Prognostic Value of Growth Differentiation Factor 15 in Kidney Donors and Recipients
Abstract
1. Introduction
2. Experimental Section
2.1. GDF15
2.2. Study Population
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
3.1. GDF15
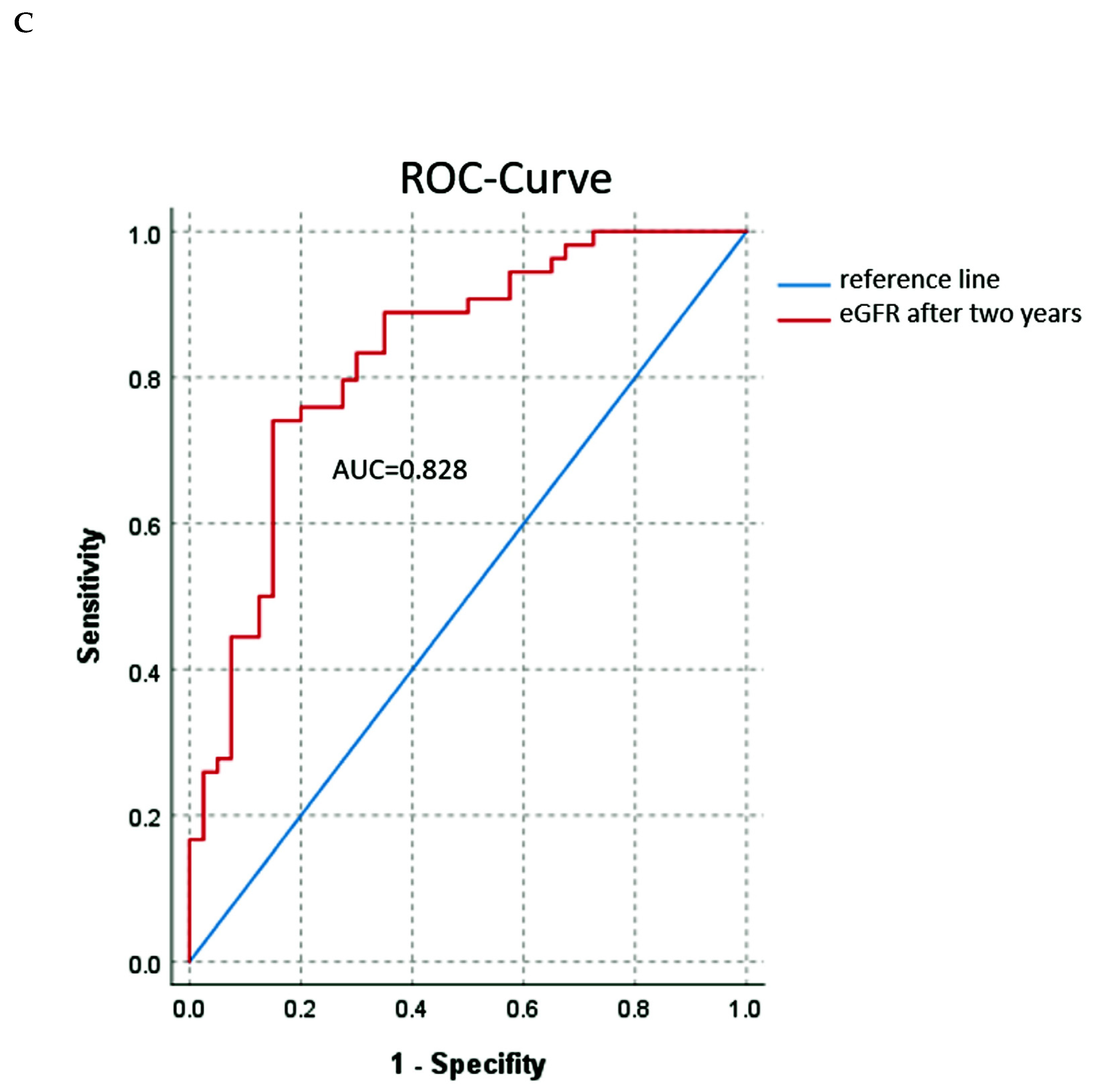
3.2. Renal Function
3.3. Rejection Episodes
3.4. Tacrolimus Trough Levels and Trough Concentration/Daily Dose (C/D) Ratio
3.5. Cardiovascular Disease (CVD)
3.6. Cytomegalovirus and BK Polyomavirus (BKPyV)
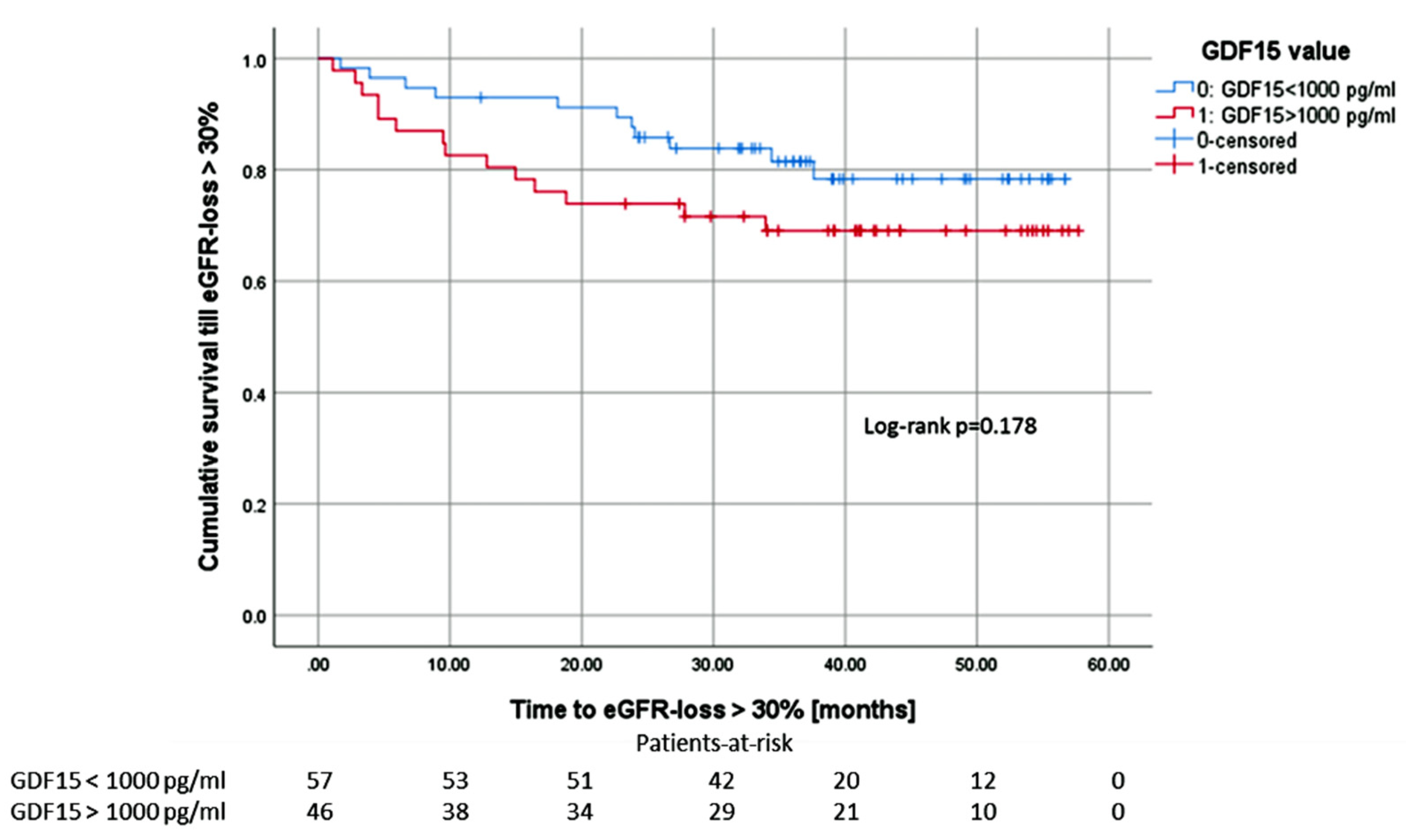
3.7. Overall Graft Survival
3.8. Adjustment
3.9. Multivariable Linear Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgment
Conflicts of Interest
References
- Bootcov, M.R.; Bauskin, A.R.; Valenzuela, S.M.; Moore, A.G.; Bansal, M.; He, X.Y.; Zhang, H.P.; Donnellan, M.; Mahler, S.; Pryor, K.; et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc. Natl. Acad. Sci. USA 1997, 94, 11514–11519. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Lu, L.; Liu, L. Growth differentiation factor-15 and the risk of cardiovascular diseases and all-cause mortality: A meta-analysis of prospective studies. Clin. Cardiol. 2019, 42, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.N.; Carrero, J.J.; Tsai, V.W.-W.; Yagoutifam, N.; Luo, W.; Kuffner, T.; Bauskin, A.R.; Wu, L.; Jiang, L.; Barany, P.; et al. Macrophage inhibitory cytokine-1 (MIC-1/GDF15) and mortality in end-stage renal disease. Nephrol. Dial. Transpl. 2012, 27, 70–75. [Google Scholar] [CrossRef] [PubMed]
- You, A.S.; Kalantar-Zadeh, K.; Lerner, L.; Nakata, T.; Lopez, N.; Lou, L.; Veliz, M.; Soohoo, M.; Jing, J.; Zaldivar, F.; et al. Association of growth differentiation factor 15 with mortality in a prospective hemodialysis cohort. Cardiorenal Med. 2017, 7, 158–168. [Google Scholar] [CrossRef]
- Tuegel, C.; Katz, R.; Alam, M.; Bhat, Z.; Bellovich, K.; de Boer, I.; Brosius, F.; Gadegbeku, C.; Gipson, D.; Hawkins, J.; et al. GDF-15, Galectin 3, Soluble ST2, and risk of mortality and cardiovascular events in CKD. Am. J. Kidney Dis. 2018, 72, 519–528. [Google Scholar] [CrossRef]
- Wollert, K.C.; Kempf, T.; Wallentin, L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin. Chem. 2017, 63, 140–151. [Google Scholar] [CrossRef]
- Corre, J.; Hébraud, B.; Bourin, P. Concise review: Growth differentiation factor 15 in pathology: A clinical role. Stem Cells Transl. Med. 2013, 2, 946–952. [Google Scholar] [CrossRef]
- Luan, H.H.; Wang, A.; Hilliard, B.K.; Carvalho, F.; Rosen, C.E.; Ahasic, A.M.; Herzog, E.L.; Kang, I.; Pisani, M.A.; Yu, S.; et al. GDF15 is an inflammation-induced central mediator of tissue tolerance. Cell 2019, 178, 1231–1244. [Google Scholar] [CrossRef]
- Mazagova, M.; Buikema, H.; van Buiten, A.; Duin, M.; Goris, M.; Sandovici, M.; Henning, R.H.; Deelman, L.E. Genetic deletion of growth differentiation factor 15 augments renal damage in both type 1 and type 2 models of diabetes. Am. J. Physiol. Renal Physiol. 2013, 305, F1249–F1264. [Google Scholar] [CrossRef]
- Zimmers, T.A.; Jin, X.; Hsiao, E.C.; McGrath, S.A.; Esquela, A.F.; Koniaris, L.G. Growth differentiation factor-15/macrophage inhibitory cytokine-1 induction after kidney and lung injury. Shock 2005, 23, 543–548. [Google Scholar]
- Liu, J.; Kumar, S.; Heinzel, A.; Gao, M.; Guo, J.; Alvarado, G.F.; Reindl-Schwaighofer, R.; Krautzberger, A.M.; Cippà, P.E.; McMahon, J.; et al. Renoprotective and Immunomodulatory Effects of GDF15 following AKI Invoked by Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Robinson-Cohen, C.; Smith, M.R.; Bellovich, K.A.; Bhat, Z.Y.; Bobadilla, M.; Brosius, F.; de Boer, I.H.; Essioux, L.; Formentini, I.; et al. Growth differentiation factor-15 and risk of CKD progression. J. Am. Soc. Nephrol. 2017, 28, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Connelly, P.W.; Yan, A.T.; Nash, M.M.; Lok, C.E.; Gunaratnam, L.; Prasad, G.V.R. Growth differentiation factor 15 is decreased by kidney transplantation. Clin. Biochem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Malyszko, J.; Koc-Zorawska, E.; Malyszko, J.S.; Glowinska, I.; Mysliwiec, M.; Macdougall, I.C. GDF15 is related to anemia and hepcidin in kidney allograft recipients. Nephron Clin. Pract. 2013, 123, 112–117. [Google Scholar] [CrossRef]
- Thorsteinsdottir, H.; Salvador, C.L.; Mjøen, G.; Lie, A.; Sugulle, M.; Tøndel, C.; Brun, A.; Almaas, R.; Bjerre, A. Growth differentiation factor 15 in children with chronic kidney disease and after renal transplantation. Dis. Markers 2020, 2020, 6162892. [Google Scholar] [CrossRef]
- Loupy, A.; Haas, M.; Solez, K.; Racusen, L.; Glotz, D.; Seron, D.; Nankivell, B.J.; Colvin, R.B.; Afrouzian, M.; Akalin, E.; et al. The Banff 2015 kidney meeting report: Current challenges in rejection classification and prospects for adopting molecular pathology. Am. J. Transpl. 2017, 17, 28–41. [Google Scholar] [CrossRef]
- Jehn, U.; Schütte-Nütgen, K.; Bautz, J.; Pavenstädt, H.; Suwelack, B.; Thölking, G.; Heinzow, H.; Reuter, S. Cytomegalovirus viremia after living and deceased donation in kidney transplantation. J. Clin. Med. 2020, 9. [Google Scholar] [CrossRef]
- Levey, A.S.; Inker, L.A.; Matsushita, K.; Greene, T.; Willis, K.; Lewis, E.; de Zeeuw, D.; Cheung, A.K.; Coresh, J. GFR decline as an end point for clinical trials in CKD: A scientific workshop sponsored by the national kidney foundation and the US food and drug administration. Am. J. Kidney Dis. 2014, 64, 821–835. [Google Scholar] [CrossRef]
- Mol, P.G.M.; Maciulaitis, R.; Vetter, T. GFR decline as an end point for clinical trials in CKD: A view from Europe. Am. J. Kidney Dis. 2014, 64, 838–840. [Google Scholar] [CrossRef]
- Ho, J.E.; Hwang, S.-J.; Wollert, K.C.; Larson, M.G.; Cheng, S.; Kempf, T.; Vasan, R.S.; Januzzi, J.L.; Wang, T.J.; Fox, C.S. Biomarkers of cardiovascular stress and incident chronic kidney disease. Clin. Chem. 2013, 59, 1613–1620. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, S.; Won, C.W.; Jeong, K.H. Association between plasma levels of growth differentiation factor-15 and renal function in the elderly: Korean frailty and aging cohort study. Kidney Blood Press. Res. 2019, 44, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Rauchman, M.; Griggs, D. Emerging strategies to disrupt the central TGF-β axis in kidney fibrosis. Transl. Res. 2019, 209, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.; Gongora, E. Correlations of GDF-15 with sST2, MMPs, and worsening functional capacity in idiopathic dilated cardiomyopathy: Can we gain new insights into the pathophysiology. J. Circ. Biomark. 2018, 7. [Google Scholar] [CrossRef]
- Arif, E.; Solanki, A.K.; Srivastava, P.; Rahman, B.; Tash, B.R.; Holzman, L.B.; Janech, M.G.; Martin, R.; Knölker, H.-J.; Fitzgibbon, W.R.; et al. The motor protein Myo1c regulates transforming growth factor-β-signaling and fibrosis in podocytes. Kidney Int. 2019, 96, 139–158. [Google Scholar] [CrossRef]
- Kim, Y.-I.; Shin, H.-W.; Chun, Y.-S.; Park, J.-W. CST3 and GDF15 ameliorate renal fibrosis by inhibiting fibroblast growth and activation. Biochem. Biophys. Res. Commun. 2018, 500, 288–295. [Google Scholar] [CrossRef]
- Berezin, A.E. Diabetes mellitus related biomarker: The predictive role of growth-differentiation factor-15. Diabetes Metab. Syndr. 2016, 10, S154–S157. [Google Scholar] [CrossRef]
- Mueller, T.F.; Luyckx, V.A. The natural history of residual renal function in transplant donors. J. Am. Soc. Nephrol. 2012, 23, 1462–1466. [Google Scholar] [CrossRef]
- de Seigneux, S.; Ponte, B.; Berchtold, L.; Hadaya, K.; Martin, P.-Y.; Pasch, A. Living kidney donation does not adversely affect serum calcification propensity and markers of vascular stiffness. Transpl. Int. 2015, 28, 1074–1080. [Google Scholar] [CrossRef]
- Melvin, A.; Lacerda, E.; Dockrell, H.M.; O'Rahilly, S.; Nacul, L. Circulating levels of GDF15 in patients with myalgic encephalomyelitis/chronic fatigue syndrome. J. Transl. Med. 2019, 17, 409. [Google Scholar] [CrossRef]
- Suwelack, B.; Wörmann, V.; Berger, K.; Gerß, J.; Wolters, H.; Vitinius, F.; Burgmer, M. Investigation of the physical and psychosocial outcomes after living kidney donation - a multicenter cohort study (SoLKiD - Safety of Living Kidney Donors). BMC Nephrol. 2018, 19, 83. [Google Scholar] [CrossRef] [PubMed]




| All (n = 104) | Living (n = 55) | Cadaveric (n = 49) | p-value | |
|---|---|---|---|---|
| Age (years, mean ± SD) | 50.39 (15.83) | 42.9 (15.2) | 58.8 (12.0) | <0.001 a |
| Male sex, n (%) | 67 (64.4) | 34 (61.8) | 33 (67.3) | 0.682 b |
| Diagnosis of ESRD, n (%) | 0.766 b | |||
| Hypertension | 13 (12.5) | 7 (12.7) | 6 (12.2) | |
| Diabetes | 3 (2.9) | 0 (0) | 3 (6.1) | |
| Polycystic kidney disease | 14 (13.5) | 6 (10.9) | 8 (16.3) | |
| Obstructive Nephropathy | 5 (4.8) | 3 (5.4) | 2 (4.1) | |
| Glomerulonephritis | 34 (32.7) | 20 (36.4) | 14 (28.6) | |
| FSGS | 7 (6.7) | 5 (9.1) | 2 (4.1) | |
| Interstitial Nephritis | 3 (2.9) | 1 (1.8) | 2 (4.1) | |
| Vasculitis | 3 (2.9) | 2 (3.6) | 1 (2) | |
| Other | 22 (21.2) | 11 (20) | 11 (22.4) | |
| Mode of dialysis, n (%) | 0.009 b | |||
| HD | 75 (72.1) | 39 (70.9) | 36 (73.5) | |
| PD | 12 (11.6) | 6 (10.9) | 6 (12.2) | |
| Both | 9 (8.7) | 2 (3.6) | 7 (14.3) | |
| Preemptive transplantation | 8 (7.7) | 8 (14.5) | 0 (0) | |
| Dialysis vintage (months, median (1st, 3rd quartile)) | 26.8 (11.41, 80.95) | 12.3 (0.75, 25.6) | 79.2 (38.0, 104.6) | <0.001 a |
| European senior program (ESP) | 17 (16.3) | 0 (0) | 17 (34.7) | <0.001 b |
| ≥1 prior kidney transplant, n (%) | 14 (13.4) | 8 (14.5) | 6 (12.2) | 0.882 b |
| HLA mismatch, n (%) | 0.088 a | |||
| 0–3 | 62 (59.6) | 32 (58.2) | 30 (61.2) | |
| 4–6 | 41 (39.4) | 22 (40.0) | 19 (38.8) | |
| Current PRA, n (%) | 0.419 a | |||
| 0–20% | 76 (73.1) | 41 (74.5) | 35 (69.4) | |
| >20% | 28 (26.9) | 14 (25.5) | 14 (30.6) | |
| Induction therapy, n (%) | <0.001 b | |||
| Basiliximab | 78 (75) | 37 (67.3) | 41 (83.7) | |
| Thymoglobulin | 6 (5.8) | 0 (0) | 6 (12.2) | |
| Basiliximab + Thymoglobulin | 1 (0.9) | 0 (0) | 1 (2.0) | |
| Rituximab + Thymoglobulin | 1 (0.9) | 1 (1.8) | 0 (0) | |
| Rituximab | 16 (15.4) | 16 (29.2) | 0 (0) | |
| Eculizumab + Basiliximab | 2 (2.9) | 1 (1.8) | 1 (2.0) | |
| Cold ischemia time (hours, mean ± SD) | 6.03 (4.4) | 2.52 (0.55) | 9.9 (3.4) | <0.001 a |
| Warm ischemia time (min, mean ± SD) | 34.6 (7.6) | 34 (7.9) | 35.4 (7.3) | 0.200 a |
| AB0i | 17 (16.3) | 17 (30.9) | 0 (0) | <0.001 b |
| All (n = 104) | Living (n = 55) | Cadaveric (n = 49) | p-value | |
|---|---|---|---|---|
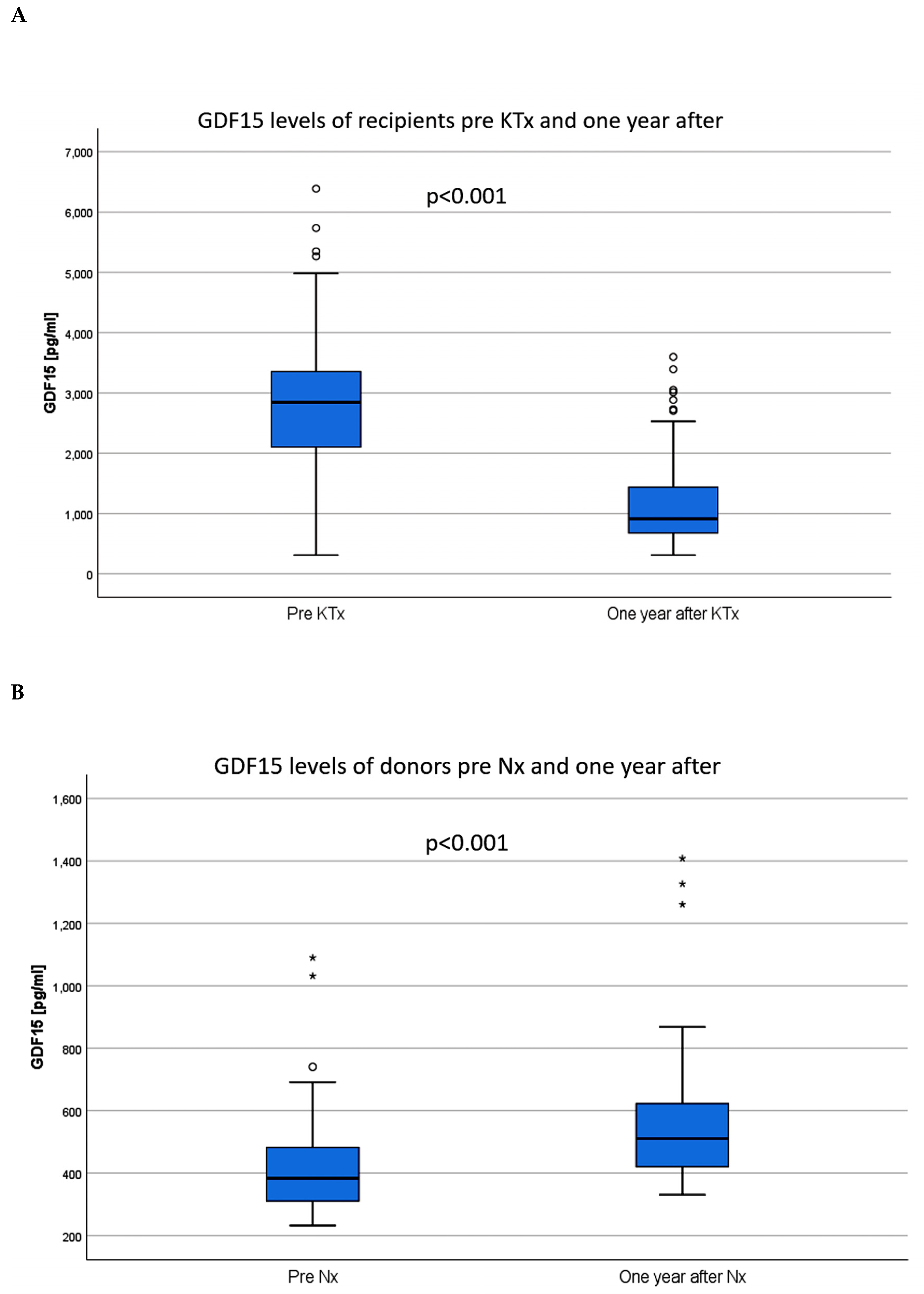
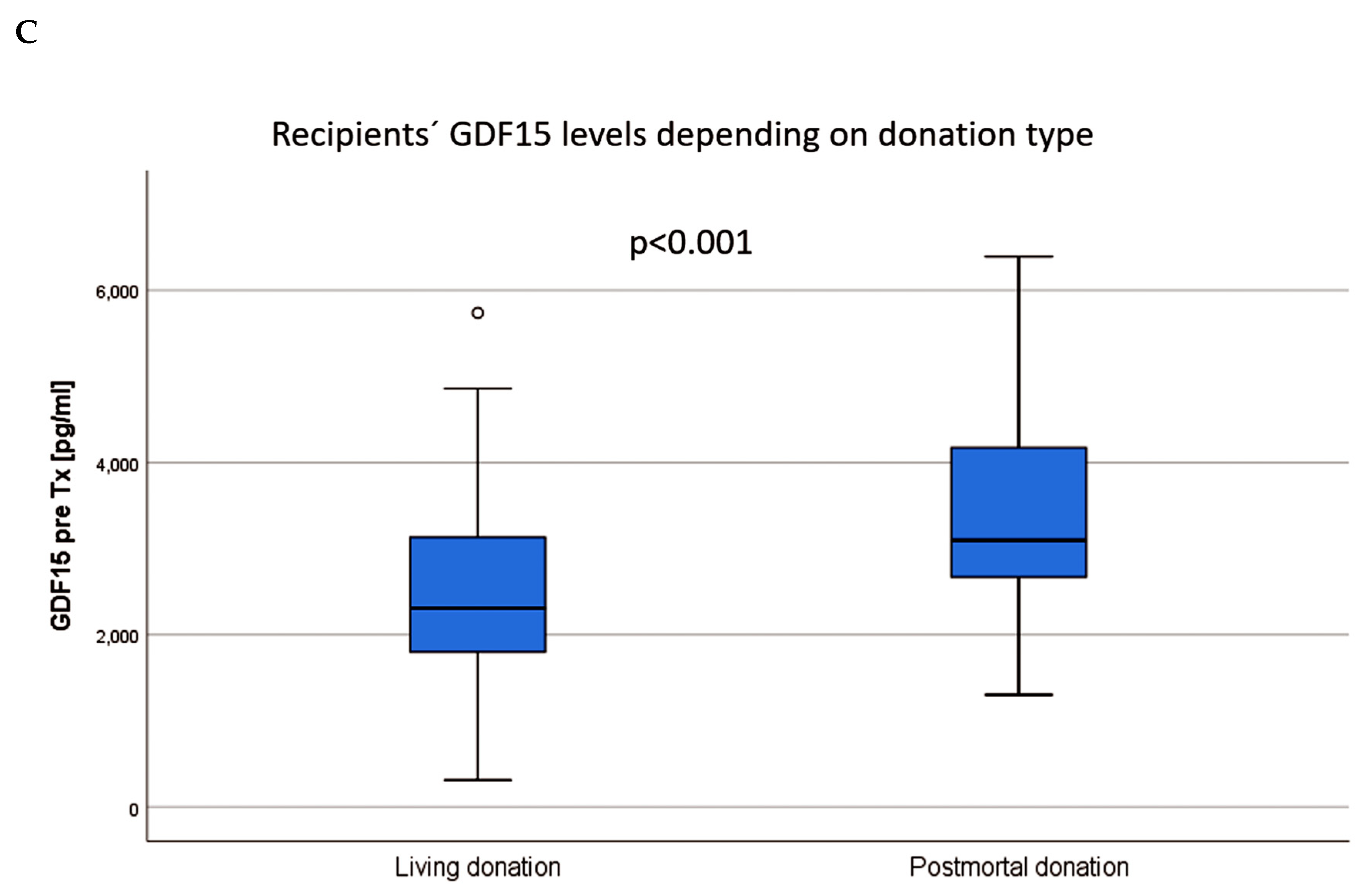
| GDF15 pre KTx (median (1st, 3rd quartile) | 2,844 (2087, 3,361) | 2,299 (1763, 3,137) | 3,096 (2,631, 4,223) | <0.001 a |
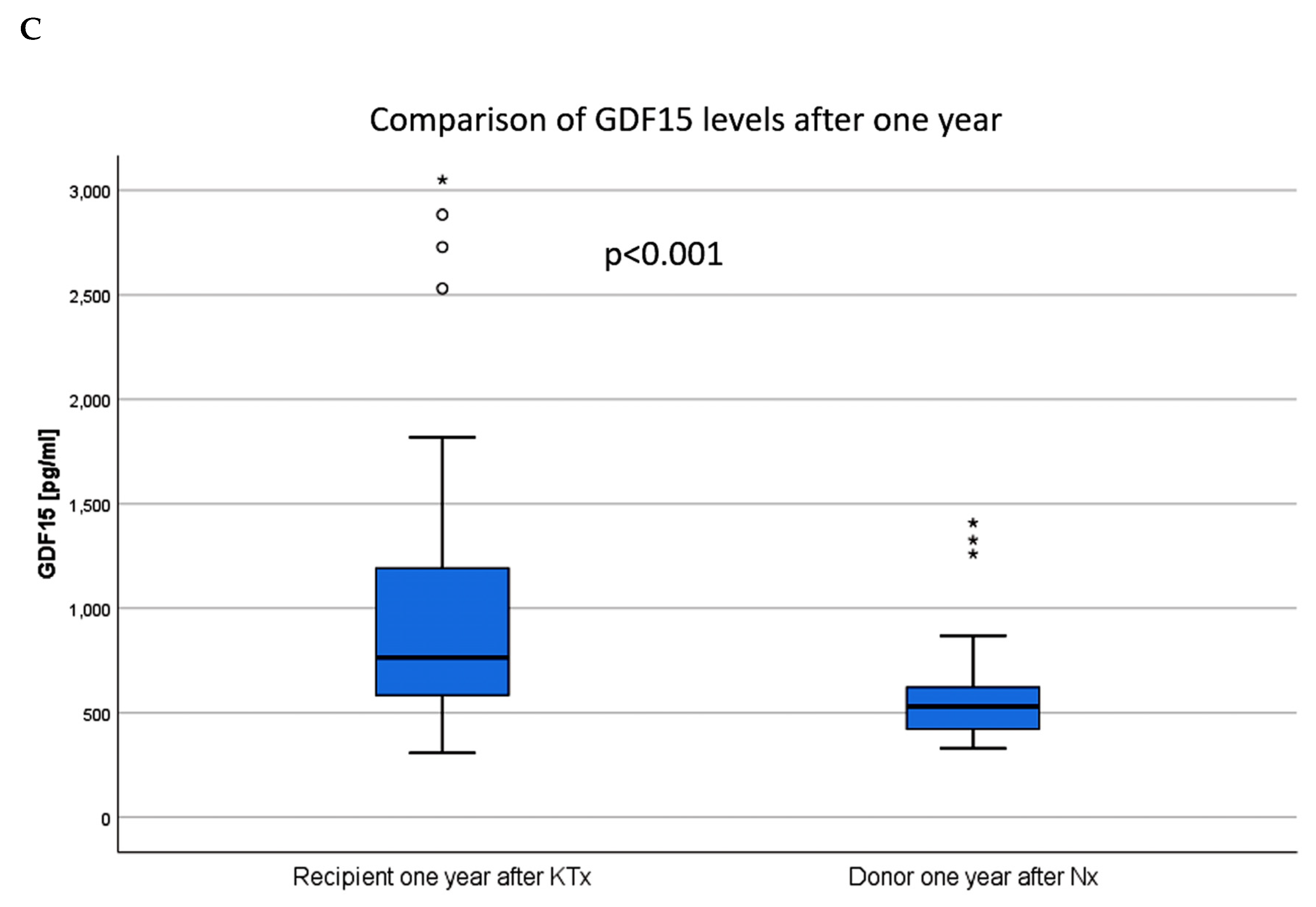
| GDF15 day 365 median (1st, 3rd quartile) | 913 (674, 1,453) | 775 (611, 1,139) | 1,141 (747, 1,636) | <0.001 a |
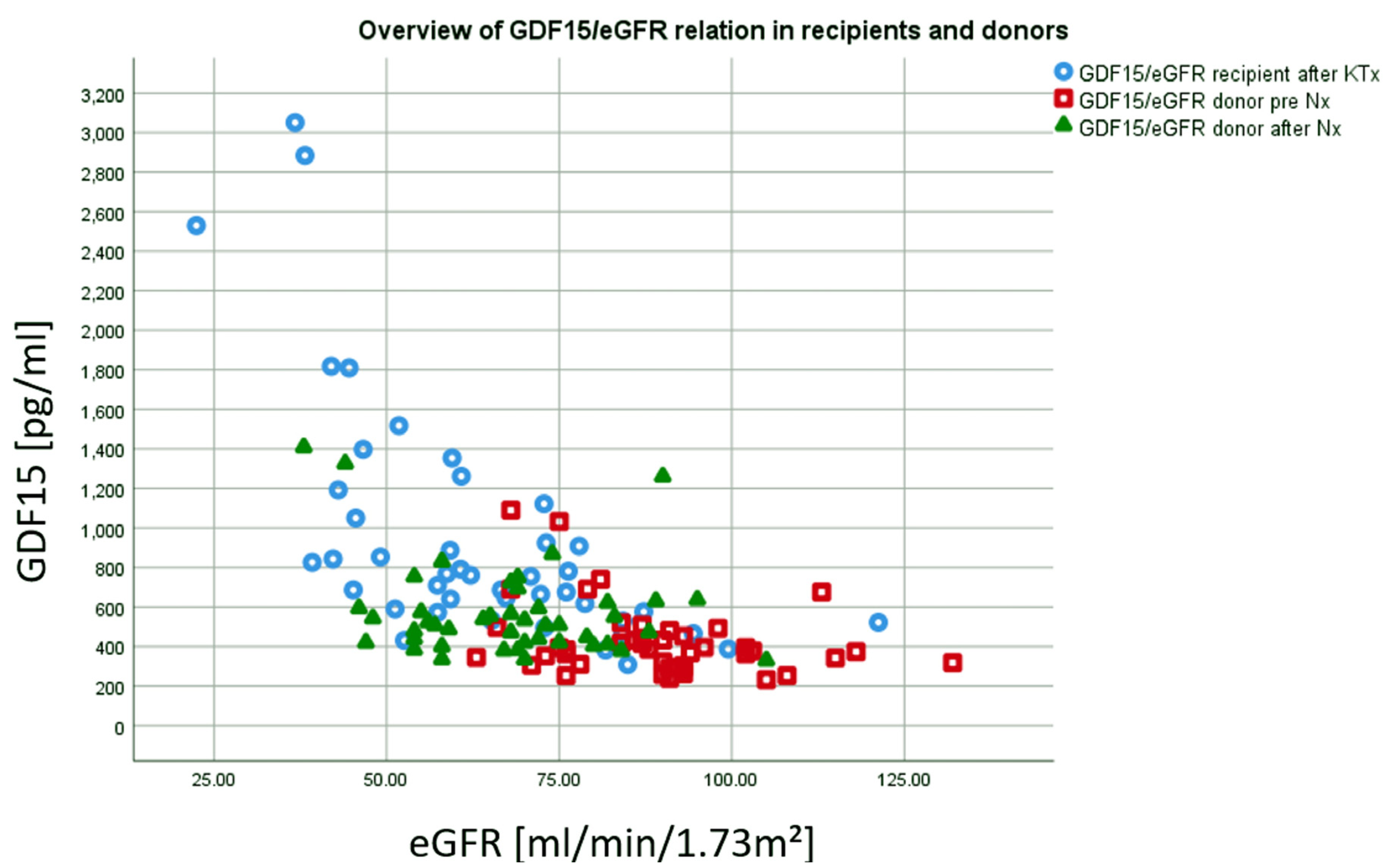
| eGFR day 365 (ml/min/1.73m², mean ± SD) | 61.1 (18.6) | 64.6 (16.9) | 57.5 (19.9) | 0.036 a |
| eGFR day 720 (ml/min/1.73m², mean ± SD) | 59.9 (19.0) | 62.5 (15.5) | 57.2 (22.0) | 0.107 a |
| eGFR day 1080 (ml/min/1.73m², mean ± SD) | 56.0 (18.1) | 58.5 (16.2) | 53.3 (19.8) | 0.068 a |
| eGFR day 1440 (ml/min/1.73m², mean ± SD) | 52.6 (15.4) | 54.7 (13.9) | 50.4 (16.7) | 0.207 a |
| UPCR day 365 (mg/g crea, mean ± SD) | 135 (114) | 131 (82) | 140 (141) | 0.832 |
| UPCR day 720 (mg/g crea, mean ± SD) | 160 (214) | 131 (106) | 191 (285) | 0.344 a |
| UPCR day 1080 (mg/g crea, mean ± SD) | 165 (208) | 167 (247) | 163 (163) | 0.594 a |
| UPCR day 1440 (mg/g crea, mean ± SD) | 169 (205) | 175 (244) | 163 (159) | 0.978 a |
| eGFR-loss > 30% from year one, n (%) | 25 (24.0) | 15 (27.3) | 10 (20.4) | 0.496 |
| Rejection, n (%) | 46 (44.2) | 28 (50.9) | 18 (36.7) | 0.170 b |
| Antibody-mediated rejection | 14 (13.5) | 10 (18.2) | 4 (8.2) | |
| T-cellular rejection | 4 (3.8) | 3 (5.5) | 1 (2.0) | |
| Combined rejection | 4 (3.8) | 2 (3.6) | 2 (4.1) | |
| T-cellular borderline rejection | 23 (22.1) | 12 (21.8) | 11 (22.4) | |
| CMV viremia, n (%) | 20 (19.6) | 4 (7.3) | 16 (32.7) | 0.001 b |
| BKPyV viremia, n (%) | 19 (18.3) | 6 (10.9) | 13 (26.5) | 0.043 b |
| NODAT, n (%) | 19 (18.3) | 6 (10.9) | 13 (26.5) | 0.046 b |
| Variables Baseline | |
|---|---|
| Patients (n) | 54 |
| Age at donation [years] (min, max) | 52.0 (35.7, 64.3) |
| Sex male (%) | 18 (28.6%) |
| BMI (kg/m2± SD) | 24.0 (6.6) |
| Variables Outcome | |
| GDF15 pre donation (pg/mL, median (1st, 3rd quartile)) | 384 (307, 487) |
| GDF15 day 365 (pg/mL, median (1st, 3rd quartile)) | 510 (420, 626) a |
| eGFR before donation [mL/min/1.73m2] | 95.9 (17.0) |
| eGFR day 365 (mL/min/1.73m2 ± SD) | 69.0 (15.8) a |
| UPCR before donation (mg/g crea± SD) | 79.5 (62.9) |
| UPCR day 365 (mg/g crea ± SD) | 86.1 (41.2) b |
| Variable | Regression-coefficient B | 95% CI | p value |
|---|---|---|---|
| GDF15 365 days | −0.005 | −0.008–−0.003 | <0.001 |
| Patient age at KTx | 0.563 | −0.871–−0.255 | <0.001 |
| No previous KTx | −3.072 | −13.819–7.674 | 0.571 |
| CMV mm D-/R- | 7.076 | −1.880–16.031 | 0.120 |
| CMV mm D+/R- | 5.799 | −6.423–18.021 | 0.348 |
| CMV mm D-/R+ | −0.254 | −9.274–8.766 | 0.955 |
| CMV mm D+/R+ | 0 a | . | . |
| Postmortal KTx | −19.184 | −34.228–−4.140 | 0.013 |
| Donor age | −0.326 | −0.697–0.046 | 0.085 |
| Dialysis vintage | −0.092 | −0.208–0.025 | 0.123 |
| Cold ischemia time | −1.012 | −2.802–0.778 | 0.264 |
| Warm ischemia time | 0.176 | −0.279–0.631 | 0.444 |
| PRA | 0.006 | −0.114–0.126 | 0.921 |
| HLA-mismatches | 1.758 | −0.880–4.396 | 0.189 |
| Variable | Regression-Coefficient B | 95% CI | p Value |
|---|---|---|---|
| GDF15 365 days | −0.015 | −0.021–−0.010 | <0.001 |
| Patient age at KTx | −0.372 | −0.670–−0.075 | 0.015 |
| No previous KTx | 5.135 | −5.061–15.330 | 0.319 |
| CMV mm D-/R- | 2.095 | −6.371–10.562 | 0.623 |
| CMV mm D+/R- | 3.239 | −9.026–15.504 | 0.600 |
| CMV mm D-/R+ | −1.445 | −9.849–6.960 | 0.733 |
| CMV mm D+/R+ | 0 a | . | . |
| Postmortal KTx | −22.898 | −37.328–−8.467 | 0.002 |
| Donor age | −0.544 | −0.898–−0.190 | 0.003 |
| Time of dialysis | −0.101 | −0.213–0.011 | 0.076 |
| Cold ischemia time | 0.969 | −2.659–0.720 | 0.257 |
| Warm ischemia time | 0.143 | −0.293–0.579 | 0.516 |
| PRA | 0.041 | −0.077–0.160 | 0.491 |
| HLA-mismatches | 1.747 | −0.814–4.308 | 0.178 |
| Regression-coefficient B | 95% CI | p value | |
|---|---|---|---|
| eGFR 365 days | −36.979 | −53.547–−20.411 | <0.001 |
| Patient age at KTx | −6.908 | −34.639–20.823 | 0.622 |
| No previous KTx | −359.404 | −2524.879–1806.070 | 0.742 |
| No CMV viremia | 192.050 | −608.566–992.665 | 0.635 |
| Postmortal KTx | −649.883 | −2037.429–737.663 | 0.354 |
| Donor age | −18.420 | −48.669–11.829 | 0.229 |
| Time of dialysis | −8.644 | −18.358–1.071 | 0.080 |
| Cold ischemia time | 0.255 | −153.523–154.034 | 0.997 |
| Warm ischemia time | 3.154 | −34.709–41.017 | 0.869 |
| PRA | −0.106 | −10.118–9.905 | 0.983 |
| HLA-mismatches | 187.076 | −34.100–408.253 | 0.096 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jehn, U.; Schütte-Nütgen, K.; Henke, U.; Bautz, J.; Pavenstädt, H.; Suwelack, B.; Reuter, S. Prognostic Value of Growth Differentiation Factor 15 in Kidney Donors and Recipients. J. Clin. Med. 2020, 9, 1333. https://doi.org/10.3390/jcm9051333
Jehn U, Schütte-Nütgen K, Henke U, Bautz J, Pavenstädt H, Suwelack B, Reuter S. Prognostic Value of Growth Differentiation Factor 15 in Kidney Donors and Recipients. Journal of Clinical Medicine. 2020; 9(5):1333. https://doi.org/10.3390/jcm9051333
Chicago/Turabian StyleJehn, Ulrich, Katharina Schütte-Nütgen, Ute Henke, Joachim Bautz, Hermann Pavenstädt, Barbara Suwelack, and Stefan Reuter. 2020. "Prognostic Value of Growth Differentiation Factor 15 in Kidney Donors and Recipients" Journal of Clinical Medicine 9, no. 5: 1333. https://doi.org/10.3390/jcm9051333
APA StyleJehn, U., Schütte-Nütgen, K., Henke, U., Bautz, J., Pavenstädt, H., Suwelack, B., & Reuter, S. (2020). Prognostic Value of Growth Differentiation Factor 15 in Kidney Donors and Recipients. Journal of Clinical Medicine, 9(5), 1333. https://doi.org/10.3390/jcm9051333





