) | 43 (18–80) | 0.095 |
| Sex (M/F) | 133/135 | 21/16 | 112/119 | 0.350 |
| Cr (mg/dL), mean | 0.83 | 0.92 | 0.82 | 0.266 |
| WIT, mean (minutes) † | 3.0 | 3.49 | 2.94 | 0.011 |
| CIT, mean (minutes) ‡ | 90.1 | 106.1 | 87.3 | 0.015 |
| Total (n = 268) | Low Dose r-ATG (n = 37) | Basiliximab (n = 231) | p-Value | |
|---|---|---|---|---|
| DGF (%) | 2 (0.7) | 0 | 2 (0.9) | 0.898 |
| Graft failure (%) | 12 (4.5) | 1 (2.7) | 11 (4.8) | 0.737 |
| Patient death (%) | 3 (1.1) | 0 | 3 (1.3) | 0.657 |
| Total BPAR (%) | 125 (46.6) | 19 (51.4) | 106 (45.9) | 0.335 |
| Clinical BPAR | ||||
| TCMR (%) | 53 (19.8) | 4 (10.8) | 49 (21.2) * | 0.355 |
| Borderline change (%) | 29 (10.8) | 9 (24.3) | 20 (8.7) | |
| Subclinical BPAR † | ||||
| TCMR (%) | 11 (4.1) | 2 (5.4) * | 9 (3.9) * | |
| Borderline change (%) | 32 (11.9) | 4 (10.8) | 28 (12.1) | |
| No rejection | 143 (53.4) | 18 (48.6) | 125 (54.1) | |
| de novo DSA (total n = 245) ‡ | 10 (4.1) | 5 (14.3) | 5 (2.4) | 0.004 |
| e-GFR, mean (mL/min/1.73m2) | 0.120 | |||
| 1 month | 68.5 | 68.2 | 68.6 | |
| 1 year | 62.2 | 63.0 | 62.1 | |
| 2 years | 67.1 | 66.5 | 67.1 | |
| 3 years | 66.0 | 65.9 | 66.0 | |
| 5 years | 64.7 | 63.0 | 64.9 |
| Univariate HR (95% CI) | p-Value | Multivariate † HR (95% CI) | p-Value | |
|---|---|---|---|---|
| Induction therapy: r-ATG/Basiliximab | 0.70 (0.09–5.49) | 0.737 | 0.64 (0.08–5.03) | 0.670 |
| Recipient age | 1.00 (0.95–1.05) | 0.903 | ||
| BMI | 1.08 (0.91–1.28) | 0.403 | ||
| sex: male/female | 1.66 (0.45–6.12) | 0.450 | ||
| DM | 1.86 (0.56–6.22) | 0.315 | 1.48 (0.44–4.98) | 0.525 |
| HLA 1 mismatch | 1.22 (0.74–2.00) | 0.442 | ||
| HLA 2 mismatch | 1.77 (0.75–4.18) | 0.192 | 1.40(0.57–3.42) | 0.462 |
| WIT | 0.95 (0.58–1.58) | 0.854 | ||
| CIT | 0.99 (0.97–1.01) | 0.375 | ||
| Donor age | 0.97 (0.93–1.02) | 0.267 | ||
| creatinine | 1.58 (0.22–10.0) | 0.686 | ||
| sex: male/female | 1.42 (0.45–4.48) | 0.552 | ||
| CMV antigenemia > 50/400,000 | 0.04 (0.00–639) | 0.523 | ||
| BKV viremia | 10.91 (0.20–4.17) | 0.908 | ||
| BPAR | 6.72 (1.47–30.8) | 0.014 | 5.89 (1.25–27.8) | 0.025 |
| Univariate HR (95% CI) | p-Value | Multivariate HR (95% CI) | p-Value | ||
|---|---|---|---|---|---|
| Biopsy proven Acute Rejection | Induction therapy: r-ATG/Basiliximab | 1.27 (0.78–2.08) | 0.335 | 1.16 (0.69–1.96) | 0.585 |
| Recipient age | 0.99 (0.98–1.01) | 0.423 | |||
| BMI | 1.02 (0.97–1.08) | 0.420 | |||
| sex: male/female | 1.39 (0.95–2.03) | 0.091 | 1.33 (0.88–1.99) | 0.172 | |
| DM | 1.29 (0.88–1.90) | 0.196 | |||
| HLA 1 mismatch | 1.17 (1.00–1.37) | 0.045 | 1.01 (0.83–1.24) | 0.896 | |
| HLA 2 mismatch | 1.71 (1.31–2.23) | <0.001 | 1.74 (1.24–2.44) | 0.001 | |
| PRA ≥ 50% | 0.28 (0.04–1.98) | 0.201 | |||
| WIT | 1.12 (0.98–1.29) | 0.091 | 1.11 (0.97–1.27) | 0.149 | |
| CIT | 1.00 (1.00–1.01) | 0.199 | |||
| Donor age | 1.01 (1.00–1.03) | 0.105 | |||
| creatinine | 1.14 (0.52–2.51) | 0.749 | |||
| sex: male/female | 0.75 (0.53–1.06) | 0.104 | |||
| de novo DSA † | Induction therapy: r-ATG/Basiliximab | 6.83 (1.87–25.0) | 0.004 | 6.78 (1.80–25.5) | 0.005 |
| Recipient age | 0.97 (0.93–1.03) | 0.313 | |||
| BMI | 0.99 (0.81–1.21) | 0.925 | |||
| sex: male/female | 0.36 (0.10–1.30) | 0.119 | |||
| DM | 0.29 (0.04–2.34) | 0.246 | |||
| HLA 1 mismatch | 1.24 (0.70–2.22) | 0.461 | |||
| HLA 2 mismatch | 2.91 (1.06–8.00) | 0.038 | 2.72 (1.02–7.29) | 0.046 | |
| WIT | 1.06 (0.64–1.75) | 0.822 | |||
| CIT | 1.08 (1.00–1.02) | 0.147 | |||
| Donor age | 0.99 (0.94–1.04) | 0.720 | |||
| creatinine | 2.19 (0.40–12.2) | 0.369 | |||
| sex: male/female | 1.59 (0.44–5.79) | 0.480 |
| Univariate Beta Coefficient (Standard Error) | p-Value | Multivariate Beta Coefficient (Standard Error) | p-Value | |
|---|---|---|---|---|
| Induction therapy (time adjusted): r-ATG/Basiliximab | 0.100 | 0.120 | ||
| Recipient age | 0.05 (0.07) | 0.505 | ||
| BMI | −0.45 (0.25) | 0.078 | −0.31 (0.20) | 0.124 |
| sex: male/female | −0.59 (1.85) | 0.749 | ||
| DM | 0.77 (1.83) | 0.672 | ||
| Dialysis duration | 1.36 (0.60) | 0.036 | 1.20 (0.50) | 0.017 |
| HLA 1 mismatch | −0.81 (0.78) | 0.302 | ||
| HLA 2 mismatch | 0.14 (1.29) | 0.910 | ||
| PRA ≥ 50% | −1.92 (4.53) | 0.671 | ||
| Donor age | −0.58 (0.06) | <0.001 | −0.57 (0.06) | <0.001 |
| creatinine | −2.93 (3.00) | 0.328 | ||
| sex: male/female | 5.86 (1.70) | 0.001 | 1.04 (1.47) | 0.478 |
| Total (n = 268) | Low Dose r-ATG (n = 37) | Basiliximab (n = 231) | p-Value | |
|---|---|---|---|---|
| CMV antigenemia | 147 (54.9) | 27 (73.0) | 120 (51.9) | 0.032 † |
| CMV antigen > 50/400,000 WBCs | 21 (7.8) | 6 (16.2) | 15 (6.5) | 0.049 |
| BK viremia | 50 (18.7) | 14 (37.8) | 36 (15.6) | 0.002 † |
| Viral pneumonia (%) | 7 (2.6) | 0 | 7 (3.0) | 0.538 |
| Bacterial infection (%) | 72 (26.9) | 12 (32.4) | 61 (26.4) | 0.446 |
| Fungal infection (%) | 3 (1.1) | 0 | 3 (1.3%) | 0.928 |
| Tuberculosis infection (%) | 4 (1.5) | 1 (2.7) | 3 (1.3) | 0.524 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.J.; Rhu, J.; Yoo, H.; Kim, K.; Lee, K.W.; Park, J.B. Outcome Comparison between Low-Dose Rabbit Anti-Thymocyte Globulin and Basiliximab in Low-Risk Living Donor Kidney Transplantation. J. Clin. Med. 2020, 9, 1320. https://doi.org/10.3390/jcm9051320
Kim SJ, Rhu J, Yoo H, Kim K, Lee KW, Park JB. Outcome Comparison between Low-Dose Rabbit Anti-Thymocyte Globulin and Basiliximab in Low-Risk Living Donor Kidney Transplantation. Journal of Clinical Medicine. 2020; 9(5):1320. https://doi.org/10.3390/jcm9051320
Chicago/Turabian StyleKim, Sang Jin, Jinsoo Rhu, Heejin Yoo, Kyunga Kim, Kyo Won Lee, and Jae Berm Park. 2020. "Outcome Comparison between Low-Dose Rabbit Anti-Thymocyte Globulin and Basiliximab in Low-Risk Living Donor Kidney Transplantation" Journal of Clinical Medicine 9, no. 5: 1320. https://doi.org/10.3390/jcm9051320
APA StyleKim, S. J., Rhu, J., Yoo, H., Kim, K., Lee, K. W., & Park, J. B. (2020). Outcome Comparison between Low-Dose Rabbit Anti-Thymocyte Globulin and Basiliximab in Low-Risk Living Donor Kidney Transplantation. Journal of Clinical Medicine, 9(5), 1320. https://doi.org/10.3390/jcm9051320





