Aggrecan Turnover in Women with Rheumatoid Arthritis Treated with TNF-α Inhibitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Serologic and Inflammatory Parameters
2.3. Immunoassay of CS846 Epitope and AGC
2.4. Immunoassay of ADAMTS-4, ADAMTS-5 and TIMP-3
2.5. Statistical Analysis
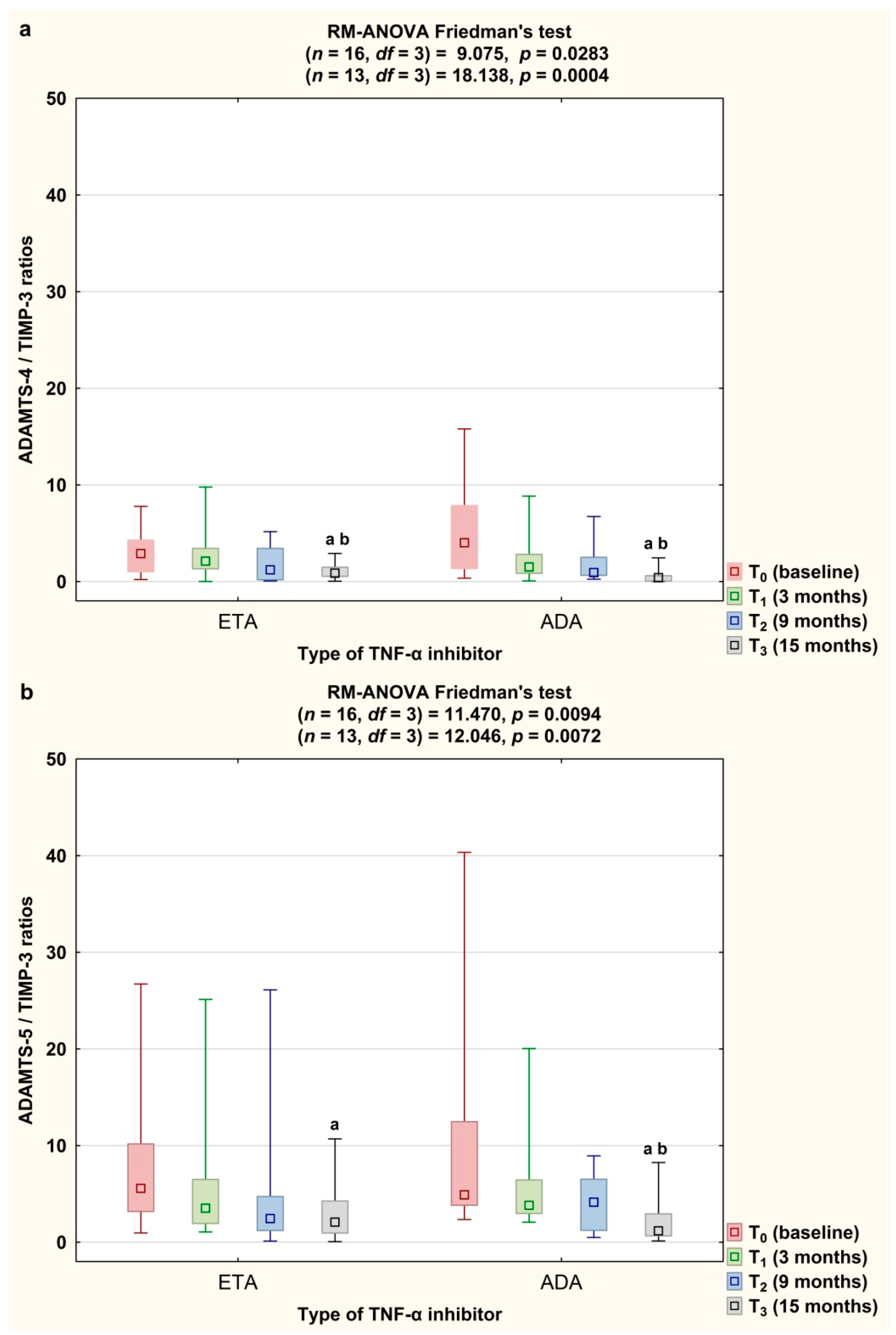
3. Results
3.1. Clinical Response
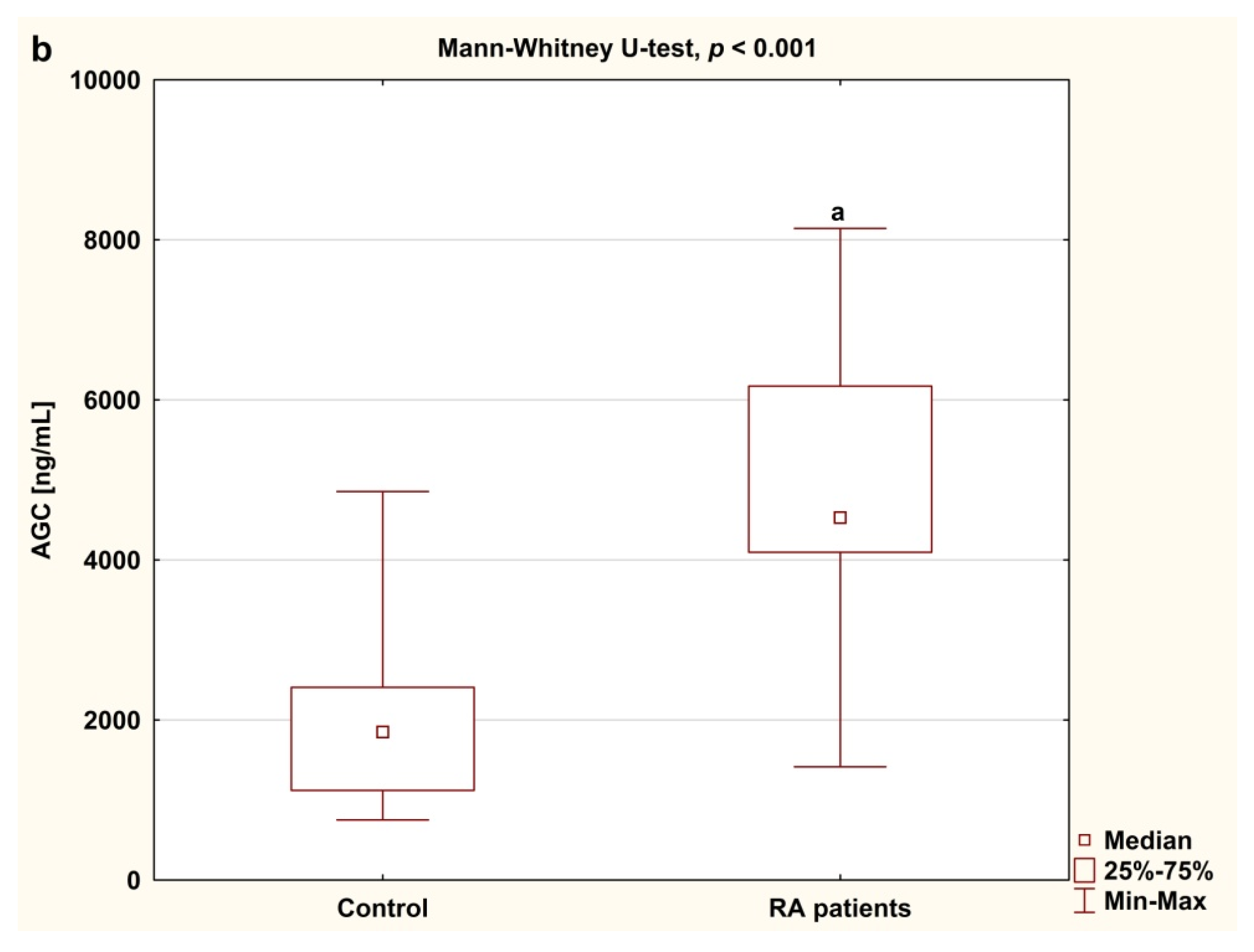
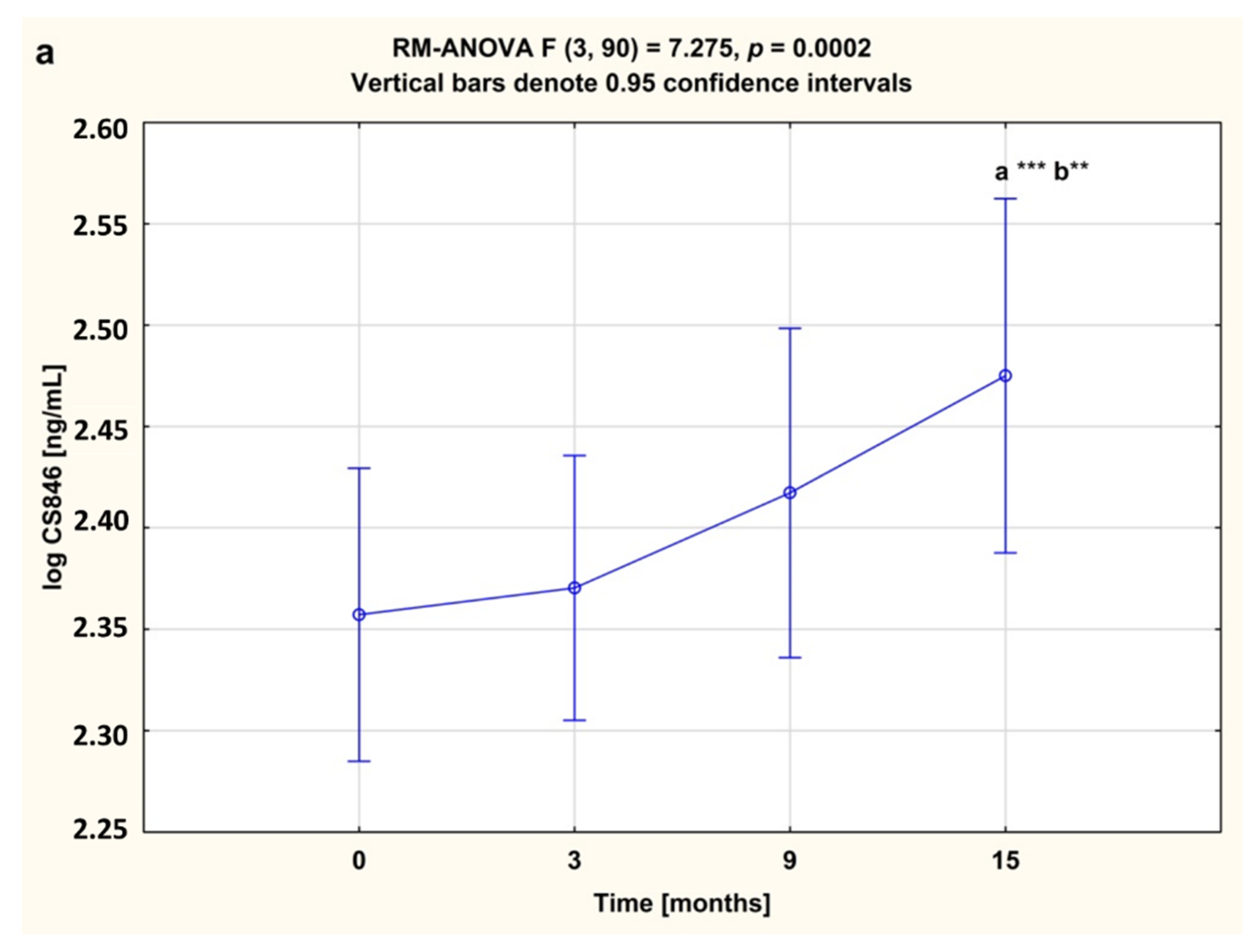
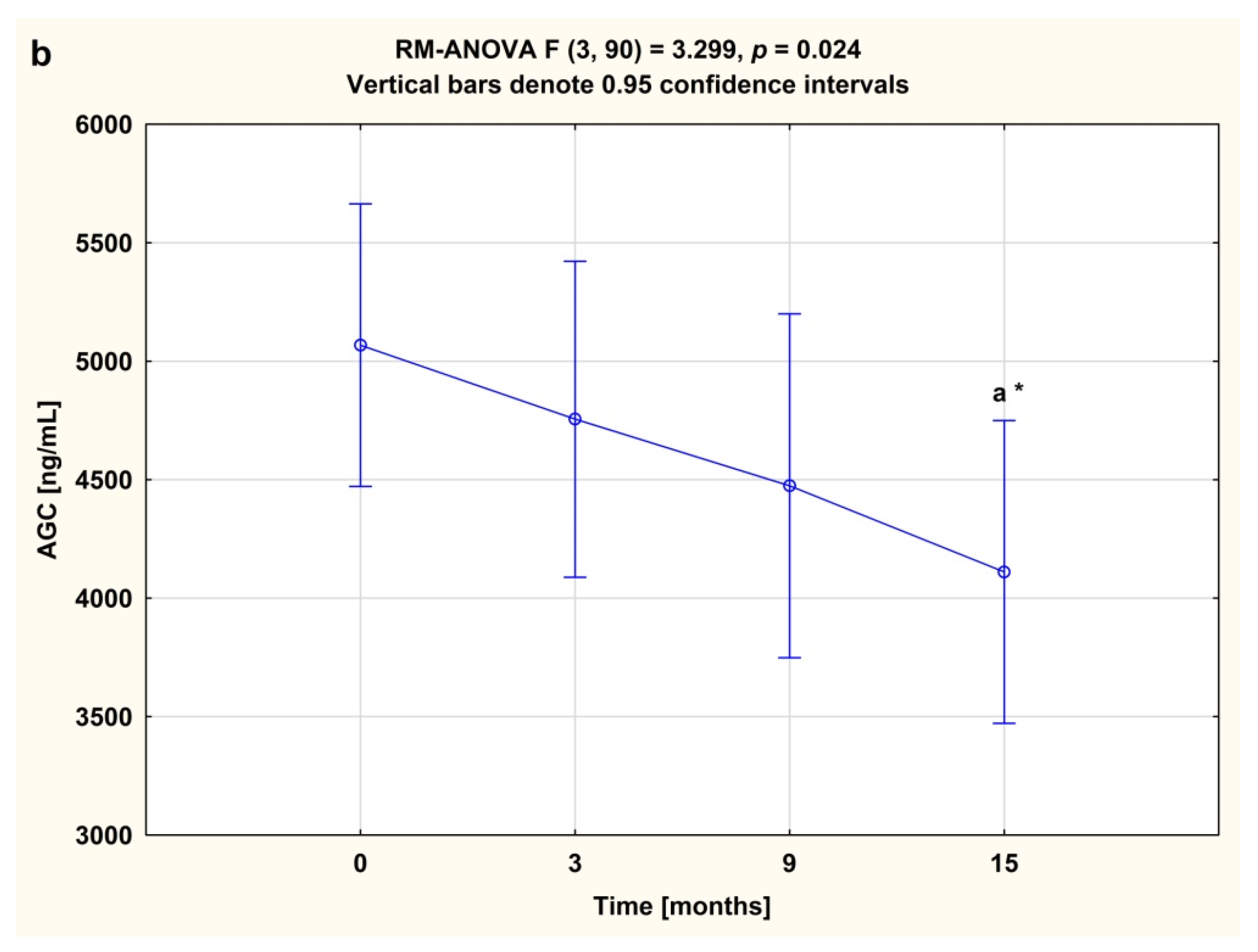
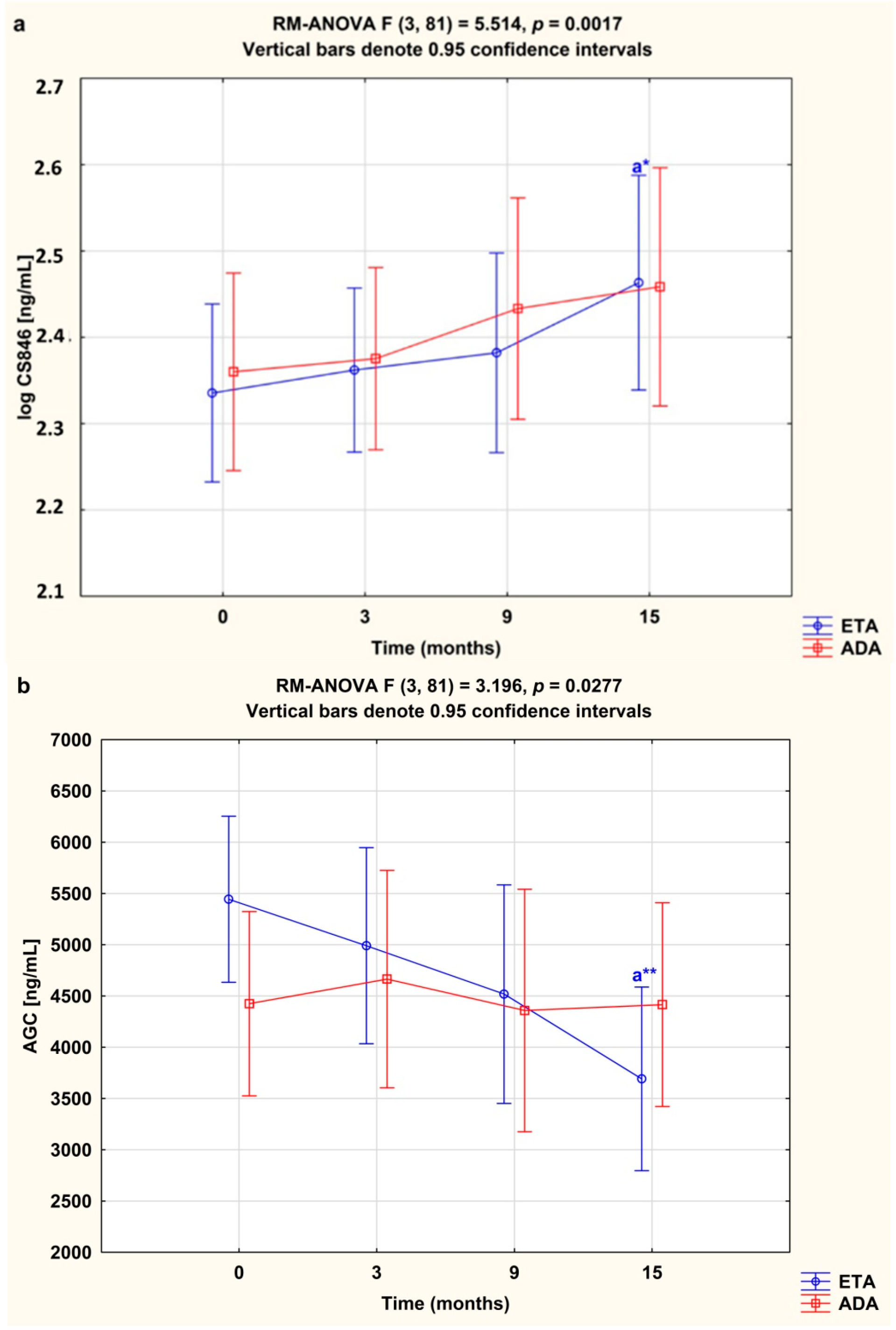
3.2. Serum Concentrations of CS846 and AGC
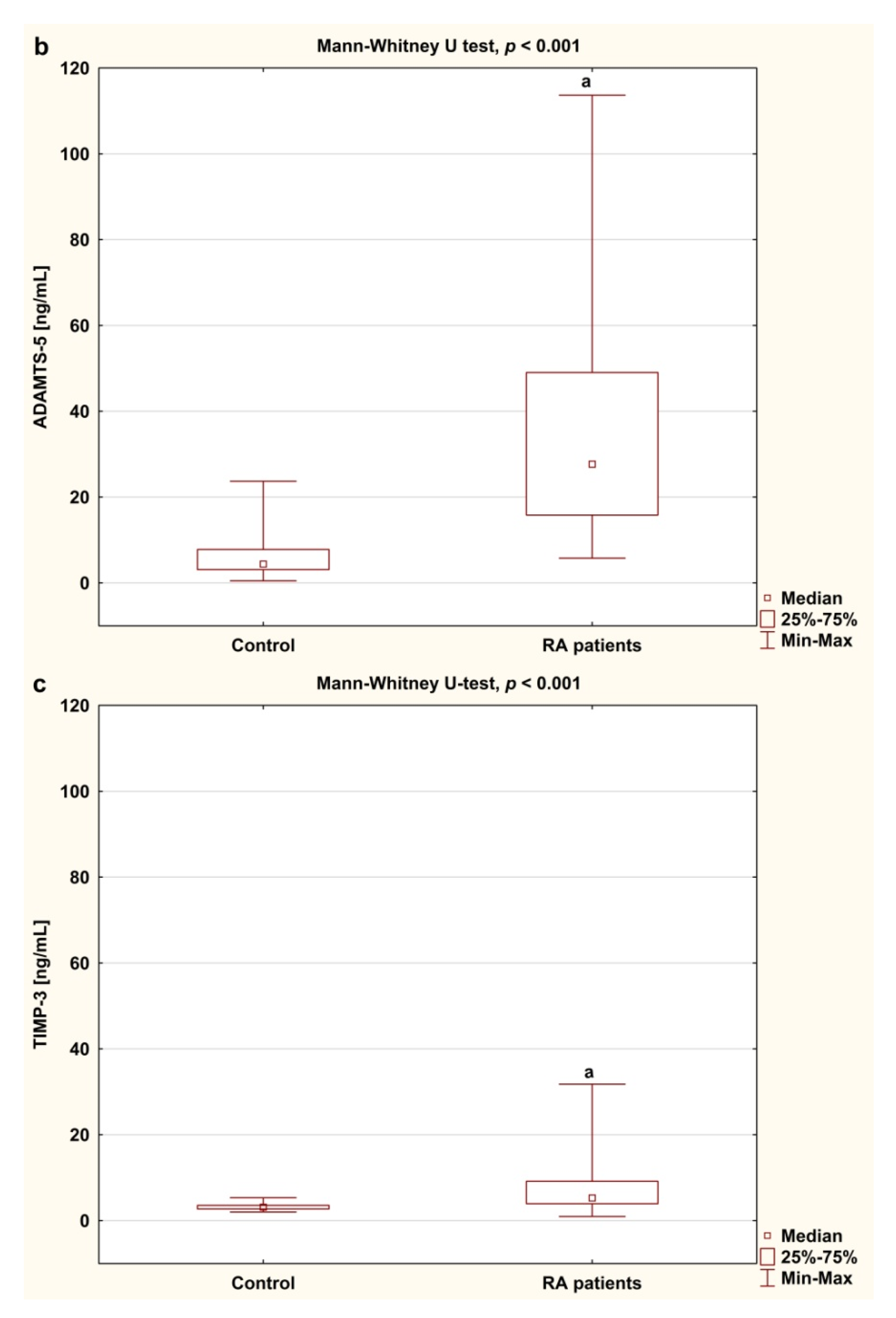
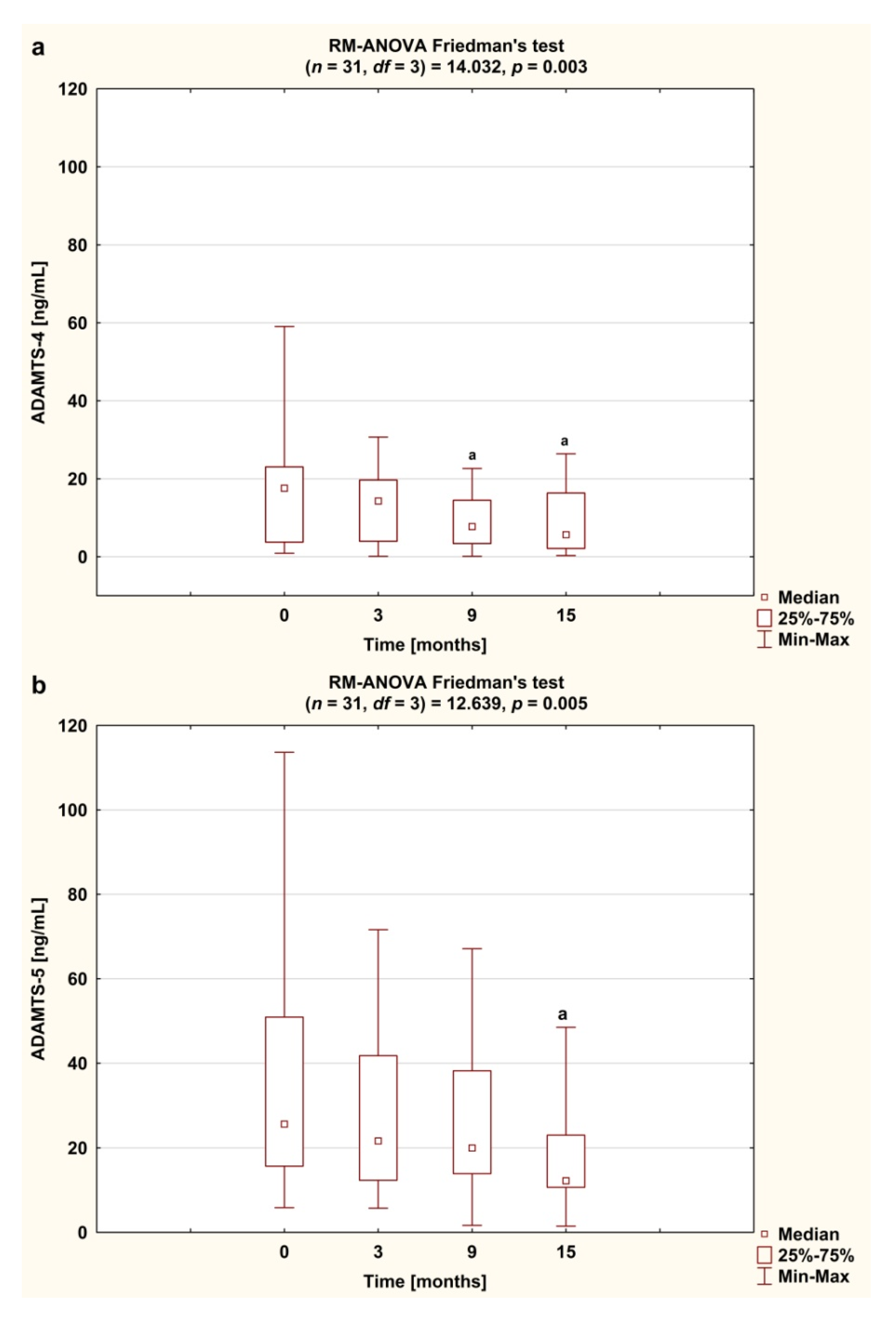
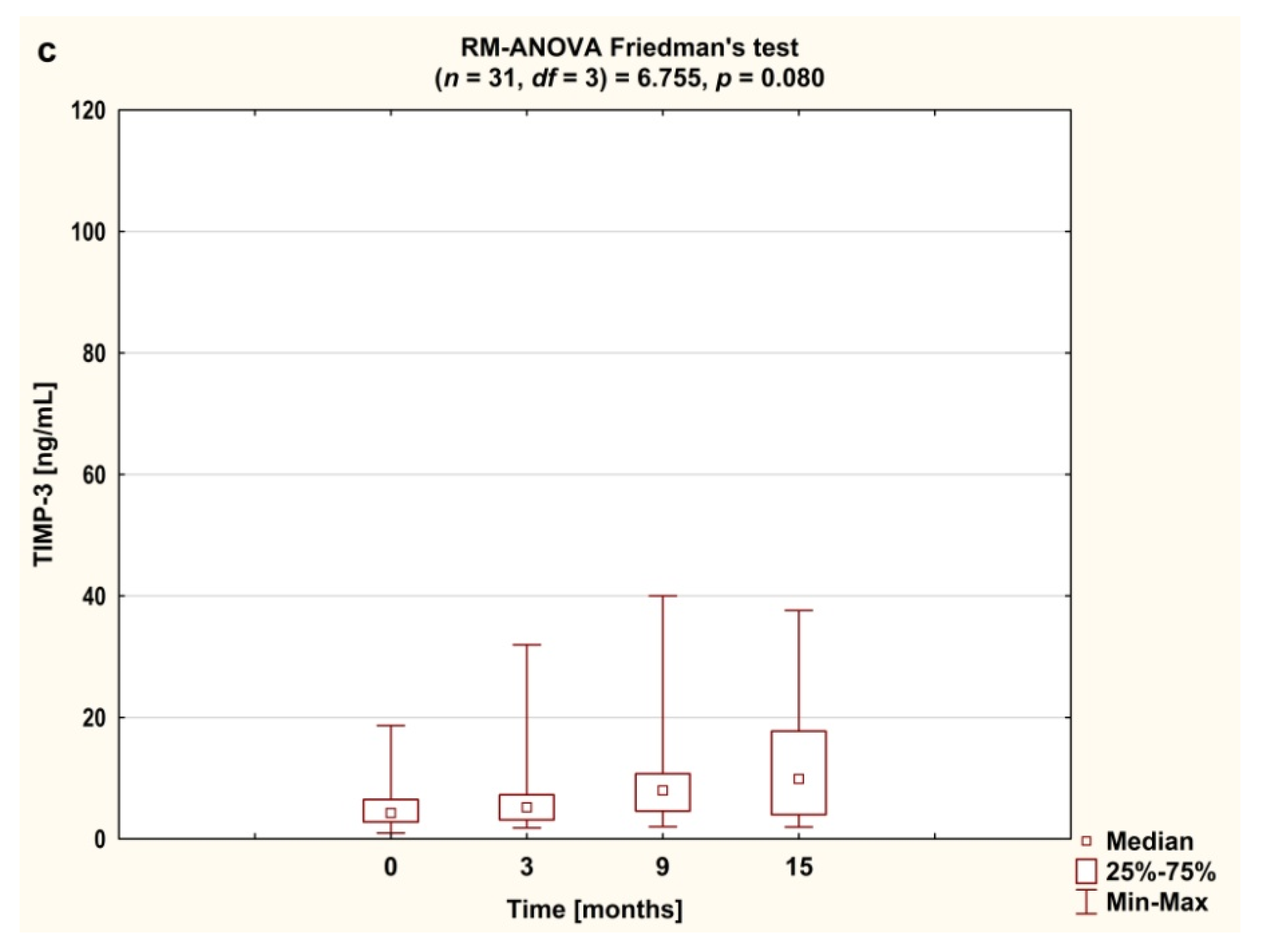
3.3. Serum Concentrations of ADAMTS and TIMP-3
3.4. Serum Concentrations of CS846 and AGC and ADAMTS/TIMP-3 Ratios Depending on the Type of TNF-α Inhibitor Used
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aletaha, D.; Smolen, J.S. Diagnosis and management of rheumatoid arthritis: A review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Jantan, I.; Bukhari, S.N.A. Rheumatoid arthritis: Recent advances on its etiology, role of cytokines and pharmacotherapy. Biomed Pharmacother. 2017, 92, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Zafar, A.; Moin, S.; Khan, A.Q.; Zubair, S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin. Chim. Acta 2016, 455, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 2009, 11, 224. [Google Scholar] [CrossRef] [Green Version]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage. Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar]
- Roughley, P.J.; Mort, J.S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 2014, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Sivan, S.S.; Wachtel, E.; Roughley, P. Structure, function, aging and turnover of aggrecan in the intervertebral disc. Biochim. Biophys. Acta 2014, 1840, 3181–3189. [Google Scholar] [CrossRef]
- Dudhia, J. Aggrecan, aging and assembly in articular cartilage. Cell. Mol. Life Sci. 2005, 62, 2241–2256. [Google Scholar] [CrossRef]
- Verma, P.; Delal, K. ADAMTS-4 and ADAMTS-5: Key enzymes in osteoarthritis. J. Cell. Biochem. 2011, 112, 3507–3514. [Google Scholar] [CrossRef]
- Nagase, H.; Kashiwagi, M. Aggrecanases and cartilage matrix degradation. Arthritis Res. 2003, 5, 94–103. [Google Scholar] [CrossRef]
- Charni-Ben Tabassi, N.; Garnero, P. Monitoring cartilage turnover. Curr. Rheumatol. Rep. 2007, 9, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Durigova, M.; Nagase, H.; Mort, J.S.; Roughley, P.J. MMPs are less efficient than ADAMTS5 in cleaving aggrecan core protein. Matrix Biol. 2011, 30, 145–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, G.; Nagase, H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: Destruction or repair? Nat. Clin. Pract. Rheumatol. 2008, 4, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Dancevic, C.M.; McCulloch, D.R. Current and emerging therapeutic strategies for preventing inflammation and aggrecanase-mediated cartilage destruction in arthritis. Arthritis Res. Ther. 2014, 16, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malemud, C.J. Inhibition of MMPs and ADAM/ADAMTS. Biochem. Pharmacol. 2019, 165, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Mead, T.J.; Apte, S.S. ADAMTS proteins in human disorders. Matrix Biol. 2018, 71, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y. Metalloproteinases in rheumatoid arthritis: Potential therapeutic targets to improve current therapies. Prog. Mol. Biol. Trans. Sci. 2017, 148, 327–338. [Google Scholar]
- Yamanishi, Y.; Boyle, D.L.; Clark, M.; Maki, R.A.; Tortorella, M.D.; Arner, E.C.; Firestein, G.S. Expression and regulation of aggrecanase in arthritis: The role of TGF-beta. J. Immunol. 2002, 168, 1405–1412. [Google Scholar] [CrossRef] [Green Version]
- Yoshitomi, H. Regulation of immune responses and chronic inflammation by fibroblast-like synoviocytes. Front. Immunol. 2019, 10, 1395. [Google Scholar] [CrossRef]
- Woitge, H.W.; Seibel, M.J. Markers of Bone and Cartilage Turnover. Exp. Clin. Endocrinol. Diabetes 2017, 125, 454–469. [Google Scholar] [CrossRef]
- Poole, A.R.; Dieppe, P. Biological markers in rheumatoid arthritis. Semin. Arthritis Rheum. 1994, 23, 17–31. [Google Scholar] [CrossRef]
- Rein, P.; Mueller, R.B. Treatment with biologicals in rheumatoid arthritis: An overview. Rheumatol. Ther. 2017, 4, 247–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., III; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- Fransen, J.; van Riel, P.L. The Disease Activity Score and the EULAR response criteria. Rheum. Dis. Clin. N. Am. 2009, 35, 745–757. [Google Scholar] [CrossRef]
- Szeremeta, A.; Jura-Półtorak, A.; Koźma, E.M.; Głowacki, A.; Kucharz, E.J.; Kopeć-Mędrek, M.; Olczyk, K. Effects of a 15-month anti-TNF-α treatment on plasma levels of glycosaminoglycans in women with rheumatoid arthritis. Arthritis Res. Ther. 2018, 20, 211. [Google Scholar] [CrossRef] [Green Version]
- Poole, A.R.; Ionescu, M.; Swan, A.; Dieppe, P.A. Changes in cartilage metabolism in arthritis are reflected by altered serum and synovial fluid levels of the cartilage proteoglycan aggrecan. Implications for pathogenesis. J. Clin. Invest. 1994, 94, 25–33. [Google Scholar] [CrossRef]
- Månsson, B.; Carey, D.; Alini, M.; Ionescu, M.; Rosenberg, L.C.; Poole, A.R. Cartilage and bone metabolism in rheumatoid arthritis. Differences between rapid and slow progression of disease identified by serum markers of cartilage metabolism. J. Clin. Invest. 1995, 95, 1071–1077. [Google Scholar] [CrossRef]
- Garnero, P. Osteoarthritis: Biological markers for the future? Joint Bone Spine 2002, 69, 525–530. [Google Scholar] [CrossRef]
- Månsson, B.; Geborek, P.; Saxne, T. Cartilage and bone macromolecules in knee joint synovial fluid in rheumatoid arthritis: Relation to development of knee or hip joint destruction. Ann. Rheum. Dis. 1997, 56, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Tracey, D.; Klareskog, L.; Sasso, E.H.; Salfeld, J.G.; Tak, P.P. Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharmacol. Ther. 2008, 117, 244–279. [Google Scholar] [CrossRef] [PubMed]
- García-Hernández, M.H.; González-Amaro, R.; Portales-Pérez, D.P. Specific therapy to regulate inflammation in rheumatoid arthritis: Molecular aspects. Immunotherapy 2014, 6, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Maksymowych, W.P.; Poole, A.R.; Hiebert, L.; Webb, A.; Ionescu, M.; Lobanok, T.; King, L.; Davis, J.C., Jr. Etanercept exerts beneficial effects on articular cartilage biomarkers of degradation and turnover in patients with ankylosing spondylitis. J. Rheumatol. 2005, 32, 1911–1917. [Google Scholar] [PubMed]
- Larsson, S.; Lohmander, L.S.; Struglics, A. Synovial fluid level of aggrecan ARGS fragments is a more sensitive marker of joint disease than glycosaminoglycan or aggrecan levels: A cross-sectional study. Arthritis Res. Ther. 2009, 11, R92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxne, T.; Glennås, A.; Kvien, T.K.; Melby, K.; Heinegård, D. Release of cartilage macromolecules into the synovial fluid in patients with acute and prolonged phases of reactive arthritis. Arthritis Rheum. 1993, 36, 20–25. [Google Scholar] [CrossRef]
- El-Arman, M.M.; El-Fayoumi, G.; El-Shal, E.; El-Boghdady, I.; El-Ghaweet, A. Aggrecan and cartilage oligomeric matrix protein in serum and synovial fluid of patients with knee osteoarthritis. HSS J. 2010, 6, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Rousseau, J.C.; Sumer, E.U.; Hein, G.; Sondergaard, B.C.; Madsen, S.H.; Pedersen, C.; Neumann, T.; Mueller, A.; Qvist, P.; Delmas, P.; et al. Patients with rheumatoid arthritis have an altered circulatory aggrecan profile. BMC Musculoskelet. Disord. 2008, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Malfait, A.M.; Liu, R.Q.; Ijiri, K.; Komiya, S.; Tortorella, M.D. Inhibition of ADAM-TS4 and ADAM-TS5 prevents aggrecan degradation in osteoarthritic cartilage. J. Biol. Chem. 2002, 277, 22201–22208. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Du, C.; Wang, H.; Zhang, C. Increased serum ADAMTS-4 in knee osteoarthritis: A potential indicator for the diagnosis of osteoarthritis in early stages. Genet. Mol. Res. 2014, 13, 9642–9649. [Google Scholar] [CrossRef]
- Tsuzaka, K.; Itami, Y.; Takeuchi, T.; Shinozaki, N.; Morishita, T. ADAMTS5 is a biomarker for prediction of response to infliximab in patients with rheumatoid arthritis. J. Rheumatol. 2010, 37, 1454–1460. [Google Scholar] [CrossRef]
- Bondeson, J.; Wainwright, S.D.; Lauder, S.; Amos, N.; Hughes, C.E. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res. Ther. 2006, 8, R187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gendron, C.; Kashiwagi, M.; Hughes, C.; Caterson, B.; Nagase, H. TIMP-3 inhibits aggrecanase-mediated glycosaminoglycan release from cartilage explants stimulated by catabolic factors. FEBS Lett. 2003, 555, 431–436. [Google Scholar] [CrossRef] [Green Version]
- Catrina, A.I.; Lampa, J.; Ernestam, S.; Klingt, E.; Bratt, J.; Klareskog, L.; Ulfgren, A.K. Anti-tumour necrosis factor (TNF)-α therapy (etanercept) down-regulates serum matrix metalloproteinase (MMP)-3 and MMP-1 in rheumatoid arthritis. Rheumatology 2002, 41, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Klimiuk, P.A.; Sierakowski, S.; Domyslawska, I.; Chwiecko, J. Effect of repeated infliximab therapy on serum matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with rheumatoid arthritis. J. Rheumatol. 2004, 31, 238–242. [Google Scholar] [PubMed]
- Hattori, Y.; Kojima, T.; Kaneko, A.; Kida, D.; Hirano, Y.; Fujibayashi, T.; Yabe, Y.; Oguchi, T.; Kanayama, Y.; Miyake, H.; et al. High rate of improvement in serum matrix metalloproteinase-3 levels at 4 weeks predicts remission at 52 weeks in RA patients treated with adalimumab. Mod. Rheumatol. 2018, 28, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, G.; Liu, X.; Song, J.; Chen, L.; Xu, H. Matrix metalloproteinase-3 and the 7-joint ultrasound score in the assessment of disease activity and therapeutic efficacy in patients with moderate to severe rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrotin, Y.; Kurz, B.; Aigner, T. Oxygen and reactive oxygen species in cartilage degradation: Friends or foes? Osteoarthritis Cartilage 2005, 13, 643–654. [Google Scholar] [CrossRef] [Green Version]
- Mateen, S.; Moin, S.; Khan, A.Q.; Zafar, A.; Fatima, N. Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PLoS ONE 2016, 11, e0152925. [Google Scholar] [CrossRef] [Green Version]
- Biniecka, M.; Kennedy, A.; Ng, C.T.; Chang, T.C.; Balogh, E.; Fox, E.; Veale, D.J.; Fearon, U.; O’Sullivan, J.N. Successful tumour necrosis factor (TNF) blocking therapy suppresses oxidative stress and hypoxia-induced mitochondrial mutagenesis in inflammatory arthritis. Arthritis Res. Ther. 2011, 13, R121. [Google Scholar] [CrossRef] [Green Version]
- Cacciapaglia, F.; Grazia Anelli, M.; Rizzo, D.; Morelli, E.; Mazzotta, D.; Scioscia, C.; Iannone, F.; Lapadula, G. Effective tumour necrosis factor-blocking therapy reduces reactive oxygen metabolite level in rheumatoid arthritis. J. Int. Med. Res. 2016, 44, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, A.; Aoki, Y.; Sonobe, M.; Takahashi, H.; Saito, M.; Nakagawa, K. Serum level of reactive oxygen metabolites (ROM) at 12 weeks of treatment with biologic agents for rheumatoid arthritis is a novel predictor for 52-week remission. Clin. Rheumatol. 2017, 36, 309–315. [Google Scholar] [CrossRef] [PubMed]
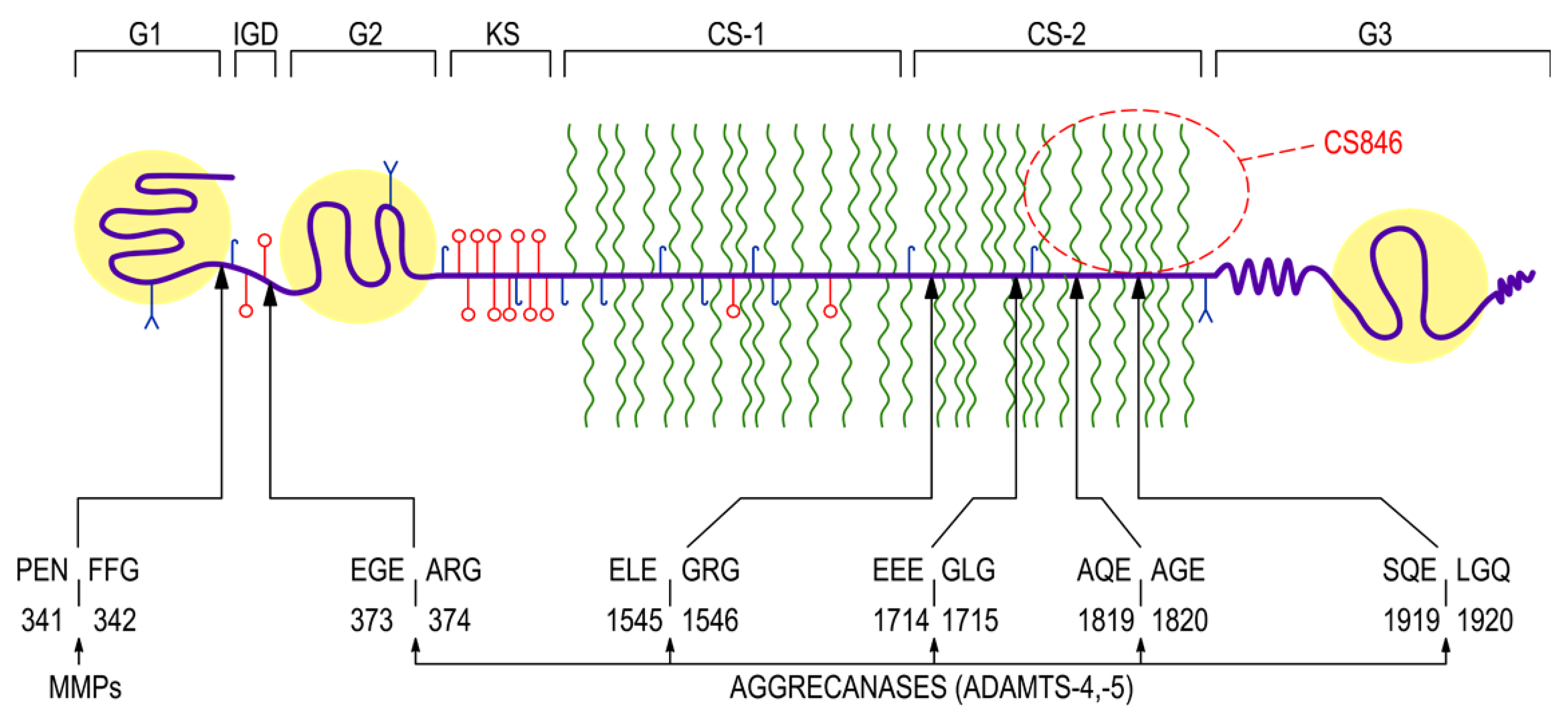


| Characteristic | Value |
|---|---|
| All women with RA, n (%) | 50 (100) |
| Age (years), mean (SD) | 47.52 (11.91) |
| Disease duration (years), median (IQR) | 6 (3–12) |
| Height (cm), mean (SD) | 163.58 (6.78) |
| Weight (kg), mean (SD) | 65.52 (14.40) |
| BMI (kg/m2), mean (SD) | 24.46 (5.17) |
| RF positive, n (%) | 44 (88) |
| Anti-CCP positive, n (%) | 43 (86) |
| SJC 28, median (IQR) | 7 (5–10) |
| TJC 28, median (IQR) | 12 (9–14) |
| VAS, median (IQR) | 80 (70–80) |
| DAS 28-ESR, mean (SD) | 5.83 (0.49) |
| ESR (mm/h), median (IQR) | 17.0 (10.0–29.0) |
| CRP (mg/L), median (IQR) | 6.37 (3.0–10.30) |
| Anti-rheumatic therapy, n (%) | |
| Methotrexate (25 mg/week) | 50 (100) |
| Prednisone (≤ 7.5 mg/day) | 50 (100) |
| Folic acid (5 mg/day), n (%) | 50 (100) |
| TNFαI therapy, n (%) | |
| Etanercept (Enbrel) | 24 (48) |
| Adalimumab (Humira) | 22 (44) |
| Certolizumab pegol (Cimzia) | 2 (4) |
| Golimumab (Simponi) | 2 (4) |
| Time after Starting Anti-TNF-α Therapy | ||||
|---|---|---|---|---|
| T0 (Baseline) | T1 (3 Months) | T2 (9 Months) | T3 (15 Months) | |
| Women with RA, n (%) | 31 (100) | |||
| Age (years), mean (SD) | 45.87 (12.28) | |||
| Disease duration (years), median (IQR) | 5 (3–11) | |||
| Growth (cm), mean (SD) | 163.77 (6.63) | |||
| Weight (kg), mean (SD) | 65.89 (14.60) | |||
| BMI (kg/m2), mean (SD) | 24.62 (5.65) | |||
| RF positive, n (%) | 28 (90.32) | |||
| Anti-CCP positive, n (%) | 26 (83.87) | |||
| SJC 28, median (IQR) | 7 (5–10) | 2 (0–3) a, c | 0 (0–0) a, b | 0 (0–0) a, b |
| TJC 28, median (IQR) | 12 (9–16) | 4 (2–7) a, c | 1 (0–2) a, b | 0 (0–0) a, b, c |
| VAS, median (IQR) | 80 (80–80) | 40 (30–50) a, c | 20 (10–30) a, b | 15 (5–20) a, b |
| DAS 28-ESR, median (IQR) | 5.78 (5.51–6.24) | 3.92 (3.08–4.42) a, c | 2.75 (2.24–3.13) a, b | 2.19 (1.75–2.51) a, b, c |
| Disease activity, n (%) | ||||
| High (>5.1) | 31 (100) | 2 (6.45) | 0 (0) | 0 (0) |
| Moderate (>3.2 and ≤5.1) | 0 (0) | 20 (64.52) | 3 (9.68) | 0 (0) |
| Low (≤3.2 and >2.6) | 0 (0) | 4 (12.91) | 14 (45.16) | 5 (16.13) |
| Remission (≤2.6) | 0 (0) | 5 (16.13) | 14 (45.16) | 26 (83.87) |
| ESR (mm/h), median (IQR) | 17.0 (10.0–34.0) | 14.0 (9.0–23.0) | 13.0 (9.0–18.0) a | 13.0 (8.0–18.0) a |
| CRP (mg/L), median (IQR) | 6.3 (3.08–14.0) | 4.0 (2.0–9.0) | 4.0 (2.0–4.3) a | 4.0 (1.5–5.1) a |
| TNFαI therapy, n (%) | ||||
| Etanercept (Enbrel) | 16 (51.62) | |||
| Adalimumab (Humira) | 13 (41.93) | |||
| Certolizumab pegol (Cimzia) | 2 (6.45) | |||
| Characteristic | Control A | RA Patients (n = 50) B | p A vs. B |
|---|---|---|---|
| ADAMTS-4/TIMP-3 | 1.99 (1.27–2.67) | 2.86 (1.29–4.57) | NS |
| ADAMTS-5/TIMP-3 | 1.34 (1.04–1.92) | 4.45 (2.61–8.40) | p < 0.001 |
| Characteristic | RA Patients (n = 31) | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time after Starting Anti-TNF-α Therapy | ||||||||||
| T0 (baseline) A | T1 (3 months) B | T2 (9 months) C | T3 (15 months) D | A vs. B | A vs. C | A vs. D | B vs. C | B vs. D | C vs. D | |
| ADAMTS-4/TIMP-3 | 3.11 (1.18–6.21) | 2.06 (0.96–3.57) | 0.79 (0.56–1.51) | 0.76 (0.17–2.22) | NS | <0.001 | <0.001 | <0.0083 | <0.0083 | NS |
| ADAMTS-5/TIMP-3 | 5.01 (3.79–12.48) | 3.73 (2.72–6.55) | 2.63 (1.23–5.64) | 1.74 (0.84–3.96) | NS | <0.0083 | <0.001 | NS | <0.001 | NS |
| Characteristic | RA Patients (n = 31) Time after Starting Anti-TNF-α Therapy | |||
|---|---|---|---|---|
| T0 (Baseline) | T3 (15 Months) | |||
| ADAMTS-4/TIMP-3 | ADAMTS-5/TIMP-3 | ADAMTS-4/TIMP-3 | ADAMTS-5/TIMP-3 | |
| SJC 28 | −0.338 NS | 0.150 NS | 0.171 NS | −0.171 NS |
| TJC 28 | 0.029 NS | −0.328 NS | 0.211 NS | −0.167 NS |
| DAS 28−ESR | −0.369 NS | −0.144 NS | 0.138 NS | 0.074 NS |
| CRP | −0.062 NS | −0.183 NS | 0.305 NS | 0.463 NS |
| ESR | −0.054 NS | −0.187 NS | −0.054 NS | 0.274 NS |
| CS846 | −0.059 NS | 0.256 NS | −0.199 NS | 0.084 NS |
| AGC | 0.154 NS | −0.054 NS | 0.405 NS | 0.231 NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szeremeta, A.; Jura-Półtorak, A.; Zoń-Giebel, A.; Kopeć-Mędrek, M.; Kucharz, E.J.; Olczyk, K. Aggrecan Turnover in Women with Rheumatoid Arthritis Treated with TNF-α Inhibitors. J. Clin. Med. 2020, 9, 1377. https://doi.org/10.3390/jcm9051377
Szeremeta A, Jura-Półtorak A, Zoń-Giebel A, Kopeć-Mędrek M, Kucharz EJ, Olczyk K. Aggrecan Turnover in Women with Rheumatoid Arthritis Treated with TNF-α Inhibitors. Journal of Clinical Medicine. 2020; 9(5):1377. https://doi.org/10.3390/jcm9051377
Chicago/Turabian StyleSzeremeta, Anna, Agnieszka Jura-Półtorak, Aleksandra Zoń-Giebel, Magdalena Kopeć-Mędrek, Eugeniusz Józef Kucharz, and Krystyna Olczyk. 2020. "Aggrecan Turnover in Women with Rheumatoid Arthritis Treated with TNF-α Inhibitors" Journal of Clinical Medicine 9, no. 5: 1377. https://doi.org/10.3390/jcm9051377





