Mortality After Delay of Adequate Empiric Antimicrobial Treatment of Bloodstream Infection
Abstract
:1. Introduction
2. Methods
2.1. Study Setting and Population
2.2. Data Collection and Microbiology Methods
2.3. Study Definitions
2.4. Statistical Methods
2.5. Ethical Approval
3. Results
3.1. Cohort Characteristics
3.2. Source of Infection and Microbiology Data
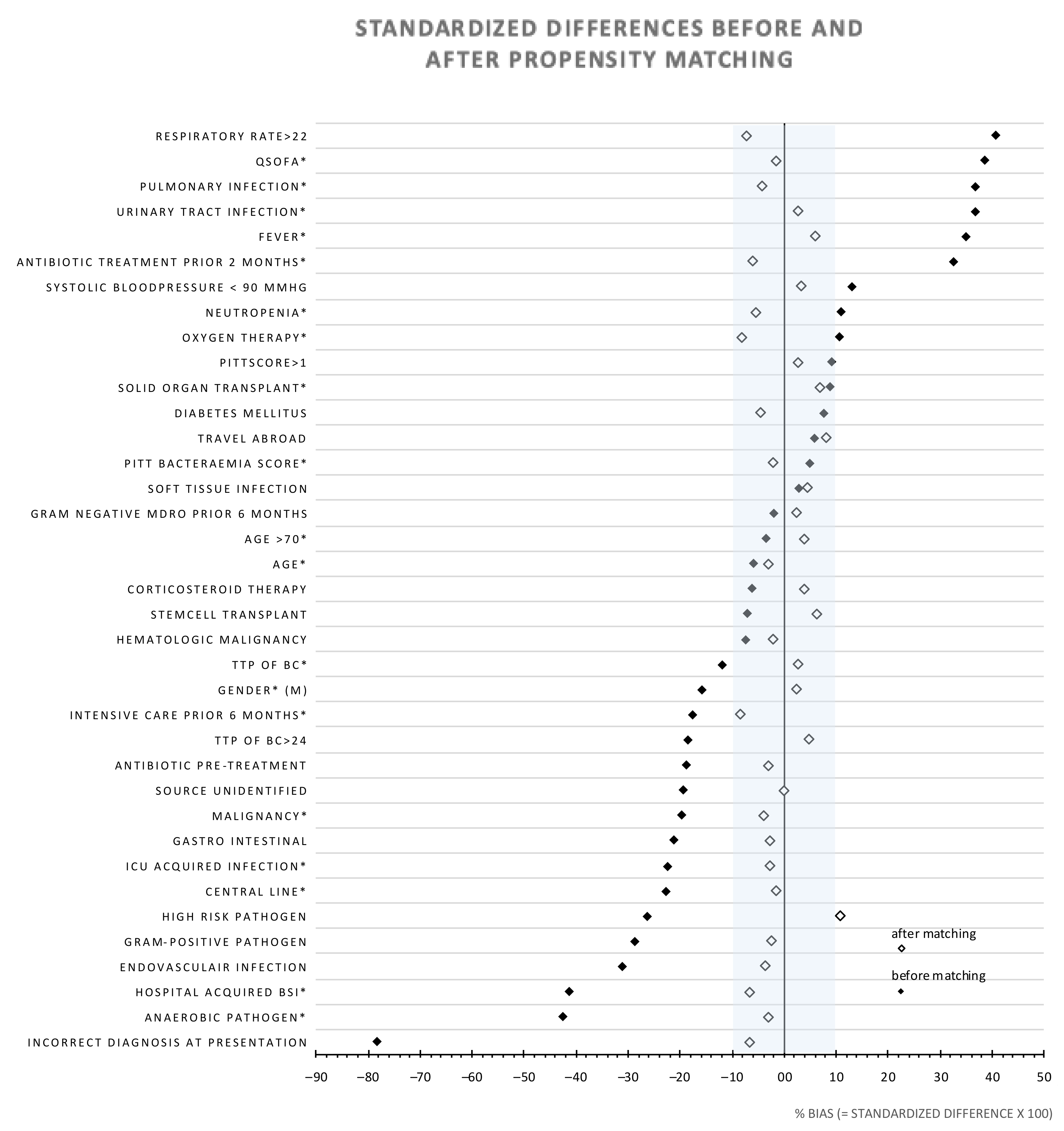
3.3. Propensity Score Matching (PSM) Analysis
4. Discussion
4.1. Key Results
4.2. Propensity of Inadequate Empiric Treatment
4.3. Study Strengths and Limitations
4.4. Generalizability and Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kontula, K.S.; Skogberg, K.; Ollgren, J.; Jarvinen, A.; Lyytikainen, O. Early deaths in bloodstream infections: A population-based case series. Infect. Dis. 2016, 48, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Prescott, H.C.; Osterholzer, J.J.; Langa, K.M.; Angus, D.C.; Iwashyna, T.J. Late mortality after sepsis: Propensity matched cohort study. BMJ 2016, 353, i2375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit. Care Med. 2017, 45, 486–552. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.A.; Murray, B.E. Antibiotic-resistant bugs in the 21st century--a clinical super-challenge. N. Engl. J. Med. 2009, 360, 439–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambregts, M.M.C.; Hendriks, B.J.C.; Visser, L.G.; Bernards, S.T.; de Boer, M.G.J. Using local clinical and microbiological data to develop an institution specific carbapenem-sparing strategy in sepsis: A nested case-control study. Antimicrob. Resist. Infect. Control 2019, 8, 19. [Google Scholar] [CrossRef]
- Coulter, S.; Roberts, J.A.; Hajkowicz, K.; Halton, K. The Use of Bloodstream Infection Mortality to Measure the Impact of Antimicrobial Stewardship Interventions: Assessing the Evidence. Infect. Dis Rep. 2017, 9, 6849. [Google Scholar] [CrossRef] [Green Version]
- Garnacho-Montero, J.; Ortiz-Leyba, C.; Herrera-Melero, I.; Aldabo-Pallas, T.; Cayuela-Dominguez, A.; Marquez-Vacaro, J.A.; Carbajal-Guerrero, J.; Garcia-Garmendia, J.L. Mortality and morbidity attributable to inadequate empirical antimicrobial therapy in patients admitted to the ICU with sepsis: A matched cohort study. J. Antimicrob. Chemother. 2008, 61, 436–441. [Google Scholar] [CrossRef]
- Kang, C.I.; Kim, S.H.; Park, W.B.; Lee, K.D.; Kim, H.B.; Kim, E.C.; Oh, M.D.; Choe, K.W. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: Risk factors for mortality and impact of inadequate initial antimicrobial therapy on outcome. Antimicrob. Agents Chemother. 2005, 49, 760–766. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.K.; Park, D.W.; Sohn, J.W.; Kim, H.Y.; Kim, Y.S.; Lee, C.S.; Lee, M.S.; Ryu, S.Y.; Jang, H.C.; Choi, Y.J.; et al. Effects of inadequate empirical antibiotic therapy on mortality in patients with healthcare-associated methicillin-resistant Staphylococcus aureus bacteremia: A propensity-matched analysis. BMC Infect. Dis. 2016, 16, 331. [Google Scholar] [CrossRef] [Green Version]
- Zaragoza, R.; Artero, A.; Camarena, J.J.; Sancho, S.; Gonzalez, R.; Nogueira, J.M. The influence of inadequate empirical antimicrobial treatment on patients with bloodstream infections in an intensive care unit. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2003, 9, 412–418. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.; Shani, V.; Muchtar, E.; Kariv, G.; Robenshtok, E.; Leibovici, L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob. Agents Chemother. 2010, 54, 4851–4863. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Park, W.B.; Lee, C.S.; Kang, C.I.; Bang, J.W.; Kim, H.B.; Kim, N.J.; Kim, E.C.; Oh, M.D.; Choe, K.W. Outcome of inadequate empirical antibiotic therapy in patients with Staphylococcus aureus bacteraemia: Analytical strategy using propensity scores. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2006, 12, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Lambregts, M.M.C.; Bernards, A.T.; van der Beek, M.T.; Visser, L.G.; de Boer, M.G. Time to positivity of blood cultures supports early re-evaluation of empiric broad-spectrum antimicrobial therapy. PLoS ONE 2019, 14, e0208819. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasan, M.N.; Baddour, L.M. Resilience of the Pitt Bacteremia Score: Three Decades and Counting. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. Soc. Am. 2019. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- The Dutch Society for Medical Microbiology. NVMM Guideline Laboratory Detection of Highly Resistant Micro-Organisms; Version 2.0; The Dutch Society for Medical Microbiology: Arnhem, The Netherlands, 2012; Available online: https://www.nvmm.nl/media/1051/2012_hrmo_mrsa_esbl.pdf (accessed on 28 March 2020).
- Retamar, P.; Portillo, M.M.; Lopez-Prieto, M.D.; Rodriguez-Lopez, F.; de Cueto, M.; Garcia, M.V.; Gomez, M.J.; Del Arco, A.; Munoz, A.; Sanchez-Porto, A.; et al. Impact of inadequate empirical therapy on the mortality of patients with bloodstream infections: A propensity score-based analysis. Antimicrob. Agents Chemother. 2012, 56, 472–478. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Dekkers, O.M.; le Cessie, S. A comparison of different methods to handle missing data in the context of propensity score analysis. Eur. J. Epidemiol. 2019, 34, 23–36. [Google Scholar] [CrossRef] [Green Version]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.I.; Wang, X.; Speicher, P.J.; Hwang, E.S.; Cheng, P.; Harpole, D.H.; Berry, M.F.; Schrag, D.; Pang, H.H. Reporting and Guidelines in Propensity Score Analysis: A Systematic Review of Cancer and Cancer Surgical Studies. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Harbarth, S.; Garbino, J.; Pugin, J.; Romand, J.A.; Lew, D.; Pittet, D. Inadequate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am. J. Med. 2003, 115, 529–535. [Google Scholar] [CrossRef]
- Harbarth, S.; Nobre, V.; Pittet, D. Does antibiotic selection impact patient outcome? Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2007, 44, 87–93. [Google Scholar] [CrossRef] [PubMed]
- McGregor, J.C.; Rich, S.E.; Harris, A.D.; Perencevich, E.N.; Osih, R.; Lodise, T.P., Jr.; Miller, R.R.; Furuno, J.P. A systematic review of the methods used to assess the association between appropriate antibiotic therapy and mortality in bacteremic patients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2007, 45, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Moehring, R.W.; Sloane, R.; Schmader, K.E.; Weber, D.J.; Fowler, V.G., Jr.; Smathers, E.; Sexton, D.J. Bloodstream infections in community hospitals in the 21st century: A multicenter cohort study. PLoS ONE 2014, 9, e91713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Q.; Xu, F.; Jiang, S. The impact of previous hospitalization in the preceding 90 days on the outcome in critically ill patients with gram-negative bloodstream infection. Diagn. Microbiol. Infect. Dis. 2014, 80, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensiv. Care Med. 2013, 39, 165–228. [Google Scholar] [CrossRef]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heuer, O.E.; et al. The global threat of antimicrobial resistance: Science for intervention. New Microb. New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Bamgbola, O. Review of vancomycin-induced renal toxicity: An update. Ther. Adv. Endocrinol. Metab. 2016, 7, 136–147. [Google Scholar] [CrossRef]
- Srigley, J.A.; Brooks, A.; Sung, M.; Yamamura, D.; Haider, S.; Mertz, D. Inadequate use of antibiotics and Clostridium difficile infection. Am. J. Infect. Control. 2013, 41, 1116–1118. [Google Scholar] [CrossRef]
- Rhee, C.; Kadri, S.S.; Dekker, J.P.; Danner, R.L.; Chen, H.; Fram, D.; Zhang, F.; Wang, R.; Klompas, M. Prevalence of antibiotic-resistant pathogens in culture-proven sepsis and outcomes associated with inadequate and broad-spectrum empiric antibiotic use. JAMA Netw. Open 2020, 3, e202899. [Google Scholar] [CrossRef]
- Lorusso, L.N. Tolerating Uncertainty—The Next Medical Revolution? Herd 2016, 375, 1713–1715. [Google Scholar] [CrossRef]
| Cohort before PS Matching Empiric Antimicrobial Treatment | Cohort after PS Matching Empiric Antimicrobial Treatment: | |||||
|---|---|---|---|---|---|---|
| Adequate (n = 574) | Inadequate (n = 319) | Adequate (n = 167) | Inadequate (n = 167) | |||
| n (%) | n (%) | p# | n (%) | n (%) | p# | |
| Demographics | ||||||
| Age, mean (range) | 62.1 (18–98) | 63.0 (18–92) | 0.41 | 62.2 (20–91) | 61.7 (18–92) | NS |
| Male | 327 (57.0) | 206 (64.6) | 0.03 | 100 (59.9) | 102 (61.1) | NS |
| Microbiology parameters | ||||||
| High risk pathogen | 257 (44.9) | 158 (58.0) | <0.01 | 82 (49.1) | 91 (54.5) | NS |
| TTP mean no. of hours (IQR) | 19.0 (13–19) | 21.0 (14–21) | <0.01 | 19.75 (13–18) | 20.17 (14–21) | 0.02 |
| Gram positive pathogen | 218 (38.0) | 166 (52.0) | <0.001 | 74 (44.3) | 43.1 | NS |
| Hospital acquired infection | 24.9% | 141 (44.2) | <0.001 | 63 (37.7) | 58 (34.7) | NS |
| Source of infection | ||||||
| Urinary tract | 180 (31.4) | 51 (16.0) | <0.001 | 35 (21.0) | 37 (22.2) | NS |
| Gastro-intestinal | 436 (76.0) | 212 (66.5) | 0.003 | 113 (67.7) | 115 (68.9) | NS |
| Pulmonary | 78 (13.6) | 11 (3.4) | <0.001 | 12 (7.2) | 10 (6.0) | NS |
| Endovasculair | 49 (8.5) | 61 (19.1) | <0.001 | 23 (13.8) | 21 (12.6) | NS |
| Soft tissue | 46 (8.0) | 23 (7.2) | 0.70 | 13 (7.8) | 15 (9.0) | NS |
| Unidentified | 42 (7.3) | 42 (13.2) | 0.006 | 19 (11.4) | 19 (11.4) | NS |
| Source correctly identified at presentation | 426 (74.3) | 120 (38.2) | <0.001 | 83 (49.7) | 88 (52.7) | NS |
| Risk factors for antimicrobial resistance | ||||||
| Antibiotic pre-treatment at presentation | 152 (26.5) | 111 (35.1) | 0.007 | 61 (36.5) | 58 (35.2) | NS |
| Antibiotic treatment in prior 2 months | 246 (44.2) | 188 (60.5) | <0.001 | 95 (56.9) | 90 (53.9) | NS |
| Gram negative MDRO in prior 6 months | 35 (6.1) | 21 (6.6) | 0.77 | 10 (6.0) | 11 (6.6) | NS |
| Intensive care unit stay in prior 6 months | 42 (7.3) | 40 (12.5) | 0.01 | 20 (12.0) | 16 (9.6) | NS |
| Medical history | ||||||
| Central intravenous catheter | 90 (15.7) | 79 (24.8) | 0.001 | 34 (20.4) | 33 (19.8) | NS |
| Corticosteroïd therapy | 171(29.8) | 104 (32.6) | 0.41 | 52 (31.1) | 55 (32.9) | NS |
| Diabetes mellitus | 126 (22.0) | 60 (18.8) | 0.30 | 38 (22.8) | 35 (21.0) | NS |
| Neutropenia | 80 (13.9) | 33 (10.3) | 0.14 | 28 (16.8) | 25 (15.0) | NS |
| Stem cell transplantation | 41 (7.1) | 29 (9.1) | 0.30 | 15 (9.0) | 18 (10.8) | NS |
| Solid organ transplantation | 80 (13.9) | 35 (11.0) | 0.21 | 20 (12.0) | 24 (14.4) | NS |
| Hematologic malignancy | 57 (9.9) | 39 (12.2) | 0.31 | 23 (13.8) | 22 (13.2) | NS |
| Malignancy (non-hematological) | 95 (16.6) | 74 (23.3) | 0.016 | 32 (19.2) | 33 (17.5) | NS |
| Clinical presentation | ||||||
| Temperature >38.5 °C | 380 (67.7) | 157 (50.8) | <0.001 | 99 (59.3) | 104 (62.3) | NS |
| Systolic bloodpressure <90 mmHg | 111 (19.3) | 46 (14.4) | 0.07 | 26 (15.6) | 28 (16.8) | NS |
| Respiratory rate >22/min | 177 (30.8) | 45 (14.1) | <0.001 | 34 (20.4) | 29 (17.4) | NS |
| Pitt bacteremia score, mean (IQR) | 1.26 (0–2) | 1.17 (0–2) | <0.003 | 1.09 (0–1) | 1.05 (0–1) | NS |
| qSOFA, median (IQR) | 1 (0–2) | 1 (0–1) | <0.001 | 1 (0–1) | 1 (0–1) | NS |
| Outcome Variable | Adequate Empiric Regimen n (%) | Inadequate Empiric Regimen n (%) | Difference n (%) | OR # | 95%CI | p ^ |
|---|---|---|---|---|---|---|
| 14-day mortality | 17/167 (10.18) | 16/167 (9.58) | 1 (0.60) | 0.77 | 0.43–1.85 | 0.45 |
| 30-day mortality | 25/167 (14.97) | 21/167 (12.57) | 4 (2.40) | 0.78 | 0.42–1.47 | 0.45 |
| Length of hospital stay in days *, median (IQR) | 10.7 (4.6–18.2) | 10.5 (4.3–20.3) | – | – | – | 0.89 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambregts, M.M.C.; Wijnakker, R.; Bernards, A.T.; Visser, L.G.; le Cessie, S.; de Boer, M.G.J. Mortality After Delay of Adequate Empiric Antimicrobial Treatment of Bloodstream Infection. J. Clin. Med. 2020, 9, 1378. https://doi.org/10.3390/jcm9051378
Lambregts MMC, Wijnakker R, Bernards AT, Visser LG, le Cessie S, de Boer MGJ. Mortality After Delay of Adequate Empiric Antimicrobial Treatment of Bloodstream Infection. Journal of Clinical Medicine. 2020; 9(5):1378. https://doi.org/10.3390/jcm9051378
Chicago/Turabian StyleLambregts, Merel M. C., Roos Wijnakker, Alexandra T. Bernards, Leo G. Visser, Saskia le Cessie, and Mark G. J. de Boer. 2020. "Mortality After Delay of Adequate Empiric Antimicrobial Treatment of Bloodstream Infection" Journal of Clinical Medicine 9, no. 5: 1378. https://doi.org/10.3390/jcm9051378
APA StyleLambregts, M. M. C., Wijnakker, R., Bernards, A. T., Visser, L. G., le Cessie, S., & de Boer, M. G. J. (2020). Mortality After Delay of Adequate Empiric Antimicrobial Treatment of Bloodstream Infection. Journal of Clinical Medicine, 9(5), 1378. https://doi.org/10.3390/jcm9051378




