Linezolid Concentrations in Plasma and Subcutaneous Tissue are Reduced in Obese Patients, Resulting in a Higher Risk of Underdosing in Critically Ill Patients: A Controlled Clinical Pharmacokinetic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Intervention
2.3. Blood and Microdialysate Samples
2.4. Microdialysis
2.5. Study Visits and Data Collection
2.6. Drug Analysis
2.7. Study Endpoints
2.8. Pharmacokinetic and Statistical Analysis
3. Results
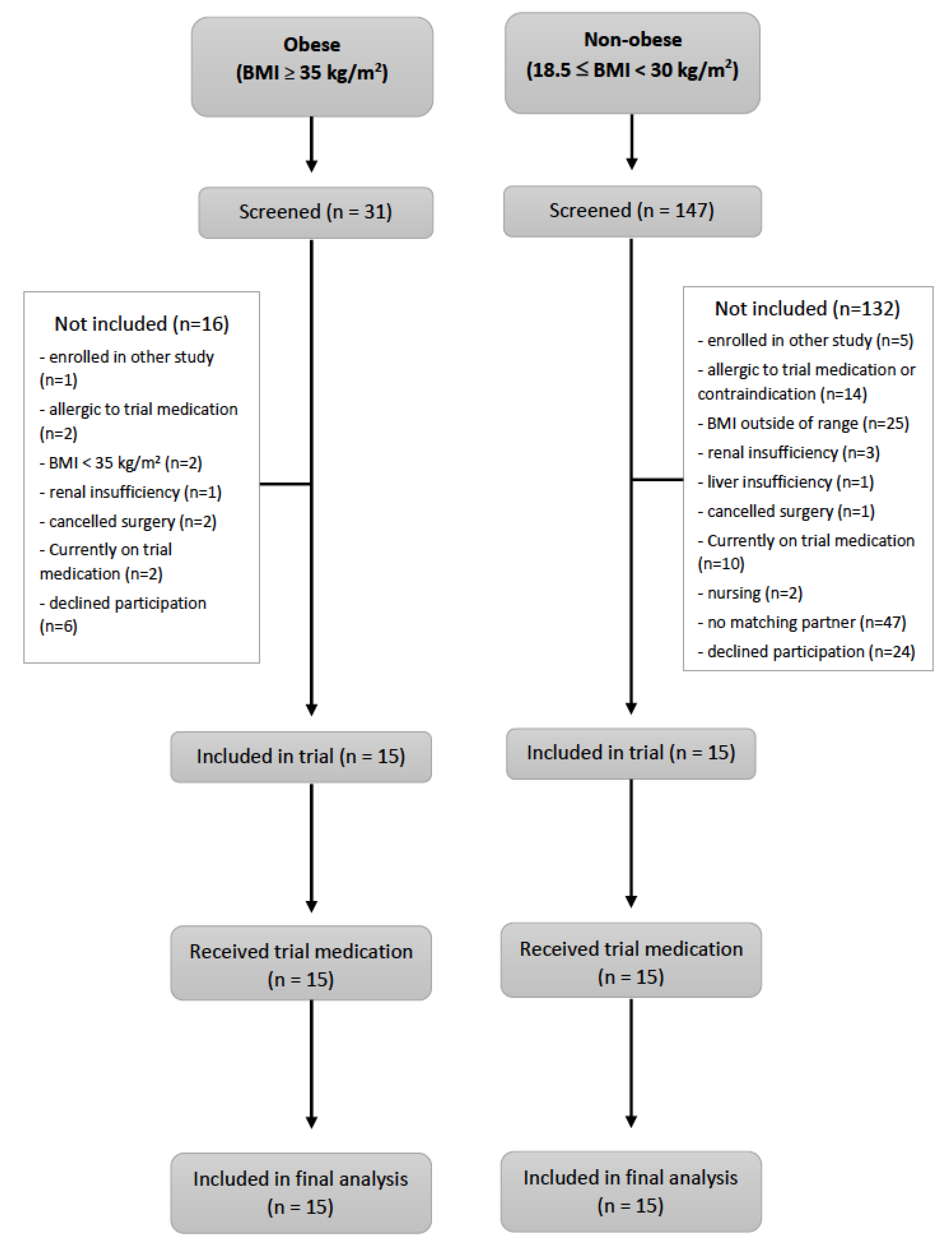
3.1. Participants
3.2. Primary (ISF) and Secondary Study Endpoint (Plasma): Correlation of AUC0–8 with Weight
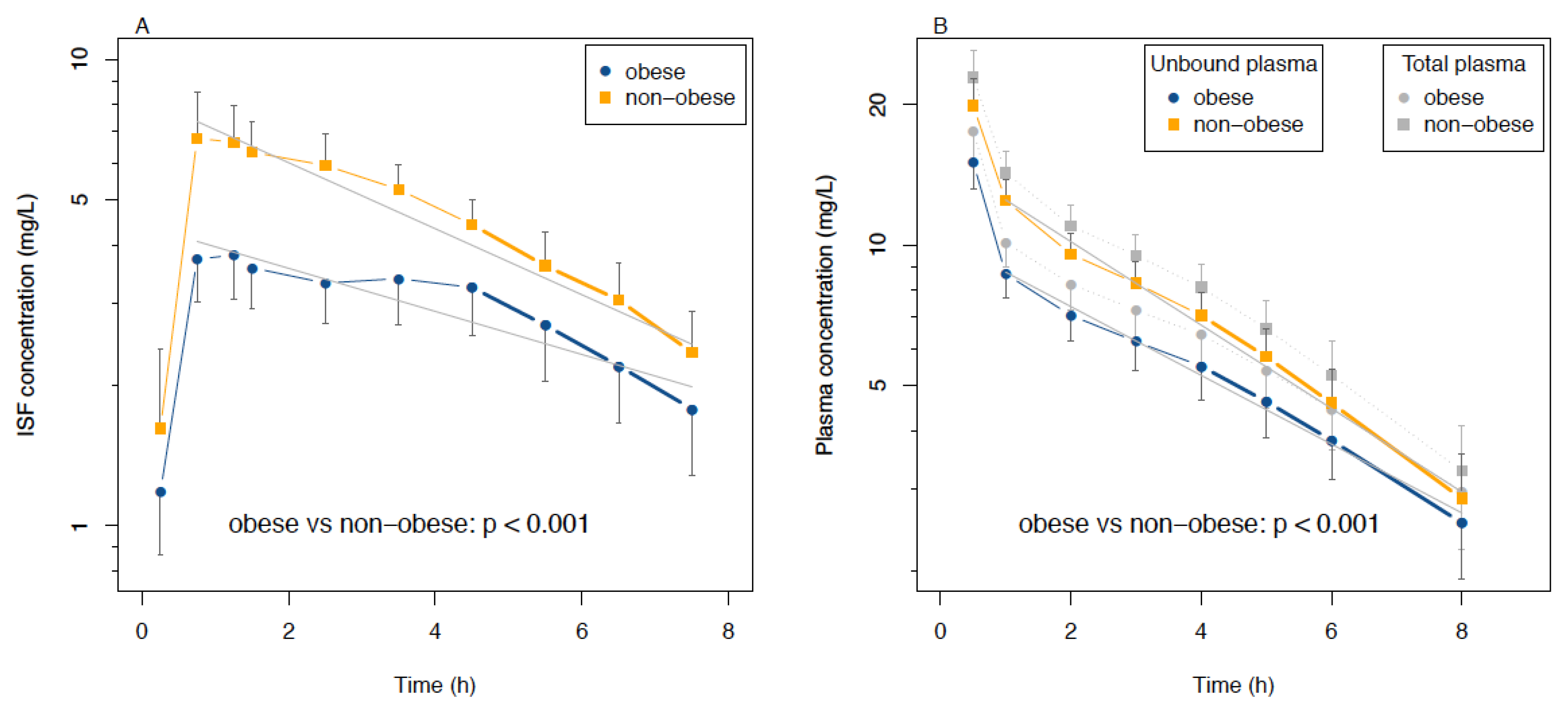
3.3. Analysis of ISF and Plasma Data as Functions of Time
3.4. PK Parameters in ISF and Plasma and Statistical Analysis
3.5. PK Relations to MIC Values for Susceptible Bacteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C. Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassetti, M.; Baguneid, M.; Bouza, E.; Dryden, M.; Nathwani, D.; Wilcox, M. European perspective and update on the management of complicated skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus after more than 10 years of experience with linezolid. Clin. Microbiol. Infect. 2014, 20, 3–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartelli, M.; Guirao, X.; Hardcastle, T.C.; Kluger, Y.; Boermeester, M.A.; Raşa, K.; Ansaloni, L.; Coccolini, F.; Montravers, P.; Abu-Zidan, F.M.; et al. 2018 WSES/SIS-E consensus conference: Recommendations for the management of skin and soft-tissue infections. World J. Emerg. Surg. 2018, 13, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnham, J.P.; Kirby, J.P.; Kollef, M.H. Diagnosis and management of skin and soft tissue infections in the intensive care unit: A review. Intensive Care Med. 2016, 42, 1899–1911. [Google Scholar] [CrossRef]
- Brinkmann, A.; Röhr, A.C.; Frey, O.R.; Krüger, W.A.; Brenner, T.; Richter, D.C.; Bodmann, K.F.; Kresken, M.; Grabein, B. S2k guidelines of the PEG on calculated parenteral initial treatment of bacterial diseases in adults: Focussed summary and supplementary information on antibiotic treatment of critically ill patients. Anaesthesist 2018, 67, 936–949. [Google Scholar] [CrossRef]
- Minichmayr, I.K.; Schaeftlein, A.; Kuti, J.L.; Zeitlinger, M.; Kloft, C. Clinical Determinants of Target Non-Attainment of Linezolid in Plasma and Interstitial Space Fluid: A Pooled Population Pharmacokinetic Analysis with Focus on Critically Ill Patients. Clin. Pharmacokinet. 2017, 56, 617–633. [Google Scholar] [CrossRef]
- Xie, F.; Mantzarlis, K.; Malliotakis, P.; Koulouras, V.; Degroote, S.; Koulenti, D.; Blot, S.; Boussery, K.; Van Bocxlaer, J.; Colin, P.; et al. Pharmacokinetic evaluation of linezolid administered intravenously in obese patients with pneumonia. J. Antimicrob. Chemother. 2019, 74, 667–674. [Google Scholar] [CrossRef]
- Dong, H.; Xie, J.; Wang, T.; Chen, L.; Zeng, X.; Sun, J.; Wang, X.; Dong, Y. Pharmacokinetic/pharmacodynamic evaluation of linezolid for the treatment of staphylococcal infections in critically ill patients. Int. J. Antimicrob. Agents 2016, 48, 259–264. [Google Scholar] [CrossRef]
- Taubert, M.; Zander, J.; Frechen, S.; Scharf, C.; Frey, L.; Vogeser, M.; Fuhr, U.; Zoller, M. Optimization of linezolid therapy in the critically ill: The effect of adjusted infusion regimens. J. Antimicrob. Chemother. 2017, 72, 2304–2310. [Google Scholar] [CrossRef]
- Cojutti, P.; Pai, M.P.; Pea, F. Population Pharmacokinetics and Dosing Considerations for the Use of Linezolid in Overweight and Obese Adult Patients. Clin. Pharmacokinet. 2018, 57, 989–1000. [Google Scholar] [CrossRef]
- Toma, O.; Suntrup, P.; Stefanescu, A.; London, A.; Mutch, M.; Kharasch, E. Pharmacokinetics and tissue penetration of cefoxitin in obesity: Implications for risk of surgical site infection. Anesth. Analg. 2011, 113, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Hollenstein, U.M.; Brunner, M.; Schmid, R.; Muller, M. Soft tissue concentrations of ciprofloxacin in obese and lean subjects following weight-adjusted dosing. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 354–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jager, N.G.L.; van Hest, R.M.; Lipman, J.; Roberts, J.A.; Cotta, M.O. Antibiotic exposure at the site of infection: Principles and assessment of tissue penetration. Expert Rev. Clin. Pharmacol. 2019, 12, 623–634. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight 2009 [Fact Sheet No. 311; Online]. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/index.html (accessed on 29 September 2009).
- Alobaid, A.S.; Hites, M.; Lipman, J.; Taccone, F.S.; Roberts, J.A. Effect of obesity on the pharmacokinetics of antimicrobials in critically ill patients: A structured review. Int. J. Antimicrob. Agents 2016, 47, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Mui, E.; Holubar, M.K.; Deresinski, S.C. Comprehensive Guidance for Antibiotic Dosing in Obese Adults. Pharmacotherapy 2017, 37, 1415–1431. [Google Scholar] [CrossRef]
- Hanley, M.J.; Abernethy, D.R.; Greenblatt, D.J. Effect of obesity on the pharmacokinetics of drugs in humans. Clin. Pharmacokinet. 2010, 49, 71–87. [Google Scholar] [CrossRef]
- Dehghanyar, P.; Bürger, C.; Zeitlinger, M.; Islinger, F.; Kovar, F.; Müller, M.; Kloft, C.; Joukhadar, C. Penetration of linezolid into soft tissues of healthy volunteers after single and multiple doses. Antimicrob. Agents Chemother. 2005, 49, 2367–2371. [Google Scholar] [CrossRef] [Green Version]
- Gee, T.; Ellis, R.; Marshall, G.; Andrews, J.; Ashby, J.; Wise, R. Pharmacokinetics and tissue penetration of linezolid following multiple oral doses. Antimicrob. Agents Chemother. 2001, 45, 1843–1846. [Google Scholar] [CrossRef] [Green Version]
- Schwameis, R.; Syré, S.; Sarahrudi, K.; Appelt, A.; Marhofer, D.; Burau, D.; Kloft, C.; Zeitlinger, M. Penetration of linezolid into synovial fluid and muscle tissue after elective arthroscopy. J. Antimicrob. Chemother. 2017, 72, 2817–2822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thallinger, C.; Buerger, C.; Plock, N.; Kljucar, S.; Wuenscher, S.; Sauermann, R.; Kloft, C.; Joukhadar, C. Effect of severity of sepsis on tissue concentrations of linezolid. J. Antimicrob. Chemother. 2008, 61, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Buerger, C.; Plock, N.; Dehghanyar, P.; Joukhadar, C.; Kloft, C. Pharmacokinetics of unbound linezolid in plasma and tissue interstitium of critically ill patients after multiple dosing using microdialysis. Antimicrob. Agents Chemother. 2006, 50, 2455–2463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pascale, G.; Fortuna, S.; Tumbarello, M.; Cutuli, S.L.; Vallecoccia, M.; Spanu, T.; Bello, G.; Montini, L.; Pennisi, M.A.; Navarra, P.; et al. Linezolid plasma and intrapulmonary concentrations in critically ill obese patients with ventilator-associated pneumonia: Intermittent vs continuous administration. Intensive Care Med. 2015, 41, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Petroff, D.; Dorn, C.; Ehmann, L.; Kloft, C.; Prettin, C.; Dietrich, A.; Zeitlinger, M.; Kees, F.; Wrigge, H. Measurement of soft tissue drug concentrations in morbidly obese and non-obese patients—A prospective, parallel group, open-labeled, controlled, phase IV, single center clinical trial. Contemp. Clin. Trials Commun. 2019, 15, 100375. [Google Scholar] [CrossRef] [PubMed]
- Bouw, M.R.; Hammarlund-Udenaes, M. Methodological Aspects of the Use of a Calibrator in In Vivo Microdialysis–Further Development of the Retrodialysis Method. Pharm. Res. 1998, 15, 1673–1679. [Google Scholar] [CrossRef]
- Burau, D.; Petroff, D.; Simon, P.; Ehmann, L.; Weise, C.; Dorn, C.; Kratzer, A.; Wrigge, H.; Kloft, C. Drug combinations and impact of experimental conditions on relative recovery in in vitro microdialysis investigations. Eur. J. Pharm. Sci. 2019, 127, 252–260. [Google Scholar] [CrossRef]
- Töpper, C.; Steinbach, C.L.; Dorn, C.; Kratzer, A.; Schleibinger, M.; Liebchen, U.; Kees, F.; Salzberger, B.; Kees, M.G. Variable Linezolid Exposure in Intensive Care Unit Patients-Possible Role of Drug-Drug Interactions. Ther. Drug Monit. 2016, 38, 573–578. [Google Scholar]
- Rayner, C.R.; Forrest, A.; Meagher, A.K.; Birmingham, M.C.; Schentag, J.J. Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use programme. Clin. Pharmacokinet. 2003, 42, 1411–1423. [Google Scholar] [CrossRef]
- Pea, F.; Furlanut, M.; Cojutti, P.; Cristini, F.; Zamparini, E.; Franceschi, L.; Viale, P. Therapeutic drug monitoring of linezolid: A retrospective monocentric analysis. Antimicrob. Agents Chemother. 2010, 54, 4605–4610. [Google Scholar] [CrossRef] [Green Version]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 9.0. 2019. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf (accessed on 1 January 2019).
- Maronna, R.A.; Yohai, V.J. The behavior of the Stahel-Donoho robust multivariate estimator. J. Am. Stat. Assoc. 1995, 90, 330–341. [Google Scholar] [CrossRef]
- Wang, J.; Zamar, R.; Marazzi, A.; Yohai, V.; Salibian-Barrera, M.; Maronna, R.; Zivot, E.; Rocke, D.; Martin, D.; Maechler, M.; et al. Robust: Port of the S+ “Robust Library” 2020. R Package Version 0.5-0.0. Available online: https://CRAN.R-project.org/package=robust (accessed on 27 March 2020).
- Bhalodi, A.A.; Papasavas, P.K.; Tishler, D.S.; Nicolau, D.P.; Kuti, J.L. Pharmacokinetics of Intravenous Linezolid in Moderately to Morbidly Obese Adults. Antimicrob. Agents Chemother. 2013, 57, 1144–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taubert, M.; Zoller, M.; Maier, B.; Frechen, S.; Scharf, C.; Holdt, L.M.; Frey, L.; Vogeser, M.; Fuhr, U.; Zander, J. Predictors of Inadequate Linezolid Concentrations after Standard Dosing in Critically Ill Patients. Antimicrob. Agents Chemother. 2016, 60, 5254–5261. [Google Scholar] [CrossRef] [Green Version]
- Meagher, A.K.; Forrest, A.; Rayner, C.R.; Birmingham, M.C.; Schentag, J.J. Population pharmacokinetics of linezolid in patients treated in a compassionate-use program. Antimicrob. Agents Chemother. 2013, 47, 548–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGee, B.; Dietze, R.; Hadah, D.J.; Molino, L.P.; Maciel, E.L.; Boom, W.H.; Palaci, M.; Johnson, J.L.; Peloquin, C.A. Population pharmacokinetics of linezolid in adults with pulmonary tuberculosis. Antimicrob. Agents Chemother. 2009, 53, 3981–3984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brill, M.J.; Houwink, A.P.; Schmidt, S.; Van Dongen, E.P.; Hazebroek, E.J.; van Ramshorst, B.; Deneer, V.H.; Mouton, J.W.; Knibbe, C.A. Reduced subcutaneous tissue distribution of cefazolin in morbidly obese versus non-obese patients determined using clinical microdialysis. J. Antimicrob. Chemother. 2014, 69, 715–723. [Google Scholar] [CrossRef] [Green Version]
- Blot, S.I.; Pea, F.; Lipman, J. The effect of pathophysiology on pharmacokinetics in the critically ill patient—Concepts appraised by the example of antimicrobial agents. Adv. Drug Deliv. Rev. 2014, 77, 3–11. [Google Scholar] [CrossRef]
- Udy, A.A.; Putt, M.T.; Boots, R.J.; Lipman, J. ARC—Augmented renal clearance. Curr. Pharm. Biotechnol. 2011, 12, 2020–2029. [Google Scholar] [CrossRef]
- Udy, A.A.; Roberts, J.A.; Boots, R.J.; Paterson, D.L.; Lipman, J. Augmented renal clearance: Implications for antibacterial dosing in the critically ill. Clin. Pharmacokinet. 2010, 49, 1–16. [Google Scholar] [CrossRef]
- Yue, J.; Dong, B.R.; Yang, M.; Chen, X.; Wu, T.; Liu, G.J. Linezolid versus vancomycin for skin and soft tissue infections. Cochrane Database Syst. Rev. 2016, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brier, M.E.; Stalker, D.J.; Aronoff, G.R.; Batts, D.H.; Ryan, K.K.; O’Grady, M.; Hopkins, N.K.; Jungbluth, G.L. Pharmacokinetics of Linezolid in Subjects with Renal Dysfunction. Antimicrob. Agents Chemother. 2003, 47, 2775–2780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Zhang, J.; Chen, Y.; Liang, X.; Guo, Y.; Yu, J.; Zhu, D.; Zhang, Y. Optimization of linezolid treatment regimens for Gram-positive bacterial infections based on pharmacokinetic/pharmacodynamic analysis. Future Microbiol. 2017, 12, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tang, Y.; Zhang, X.; Wu, C.; Kong, L. Pharmacokinetics and pharmacodynamics of linezolid in plasma/cerebrospinal fluid in patients with cerebral hemorrhage after lateral ventricular drainage by Monte Carlo simulation. Drug Des. Dev. Ther. 2018, 12, 1679–1684. [Google Scholar] [CrossRef] [Green Version]
- Pfaller, M.A.; Cormican, M.; Flamm, R.K.; Mendes, R.E.; Jones, R.N. Temporal and Geographic Variation in Antimicrobial Susceptibility and Resistance Patterns of Enterococci: Results From the SENTRY Antimicrobial Surveillance Program, 1997–2016. Open Forum Infect. Dis. 2019, 6, 54–62. [Google Scholar] [CrossRef]
- Tsilipounidaki, K.; Gerontopoulos, A.; Papagiannitsis, C.; Petinaki, E. First detection of an optrA-positive, linezolid-resistant ST16 Enterococcus faecalis from human in Greece. New Microbes New Infect. 2019, 29, 100515. [Google Scholar] [CrossRef] [PubMed]
- Sassi, M.; Guérin, F.; Zouari, A.; Beyrouthy, R.; Auzou, M.; Fines-Guyon, M.; Potrel, S.; Dejoies, L.; Collet, A.; Boukthir, S.; et al. Emergence of optrA-mediated linezolid resistance in enterococci from France, 2006–16. J. Antimicrob. Chemother. 2019, 74, 1469–1472. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, L.M.; Castanheira, M.; Flamm, R.K.; Mendes, R.E. Evolving oxazolidinone resistance mechanisms in a worldwide collection of enterococcal clinical isolates: Results from the SENTRY Antimicrobial Surveillance Program. J. Antimicrob. Chemother. 2018, 73, 2314–2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pea, F.; Cojutti, P.G.; Baraldo, M. A 10-Year Experience of Therapeutic Drug Monitoring (TDM) of Linezolid in a Hospital-wide Population of Patients Receiving Conventional Dosing: Is there Enough Evidence for Suggesting TDM in the Majority of Patients. Basic Clin. Pharmacol. Toxicol. 2017, 121, 303–308. [Google Scholar] [CrossRef]
- Zoller, M.; Maier, B.; Hornuss, C.; Neugebauer, C.; Döbbeler, G.; Nagel, D.; Holdt, L.M.; Bruegel, M.; Weig, T.; Grabein, B.; et al. Variability of linezolid concentrations after standard dosing in critically ill patients: A prospective observational study. Crit. Care 2014, 18, R148. [Google Scholar] [CrossRef] [Green Version]
- Stalker, D.J.; Jungbluth, G.L.; Hopkins, N.K.; Batts, D.H. Pharmacokinetics and tolerance of single- and multiple-dose oral or intravenous linezolid, an oxazolidinone antibiotic, in healthy volunteers. J. Antimicrob. Chemother. 2003, 51, 1239–1246. [Google Scholar] [CrossRef] [Green Version]
- Ehmann, L.; Simon, P.; Busse, D.; Petroff, D.; Dorn, C.; Huisinga, W.; Dietrich, A.; Zeitlinger, M.; Wrigge, H.; Kloft, C. Risk of target non-attainment in obese compared to non-obese patients in calculated linezolid therapy. Clin. Microbiol. Infect. 2020. under review. [Google Scholar]
- Green, B.; Duffull, S.B. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br. J. Clin. Pharmacol. 2004, 58, 119–133. [Google Scholar] [CrossRef] [Green Version]
- Traunmueller, F.; Schintler, M.V.; Spendel, S.; Popovic, M.; Mauric, O.; Scharnagl, E.; Joukhadar, C. Linezolid concentrations in infected soft tissue and bone following repetitive doses in diabetic patients with bacterial foot infections. Int. J. Antimicrob. Agents 2010, 36, 84–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eslam, R.B.; Burian, A.; Vila, G.; Sauermann, R.; Hammer, A.; Frenzel, D.; Minichmayr, I.K.; Kloft, C.; Matneller, P.; Oesterreicher, Z.; et al. Target Site Pharmacokinetics of Linezolid After Single and Multiple Doses in Diabetic Patients with Soft Tissue Infection. J. Clin. Pharmacol. 2014, 54, 1058–1062. [Google Scholar] [CrossRef] [PubMed]

| Obese (n = 15) | Non-Obese (n = 15) | |
|---|---|---|
| Number of females (n (%)) | 13 (87%) | 13 (87%) |
| Age (years) | 50.3 ± 9.5 | 49.5 ± 10.0 |
| Weight (kg) | 134.3 ± 34.3 | 67.9 ± 8.8 |
| Height (m) | 1.66 ± 0.07 | 1.68 ± 0.07 |
| BMI (kg/m2) | 48.7 ± 11.2 | 23.9 ± 2.1 |
| Creatinine (µmol/L) | 86.4 ± 27.2 | 75.3 ± 18.9 |
| Type of surgery (n (%)) | ||
| laparoscopic | 15 (100%) | 3 (20%) |
| open | 0 (0%) | 12 (80%) |
| ASA classification (n (%)) | ||
| I | 1 | 2 |
| II | 4 | 11 |
| III | 10 | 2 |
| All Patients (n = 30) | Pearson’s Correlation Coefficient with Weight | Obese (n = 15) | Non-Obese (n = 15) | p-Value | |
|---|---|---|---|---|---|
| Cmax (mg/L) | |||||
| ISF | 5.5 [3.8, 8.5] | −0.70 (−0.85 to −0.45) | 3.8 [3.2, 5.3] | 8.3 [5.7, 10.2] | <0.001 |
| Plasma (total) | 21.9 [17.2, 24.4] | −0.63 (−0.81 to −0.36) | 19.2 [16.1, 22.1] | 24.4 [18.9, 26.9] | 0.013 |
| Plasma (unbound) | 18.5 [14.4, 21.1] | −0.66 (−0.82 to −0.39) | 15.9 [13.3, 18.7] | 21.1 [16.6, 23.3] | 0.007 |
| t1/2 (hours) | |||||
| ISF | 3.9 [2.8, 4.8] | 0.11 (−0.26 to 0.45) | 3.9 [2.7, 5.1] | 3.8 [3.0, 4.2] | 0.664 |
| Plasma (total and unbound) | 3.4 [2.6, 4.4] | 0.52 (0.20 to 0.74) | 3.8 [3.0, 4.8] | 3.0 [2.5, 3.6] | 0.088 |
| Vss (L), plasma | 37.7 [31.3, 44.6] | 0.87 (0.75 to 0.94) | 41.0 [38.4, 57.9] | 30.8 [27.9, 35.5] | <0.001 |
| CL (L/h), plasma | 7.2 [6.3, 9.3] | 0.21 (−0.16 to 0.53) | 8.2 [6.2, 10.8] | 7.0 [6.4, 8.2] | 0.170 |
| MRT (h), plasma | 5.0 [3.8, 6.3] | 0.47 (0.13 to 0. 71) | 5.7 [4.5, 6.9] | 4.4 [3.8, 5.4] | 0.079 |
| Tmax, ISF 30–60/60–90/>90 min (number of patients) | 17/3/10 | — | 7/3/5 | 10/0/5 | 0.252 |
| AUC0-8, (mg*h/L) | |||||
| ISF | 29.4 [23.5, 37.8] | −0.61 (−0.80 to −0.32) | 23.7 [20.2, 28.1] | 34.4 [29.4, 45.5] | 0.004 |
| Plasma (total) | 63.4 [52.8, 71.1] | −0.66 (−0.82 to −0.39) | 57.9 [45.4, 65.6] | 67.8 [61.5, 80.5] | 0.011 |
| Plasma (unbound) | 54.5 [45.6, 61.5] | −0.68 (−0.84 to −0.43) | 47.7 [39.7, 56.7] | 59.6 [51.7, 70.8] | 0.005 |
| AUC0–8 ISF/fAUC0–8 plasma | 0.53 [0.46, 0.65] | −0.28 (−0.58 to 0.09) | 0.53 [0.45, 0.55] | 0.62 [0.53, 0.68] | 0.080 |
| All Patients (n = 30) | Pearson’s Correlation Coefficient with Weight | Obese (n = 15) | Non-Obese (n = 15) | p-Value | ||
|---|---|---|---|---|---|---|
| MIC (mg/L) | ||||||
| fAUC24/MIC | ||||||
| ISF | 0.5 | 176.8 [125.4, 218.2] | −0.47 (−0.71 to −0.13) | 140.0 [105.6, 207.0] | 182.4 [165.4, 239.6] | 0.033 |
| 1 | 88.4 [62.7, 109.1] | 70.0 [52.8, 103.5] | 91.2 [82.7, 119.8] | |||
| 2 | 44.2 [31.3, 54.6] | 35.0 [26.4, 51.7] | 45.6 [41.3, 59.9] | |||
| 4 | 22.1 [15.7, 27.3] | 17.5 [13.2, 25.9] | 22.8 [20.7, 30.0] | |||
| plasma | 0.5 | 286.6 [220.8, 324.0] | −0.42 (−0.68 to −0.08) | 237.6 [199.8, 321.8] | 289.4 [250.0, 324.0] | 0.175 |
| 1 | 143.3 [110.4, 162.0] | 118.8 [99.9, 160.9] | 144.7 [125.0, 162.0] | |||
| 2 | 71.7 [55.2, 81.0] | 59.4 [49.9, 80.5] | 72.4 [62.5, 81.0] | |||
| 4 | 35.8 [27.6, 40.4] | 29.7 [25.0, 40.2] | 36.2 [31.3, 40.5] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, P.; Busse, D.; Petroff, D.; Dorn, C.; Ehmann, L.; Hochstädt, S.; Girrbach, F.; Dietrich, A.; Zeitlinger, M.; Kees, F.; et al. Linezolid Concentrations in Plasma and Subcutaneous Tissue are Reduced in Obese Patients, Resulting in a Higher Risk of Underdosing in Critically Ill Patients: A Controlled Clinical Pharmacokinetic Study. J. Clin. Med. 2020, 9, 1067. https://doi.org/10.3390/jcm9041067
Simon P, Busse D, Petroff D, Dorn C, Ehmann L, Hochstädt S, Girrbach F, Dietrich A, Zeitlinger M, Kees F, et al. Linezolid Concentrations in Plasma and Subcutaneous Tissue are Reduced in Obese Patients, Resulting in a Higher Risk of Underdosing in Critically Ill Patients: A Controlled Clinical Pharmacokinetic Study. Journal of Clinical Medicine. 2020; 9(4):1067. https://doi.org/10.3390/jcm9041067
Chicago/Turabian StyleSimon, Philipp, David Busse, David Petroff, Christoph Dorn, Lisa Ehmann, Sophie Hochstädt, Felix Girrbach, Arne Dietrich, Markus Zeitlinger, Frieder Kees, and et al. 2020. "Linezolid Concentrations in Plasma and Subcutaneous Tissue are Reduced in Obese Patients, Resulting in a Higher Risk of Underdosing in Critically Ill Patients: A Controlled Clinical Pharmacokinetic Study" Journal of Clinical Medicine 9, no. 4: 1067. https://doi.org/10.3390/jcm9041067





