Use of Biosimilar Follicle-Stimulating Hormone in Asthenozoospermic Infertile Patients: A Multicentric Study
Abstract
:1. Introduction
2. Experimental Section
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Stamatiades, G.A.; Kaiser, U.B. Gonadotropin regulation by pulsatile GnRH: Signaling and gene expression. Mol. Cell Endocrinol. 2018, 463, 131–141. [Google Scholar] [CrossRef]
- Matsumoto, A.M.; Karpas, A.E.; Bremmer, W.J. Chronic human chorionic gonadotropin administration in normal men: Evidence that follicle stimulating hormone is necessary for the maintenance of quantitative normal spermatogenesis in men. J. Clin. Endocrinol. Metab. 1986, 62, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, R.I.; Wreford, N.G.; de Kretser, D.M.; Robertson, D.M. The effects of recombinant follicle-stimulating hormone on restoration of spermatogenesis in gonadotropin-releasing hormone-immunized adult rat. Endocrinology 1995, 136, 4035–4043. [Google Scholar] [CrossRef]
- Marshall, G.R.; Zorub, D.S.; Plant, T.M. Follicle-stimulating hormone amplifies the population of differentiated spermatogonial in the hypophysectomised testosterone-replaced adult rhesus monkey (Macaca mulatta). Endocrinology 1995, 136, 3504–3511. [Google Scholar] [CrossRef] [PubMed]
- Moudgal, N.R.; Murthy, G.S.; Prasanna, K.M.; Martin, F.; Suresh, R.; Medhamurthy, R.; Patil, S.; Sehgal, S.; Saxena, B.N. Responsiveness of human male volunteers to immunization with ovine follicle stimulating hormone vaccine: Results of a pilot study. Hum. Reprod. 1997, 12, 457–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapanainen, J.S.; Aittomaki, K.; Min, J.; Vaskivuo, T.; Huhtaniemi, I.T. Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nat. Genet. 1997, 15, 205–206. [Google Scholar] [CrossRef]
- Santi, D.; Crépieux, P.; Reiter, E.; Spaggiari, G.; Brigante, G.; Casarini, L.; Rochira, V.; Simoni, M. Follicle-Stimulating deHormone (FSH) action on spermatogenesis: A focus on physiological and therapeutic roles. J. Clin. Med. 2020, 9, 1014. [Google Scholar] [CrossRef] [Green Version]
- Kangasniemi, M.; Kaipia, A.; Toppari, J.; Perheentupa, A.; Huhtaniemi, I.; Parvinen, M. Cellular regulation of follicle-stimulating hormone (FSH) binding in rat seminiferous tubules. J. Androl. 1990, 11, 336–343. [Google Scholar]
- Mruk, D.D.; Cheng, C.Y. The Mammalian Blood-Testis Barrier: Its Biology and Regulation. Endocr. Rev. 2015, 36, 564–591. [Google Scholar] [CrossRef]
- Simoni, M.; Gromoll, J.; Nieschlag, E. The follicle-stimulating hormone receptor: Biochemistry, molecular biology, physiology, and pathophysiology. Endocr. Rev. 1997, 18, 739–773. [Google Scholar] [CrossRef]
- Casarini, L.; Crépieux, P. Molecular Mechanisms of Action of FSH. Front. Endocrinol. (Lausanne) 2019, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Chappel, S.C.; Ulloa-Aguirre, A.; Coutifaris, C. Biosynthesis and secretion of follicle-stimulating hormone. Endocr. Rev. 1983, 4, 179–211. [Google Scholar] [CrossRef] [PubMed]
- Wide, L.; Wide, M. Higher plasma disappearance rate in the mouse for pituitary follicle-stimulating hormone of young women compared to that of men and elderly women. J. Clin. Endocrinol. Metab. 1984, 58, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Bousfield, G.R.; May, J.V.; Davis, J.S.; Dias, J.A.; Kumar, T.R. In Vivo and In Vitro Impact of Carbohydrate Variation on Human Follicle-Stimulating Hormone Function. Front. Endocrinol. (Lausanne) 2018, 9, 216. [Google Scholar] [CrossRef]
- Bousfield, G.R.; Harvey, D.J. Follicle-Stimulating Hormone Glycobiology. Endocrinology 2019, 160, 1515–1535. [Google Scholar] [CrossRef]
- Wang, H.; May, J.; Butnev, V.; Shuai, B.; May, J.V.; Bousfield, G.R.; Kumar, T.R. Evaluation of in vivo bioactivities of recombinant hypo-(FSH21/18) and fully-(FSH24) glycosylated human FSH glycoforms in Fshb null mice. Mol. Cell Endocrinol. 2016, 437, 224–236. [Google Scholar] [CrossRef] [Green Version]
- Bouloux, P.; Warne, D.W.; Loumaye, E. Efficacy and safety of recombinant human follicle-stimulating hormone in men with isolated hypogonadotropic hypogonadism. Fertil. Steril. 2002, 77, 270–273. [Google Scholar] [CrossRef]
- Rohayem, J.; Sinthofen, N.; Nieschlag, E.; Kliesch, S.; Zitzmann, M. Causes of hypogonadotropic hypogonadism predict response to gonadotropin substitution in adults. Andrology 2016, 4, 87–94. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Marshall, G.R.; McNeilly, A.S.; Plant, T.M. Dynamics of the follicle-stimulating hormone (FSH)-inhibin B feedback loop and its role in regulating spermatogenesis in the adult male rhesus monkey (Macaca mulatta) as revealed by unilateral orchidectomy. Endocrinology 2000, 141, 18–27. [Google Scholar] [CrossRef]
- van Alphen, M.M.; van de Kant, H.J.; de Rooij, D.G. Follicle-stimulating hormone stimulates spermatogenesis in the adult monkey. Endocrinology 1988, 123, 1449–1455. [Google Scholar] [CrossRef]
- Simorangkir, D.R.; Ramaswamy, S.; Marshall, G.R.; Pohl, C.R.; Plant, T.M. A selective monotropic elevation of FSH, but not that of LH, amplifies the proliferation and differentiation of spermatogonia in the adult rhesus monkey (Macaca mulatta). Hum. Reprod. (Oxf. Engl.) 2009, 24, 1584–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bercovici, J.P.; Nahoul, K.; Ducasse, M.; Tater, D.; Kerlan, V.; Scholler, R. Leydig cell tumor with gynecomastia: Further studies–the recovery after unilateral orchidectomy. J. Clin. Endocrinol. Metab. 1985, 61, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; La Vignera, S.; Condorelli, R.A.; Mongioì, L.M.; Calogero, A.E. FSH dosage effect on conventional sperm parameters: A meta-analysis of randomized controlled studies. Asian J. Androl. 2020, 22, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Bettella, A.; Ferlin, A.; Garolla, A.; Rossato, M. Evidence for a stimulatory role of follicle-stimulating hormone on the spermatogonial population in adult males. Fertil. Steril. 1998, 69, 636–642. [Google Scholar] [CrossRef]
- Foresta, C.; Bettella, A.; Merico, M.; Garolla, A.; Ferlin, A.; Rossato, M. Use of recombinant human follicle-stimulating hormone in the treatment of male factor infertility. Fertil. Steril. 2002, 77, 238–244. [Google Scholar] [CrossRef]
- Casamonti, E.; Vinci, S.; Serra, E.; Fino, M.G.; Brilli, S.; Lotti, F.; Maggi, M.; Coccia, M.E.; Forti, G.; Krausz, C. Short-term FSH treatment and sperm maturation: A prospective study in idiopathic infertile men. Andrology 2017, 5, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Foresta, C.; Bettella, A.; Garolla, A.; Ambrosini, G.; Ferlin, A. Treatment of male idiopathic infertility with recombinant human follicle-stimulating hormone: A prospective, controlled, randomized clinical study. Fertil. Steril. 2005, 84, 654–661. [Google Scholar] [CrossRef]
- Garolla, A.; Selice, R.; Engl, B.; Bertoldo, A.; Menegazzo, M.; Finos, L.; Lenzi, A.; Foresta, C. Spermatid count as a predictor of response to FSH therapy. Reprod. Biomed. Online 2014, 29, 102–112. [Google Scholar] [CrossRef] [Green Version]
- Selice, R.; Garolla, A.; Pengo, M.; Caretta, N.; Ferlin, A.; Foresta, C. The response to FSH treatment in oligozoospermic men depends on FSH receptor gene polymorphisms. Int. J. Androl. 2011, 34, 306–312. [Google Scholar] [CrossRef]
- Colacurci, N.; Monti, M.G.; Fornaro, F.; Izzo, G.; Izzo, P.; Trotta, C.; Mele, D.; De Franciscis, P. Recombinant human FSH reduces sperm DNA fragmentation in men with idiopathic oligoasthenoteratozoospermia. J. Androl. 2012, 33, 588–593. [Google Scholar] [CrossRef]
- Garolla, A.; Ghezzi, M.; Cosci, I.; Sartini, B.; Bottacin, A.; Engl, B.; Di, Nisio, A.; Foresta, C. FSH treatment in infertile males candidate to assisted reproduction improved sperm DNA fragmentation and pregnancy rate. Endocrine 2017, 56, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Santi, D.; Granata, A.R.; Simoni, M. FSH treatment of male idiopathic infertility improves pregnancy rate: A meta-analysis. Endocr. Connect. 2015, 4, 46–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attia, A.M.; Abou-Setta, A.M.; Al-Inany, H.G. Gonadotrophins for idiopathic male factor subfertility. Cochrane Database Syst. Rev. 2013, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FDA. Available online: https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/biosimilars (accessed on 1 February 2020).
- Weise, M.; Bielsky, M.C.; De Smet, K.; Ehmann, F.; Ekman, N.; Giezen, T.J.; Gravanis, I.; Heim, H.K.; Heinonen, E.; Ho, K. Biosimilars: What clinicians should know. Blood 2012, 120, 5111–5117. [Google Scholar] [CrossRef] [Green Version]
- Ledford, H. ‘Biosimilar’ drugs poised to penetrate market. Nature 2010, 468, 18–19. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP); Bemfola Assessment Report; European Medicines Agency: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Assessment Report for Bemfola (R)—Procedure No:EMEA/H/C/002615; European Medicines Agency: Amsterdam, The Netherlands, 2014.
- Sanghvi, S. Assessment of Bemfola: Biosimilar follitropin alfa. J. Form. Med. Man. 2015, 1, 4–6. [Google Scholar]
- Rettenbacher, M.; Andersen, A.N.; Garcia-Velasco, J.A.; Sator, M.; Barri, P.; Lindenberg, S.; van der Ven, K.; Khalaf, Y.; Bentin-Ley, U.; Obruca, A. A multi-centre phase 3 study comparing efficacy and safety of Bemfola® versus Gonal-f in women undergoing ovarian stimulation for IVF. Reprod. Biomed. Online 2015, 30, 504–513. [Google Scholar] [CrossRef] [Green Version]
- Mastrangeli, R.; Satwekar, A.; Cutillo, F.; Ciampolillo, C.; Palinsky, W.; Longobardi, S. In-vivo biological activity and glycosylation analysis of a biosimilar recombinant human follicle-stimulating hormone product (Bemfola) compared with its reference medicinal product (GONAL-f). PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Riccetti, L.; Sperduti, S.; Lazzaretti, C.; Klett, D.; De Pascali, F.; Paradiso, E.; Limoncella, S.; Potì, F.; Tagliavini, S.; Trenti, T.; et al. Glycosylation Pattern and in vitro Bioactivity of Reference Follitropin alfa and Biosimilars. Front. Endocrinol. (Lausanne) 2019, 10, 503. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.K.; Sabanegh, E.; Mahfouz, R.; Gupta, S.; Thiyagarajan, A.; Agarwal, A. TUNEL as a test for sperm DNA damage in the evaluation of male infertility. Urology 2010, 76, 1380–1386. [Google Scholar] [CrossRef]
- Ding, Y.M.; Zhang, X.J.; Li, J.P.; Chen, S.S.; Zhang, R.T.; Tan, W.L.; Shi, X.J. Treatment of idiopathic oligozoospermia with recombinant human follicle-stimulating hormone: A prospective, randomized, double-blind, placebo-controlled clinical study in Chinese population. Clin. Endocrinol. 2015, 83, 866–871. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | Controls (N = 50) | Cases (N = 97) | * Time Group | ||||
|---|---|---|---|---|---|---|---|
| T0 | T1 | p | T0 | T1 | p | p | |
| Semen volume (mL) | 2.9 ± 1.1 | 2.6 ± 1.2 | 0.105 | 2.7 ± 1.4 | 2.8 ± 1.3 | 0.054 | 0.008 |
| Sperm concentration (106/mL) | 11.1 ± 6.7 | 13.4 ± 5.5 | 0.058 | 9.1 ± 10.2 | 12.9 ± 9.2 | <0.001 | 0.711 |
| Sperm total count (106) | 24.2 ± 16.5 | 26.9 ± 14.9 | 0.276 | 23.4 ± 19.8 | 34.4 ± 29.8 | <0.001 | 0.013 |
| Progressive motility (%) | 14.9 ± 6.0 | 15.4 ± 5.2 | 0.482 | 12.6 ± 8.5 | 19.4 ± 12.0 | <0.001 | <0.001 |
| Non-progressive motility (%) | 10.0 ± 4.6 | 10.5 ± 4.1 | 0.237 | 9.0 ± 6.0 | 12.4 ± 6.9 | <0.001 | 0.003 |
| Immotile (%) | 75.5 ± 7.6 | 74.8 ± 6.5 | 0.270 | 76.8 ± 15.3 | 51.0 ± 31.3 | <0.001 | <0.001 |
| Total motile sperm count (106) | 7.2 ± 4.9 | 8.2 ± 4.2 | 0.230 | 5.5 ± 6.7 | 11.5 ± 13.6 | <0.001 | <0.001 |
| Normal morphology (%) | 6.0 ± 3.8 | 6.1 ± 3.2 | 0.578 | 5.8 ± 3.9 | 8.2 ± 4.2 | <0.001 | 0.048 |
| Anti-sperm antibodies (%) | 4.9 ± 16.0 | 4.8 ± 15.2 | 0.695 | 3.1 ± 11.8 | 2.7 ± 10.3 | 0.368 | 0.394 |
| FSH (U/L) | 5.6 ± 1.8 | 5.5 ± 1.5 | 0.772 | 4.9 ± 1.9 | 8.2 ± 6.7 | <0.001 | 0.032 |
| LH (U/L) | 5.3 ± 1.7 | 5.2 ± 1.6 | 0.050 | 4.4 ± 1.8 | 4.4 ± 1.7 | 0.860 | 0.385 |
| Testosterone (nmol/L) | 14.4 ± 2.7 | 14.5 ± 2.8 | 0.432 | 15.4 ± 4.1 | 15.4 ± 4.0 | 0.694 | 0.852 |
| Right testicular volume (cc) | 13.8 ± 2.7 | 13.8 ± 2.6 | 0.083 | 14.5 ± 5.3 | 15.1 ± 5.4 | <0.001 | <0.001 |
| Left testicular volume (cc) | 12.2 ± 2.6 | 12.3 ± 2.6 | 0.051 | 14.3 ± 5.4 | 14.6 ± 5.6 | 0.004 | 0.247 |
| Total testicular volume (cc) | 25.9 ± 4.2 | 26.1 ± 4.2 | 0.068 | 28.8 ± 10.4 | 29.7 ± 10.6 | <0.001 | 0.001 |
| DNA fragmentation, TUNEL test (%) § | 25.8 ± 7.4 | 26.1 ± 6.9 | 0.188 | 24.2 ± 9.7 | 19.3 ± 8.0 | <0.001 | <0.001 |
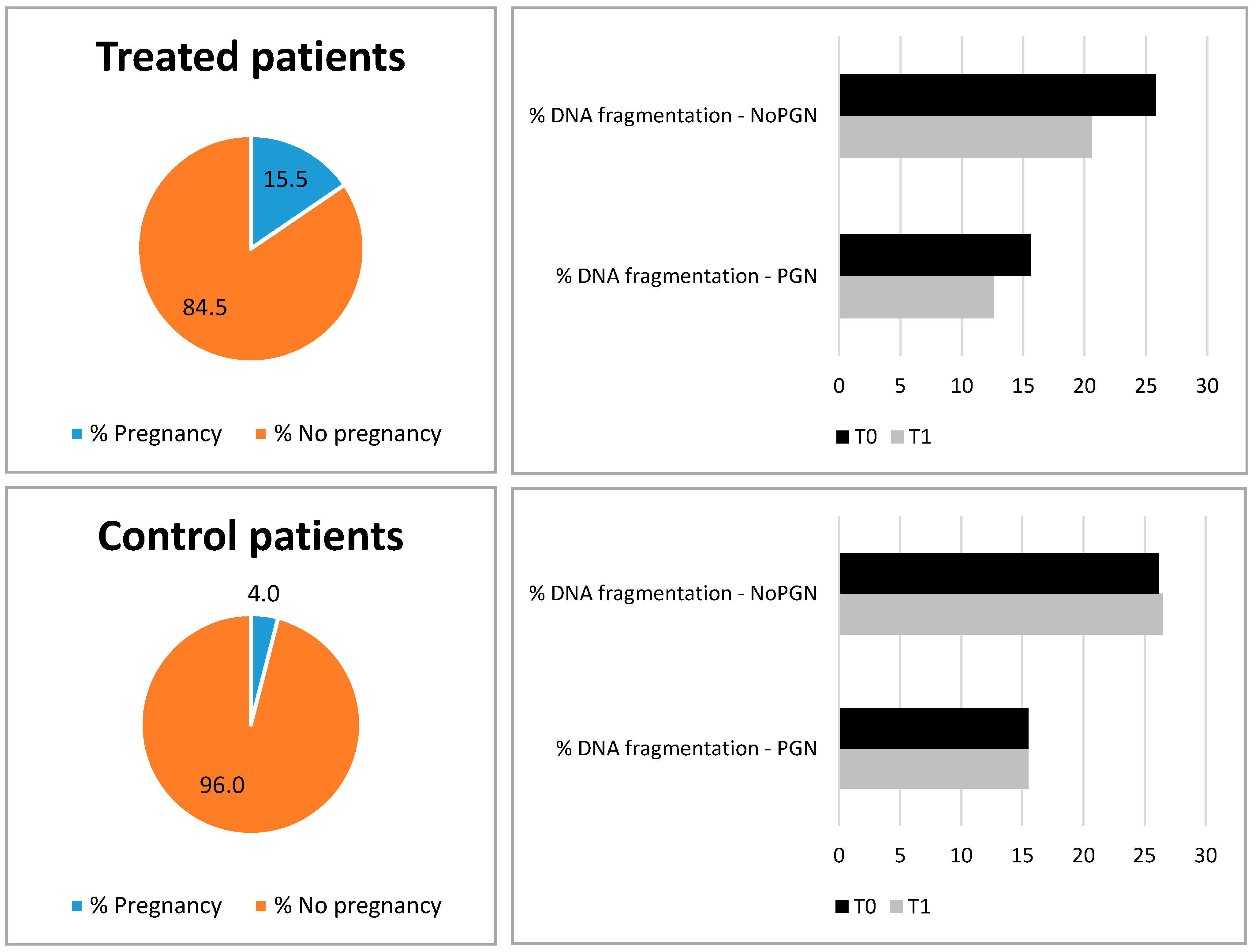
| Pregnancy Outcome in Cases and Controls | DNA Fragmentation (%) | |||||
|---|---|---|---|---|---|---|
| T0 | T1 | |||||
| PGN | NoPGN | p | PGN | NoPGN | p | |
| Treated patients, N = 65 (PGN = 10, NoPGN = 55) | 15.6 | 25.8 | 0.002 | 12.6 | 20.6 | 0.003 |
| Control patients, N = 50 (PGN = 2, NoPGN = 48) | 15.5 | 26.2 | 0.043 | 15.5 | 26.5 | 0.027 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Rocco Ponce, M.; Foresta, C.; Rago, R.; Dal Lago, A.; Balercia, G.; Calogero, A.E.; La Vignera, S.; Cosci, I.; Di Nisio, A.; Garolla, A. Use of Biosimilar Follicle-Stimulating Hormone in Asthenozoospermic Infertile Patients: A Multicentric Study. J. Clin. Med. 2020, 9, 1478. https://doi.org/10.3390/jcm9051478
De Rocco Ponce M, Foresta C, Rago R, Dal Lago A, Balercia G, Calogero AE, La Vignera S, Cosci I, Di Nisio A, Garolla A. Use of Biosimilar Follicle-Stimulating Hormone in Asthenozoospermic Infertile Patients: A Multicentric Study. Journal of Clinical Medicine. 2020; 9(5):1478. https://doi.org/10.3390/jcm9051478
Chicago/Turabian StyleDe Rocco Ponce, Maurizio, Carlo Foresta, Rocco Rago, Alessandro Dal Lago, Giancarlo Balercia, Aldo Eugenio Calogero, Sandro La Vignera, Ilaria Cosci, Andrea Di Nisio, and Andrea Garolla. 2020. "Use of Biosimilar Follicle-Stimulating Hormone in Asthenozoospermic Infertile Patients: A Multicentric Study" Journal of Clinical Medicine 9, no. 5: 1478. https://doi.org/10.3390/jcm9051478
APA StyleDe Rocco Ponce, M., Foresta, C., Rago, R., Dal Lago, A., Balercia, G., Calogero, A. E., La Vignera, S., Cosci, I., Di Nisio, A., & Garolla, A. (2020). Use of Biosimilar Follicle-Stimulating Hormone in Asthenozoospermic Infertile Patients: A Multicentric Study. Journal of Clinical Medicine, 9(5), 1478. https://doi.org/10.3390/jcm9051478








