Hormonal Treatment Strategies Tailored to Non-Binary Transgender Individuals
Abstract
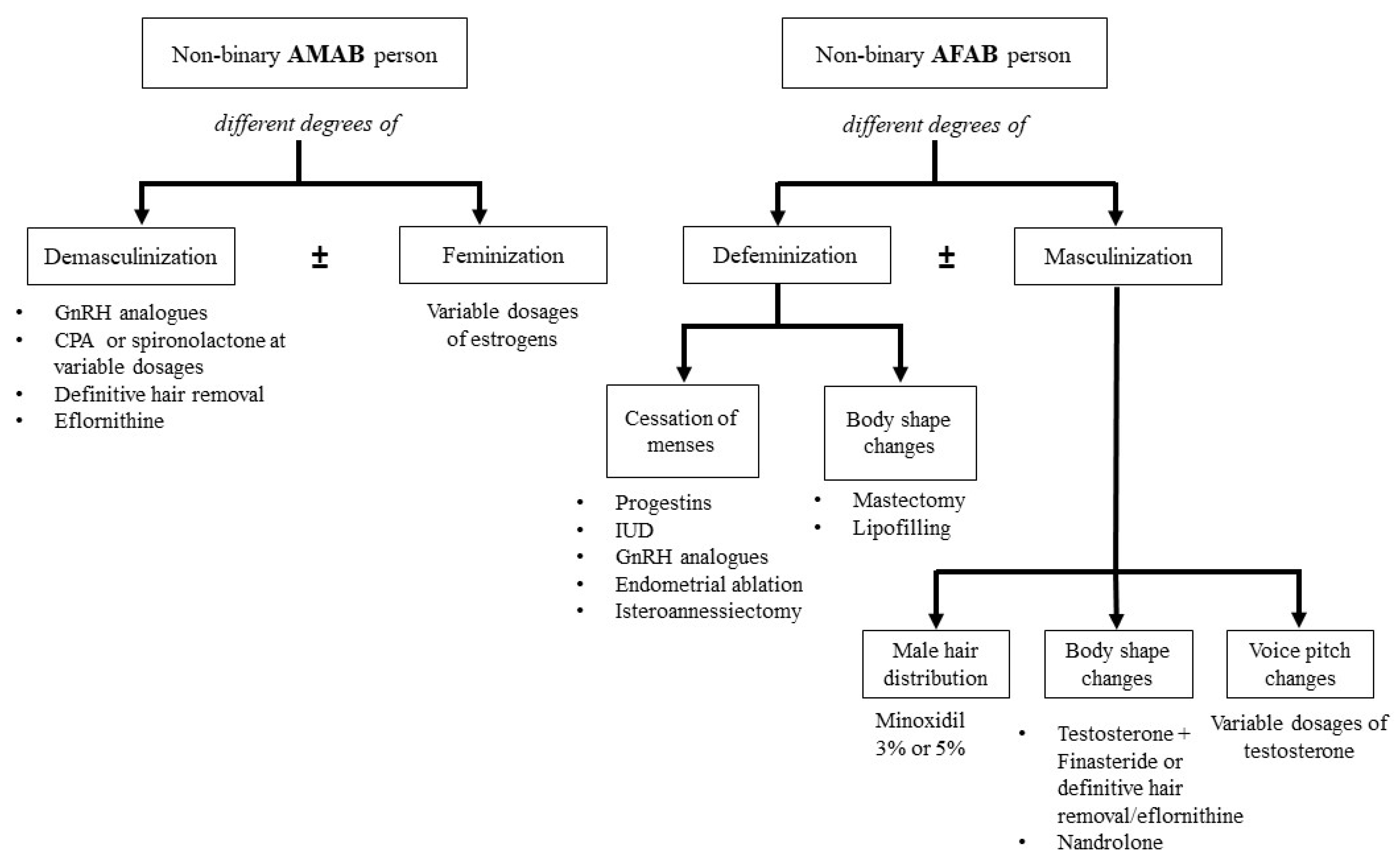
:1. Introduction
2. Methods
3. Hormonal Compounds
3.1. Estrogens
3.2. Androgens
3.3. Progestogens
3.4. Other Androgen Lowering Therapies
4. Non-Hormonal Compounds and Other Strategies
5. Conclusions
Author Contributions
Disclosure
Conflicts of Interest
References
- Arcelus, J.; Bouman, W.P. Language and terminology. In The Transgender Handbook: A Guide for Transgender People, Their Families and Professionals; Bouman, W.P., Arcelus, J., Eds.; Nova: New York, NY, USA, 2017. [Google Scholar]
- Coleman, E.; Bockting, W.; Botzer, M.; Cohen-Kettenis, P.; DeCuypere, G.; Feldman, J.; Fraser, L.; Green, J.; Knudson, G.; Meyer, W.J.; et al. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int. J. Transgenderism 2012, 13, 165–232. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5); American Psychiatric Publishers: Arlington, VA, USA, 2013. [Google Scholar]
- Hembree, W.C.; Cohen-Kettenis, P.T.; Gooren, L.; Hannema, S.E.; Meyer, W.J.; Murad, M.H.; Rosenthal, S.M.; Safer, J.D.; Tangpricha, V.; T’Sjoen, G.G. Endocrine treatment of gender-dysphoric/gender-incongruent persons: An Endocrine Society clinical practice guideline. Endocr. Pract. 2017, 23, 1437. [Google Scholar] [CrossRef] [PubMed]
- Van Caenegem, E.; Wierckx, K.; Elaut, E.; Buysse, A.; Dewaele, A.; Van Nieuwerburgh, F.; De Cuypere, G.; T’Sjoen, G. Prevalence of gender nonconformity in Flander, Belgium. Arch. Sex. Behav. 2015, 44, 1281–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahs, J.W.; Dhejne, C.; Magnusson, C.; Dal, H.; Lundin, A.; Arver, A.; Dalman, C.; Kosidou, K. Proportion of adults in the general population of Stockholm County who want gender-affirming medical treatment. PLoS ONE 2018, 13, e0204606. [Google Scholar] [CrossRef] [Green Version]
- Koehler, A.; Eyssel, J.; Nieder, T.O. Genders and individual treatment progress in (non-)binary trans individuals. J. Sex. Med. 2018, 15, 102–113. [Google Scholar] [CrossRef] [Green Version]
- T’Sjoen, G.; Arcelus, J.; Gooren, L.; Klink, D.T.; Tangpricha, V. Endocrinology of transgender medicine. Endocr. Rev. 2019, 40, 97–117. [Google Scholar] [CrossRef] [Green Version]
- T’Sjoen, G.; Arcelus, J.; De Vries, A.L.; Fisher, A.D.; Nieder, T.O.; Özer, M.; Motmans, J. European Society for Sexual Medicine position statement “Assessment and hormonal management in adolescent and adult trans people, with attention for sexual function and satisfaction”. J. Sex. Med. 2020, 17, 570–584. [Google Scholar] [CrossRef]
- Toorians, A.W.; Thomassen, M.C.; Zweegman, S.; Magdeleyns, E.J.; Tans, G.; Gooren, L.J.; Rosing, J. Venous thrombosis and changes of hemostatic variables during cross-sex hormone treatment in transsexual people. J. Clin. Endocrinol. Metab. 2003, 88, 5723–5729. [Google Scholar] [CrossRef]
- Asscheman, H.; Giltay, E.J.; Megens, J.A.; de Ronde, W.P.; van Trotsenburg, M.A.; Gooren, L.J. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur. J. Endocrinol. 2011, 164, 635–642. [Google Scholar] [CrossRef] [Green Version]
- Pelusi, C.; Costantino, A.; Martelli, V.; Lambertini, M.; Bazzocchi, A.; Ponti, F.; Battista, G.; Venturoli, S.; Meriggiola, M.C. Effects of three different testosterone formulations in female-to-male transsexual persons. J. Sex. Med. 2014, 11, 3002–3011. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Chen, W.C.; Thornton, M.J.; Qin, K.; Rosenfield, R. Sexual hormones in human skin. Horm. Metab. Res. 2007, 39, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Unluhizarci, K.; Ozel, D.; Tanriverdi, F.; Karaca, Z.; Kelestimur, F. A comparison between finasteride, flutamide, and finasteride plus flutamide combination in the treatment of hirsutism. J. Endocrinol. Invest. 2009, 32, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Bergink, E.W.; Janssen, P.S.; Turpijn, E.W.; van der Vies, J. Comparison of the receptor binding properties of nandrolone and testosterone under in vitro and in vivo conditions. J. Steroid Biochem. 1985, 22, 831–836. [Google Scholar] [CrossRef]
- Kicman, A.T. Pharmacology of anabolic steroids. Br. J. Pharmacol. 2008, 154, 502–521. [Google Scholar] [CrossRef] [PubMed]
- Alsiö, J.; Birgner, C.; Björkblom, L.; Isaksson, P.; Bergström, L.; Schiöth, H.B.; Lindblom, J. Impact of nandrolone decanoate on gene expression in endocrine systems related to the adverse effects of anabolic androgenic steroids. Basic Clin. Pharmacol. Toxicol. 2009, 105, 307–314. [Google Scholar] [CrossRef]
- Sagoe, D.; McVeigh, J.; Bjørnebekk, A.; Essilfie, M.S.; Andreassen, C.S.; Pallesen, S. Polypharmacy among anabolic-androgenic steroid users: A descriptive metasynthesis. Subst. Abuse Treat. Prev. Policy 2015, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Baggish, A.L.; Weiner, R.B.; Kanayama, G.; Hudson, J.I.; Lu, M.T.; Hoffmann, U.; Pope, H.G., Jr. Cardiovascular toxicity of illicit anabolic-androgenic steroid use. Circulation 2017, 135, 1991–2002. [Google Scholar] [CrossRef] [Green Version]
- Tofighi, A.; Shirpoor, M.; Ansari, M.H.K.; Shirpoor, A.; Zerehpoosh, M. The effect of nandrolone treatment with and without enforced swimming on histological and biochemical changes in the heart and coronary artery of male rats. Anatol. J. Cardiol. 2017, 17, 176–183. [Google Scholar] [CrossRef]
- Imperato-McGinley, J.; Zhu, Y.S. Androgens and male physiology the syndrome of 5α-reductase-2 deficiency. Mol. Cell Endocrinol. 2002, 30, 51–59. [Google Scholar] [CrossRef]
- Prior, J.C. Progesterone is important for transgender women’s therapy—Applying evidence for the benefits of progesterone in ciswomen. J. Clin. Endocrinol. Metab. 2019, 104, 1181–1186. [Google Scholar] [CrossRef]
- Robinson, G.W.; Hennighausen, L.; Johnson, P.F. Side-branching in the mammary gland: The progesterone-Wnt connection. Genes Dev. 2000, 14, 889–894. [Google Scholar] [PubMed]
- Gava, G.; Cerpolini, S.; Martelli, V.; Battista, G.; Seracchioli, R.; Meriggiola, M.C. Cyproterone acetate vs leuprolide acetate in combination with transdermal oestradiol in transwomen: A comparison of safety and effectiveness. Clin. Endocrinol. 2016, 85, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Hsia, J.; Johnson, K.C.; Rossouw, J.E.; Assaf, A.R.; Lasser, N.L.; Trevisan, M.; Black, H.R.; Heckbert, S.R.; Detrano, R.; et al. Estrogen plus progestin and the risk of coronary heart disease. N. Engl. J. Med. 2003, 349, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Barsoum, M.K.; Heit, J.A.; Ashrani, A.A.; Leibson, C.L.; Petterson, T.M.; Bailey, K.R. Is progestin an independent risk factor for incident venous thromboembolism? A population-based case-control study. Thromb. Res. 2010, 126, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Gooren, L.J. Clinical practice. Care of transsexual persons. N. Eng. J. Med. 2011, 364, 1251–1257. [Google Scholar] [CrossRef]
- Dean, J.; Kramer, K.J.; Akbary, F.; Wade, S.; Hutteman, M.; Berman, J.M.; Recanati, M. Norethindrone is superior to combined oral contraceptive pills in short-term delay of menses and onset of breakthrough bleeding: A randomized trial. BMC Womens Health 2019, 19, 70. [Google Scholar] [CrossRef] [Green Version]
- Dickersin, K.; Munro, M.G.; Clark, M.; Langenberg, P.; Scherer, R.; Frick, K.; Zhu, Q.; Hallock, L.; Nichols, J.; Yalcinkaya, T.M.; et al. Hysterectomy compared with endometrial ablation for dysfunctional uterine bleeding: A randomized controlled trial. OBSTET Gynecol. 2007, 110, 1279–1289. [Google Scholar] [CrossRef]
- Francis, A.; Jasani, S.; Bachmann, G. Contraceptive challenges and the transgender individual. Womens Midlife Health 2018, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Fisher, A.D.; Gooren, L. Hormonal treatment of transgender male to female. In Encyclopedia of Endocrine Diseases, 2nd ed.; Huhtaniemi, I., Martini, L., Eds.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Tangpricha, V.; den Heijer, M. Oestrogen and anti-androgen therapy for transgender women. Lancet Diabetes Endocrinol. 2017, 5, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Gil, M.; Oliva, B.; Timoner, J.; Maciá, M.A.; Bryant, V.; de Abajo, F.J. Risk of meningioma among users of high doses of cyproterone acetate as compared with the general population: Evidence from a population-based cohort study. Br. J. Clin. Pharmacol. 2011, 72, 965–968. [Google Scholar] [CrossRef] [Green Version]
- Ter Wengel, P.V.; Martin, E.; Gooren, L.; Den Heijer, M.; Peerdeman, S.M. Meningiomas in three male-to-female transgender subjects using oestrogens/progestogens and review of the literature. Andrologia 2016, 48, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Wierckx, K.; Van Caenegem, E.; Schreiner, T.; Haraldsen, I.; Fisher, A.D.; Toye, K.; Kaufman, J.M.; T’Sjoen, G. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: Results from the European network for the investigation of gender incongruence. J. Sex. Med. 2014, 11, 1999–2011. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.D.; Castellini, G.; Ristori, J.; Casale, H.; Cassioli, E.; Sensi, C.; Fanni, E.; Amato, A.M.; Bettini, E.; Mosconi, M.; et al. Cross-sex hormone treatment and psychobiological changes in trassexual persons: Two-year follow up data. J. Clin. Endocrinol. Metab. 2016, 101, 4260–4269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stripp, B.; Taylor, A.A.; Bartter, F.C.; Gillette, J.R.; Loriaux, D.L.; Easley, R.; Menard, R.H. Effect of spironolactone on sex hormones in man. J. Clin. Endocrinol. Metab. 1975, 41, 777–781. [Google Scholar] [CrossRef]
- Liang, J.J.; Jolly, D.; Chan, K.J.; Safer, J.D. Testosterone levels achieved by medically treated transgender women in a United States endocrinoly clinic cohort. Endocr. Pract. 2018, 24, 135–142. [Google Scholar] [CrossRef]
- Leinung, M.C.; Feustel, P.J.; Joseph, J. Hormonal treatment of transgender women with oral estradiol. Transgend. Health 2018, 3, 74–81. [Google Scholar] [CrossRef]
- Gulmez, S.E.; Lassen, A.T.; Aalykke, C.; Dall, M.; Andries, A.; Andersen, B.S.; Hansen, J.M.; Andersen, M.; Hallas, J. Spironolactone use and the risk of upper gastrointestinal bleeding: A population-based case-control study. Br. J. Clin. Pharmacol. 2008, 66, 294–299. [Google Scholar] [CrossRef] [Green Version]
- Giorgetti, R.; di Muzio, M.; Giorgetti, A.; Girolami, D.; Borgia, L.; Tagliabracci, A. Flutamide-induced hepatotoxicity: Ethical and scientific issues. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 69–77. [Google Scholar]
- Irwig, M.S. Safety concerns regarding 5a reductase inhibitors for the treatment of androgenetic alopecia. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 248–253. [Google Scholar] [CrossRef]
- Basaria, S.; Jasuja, R.; Huang, G.; Wharton, W.; Pan, H.; Pencina, K.; Li, Z.; Travison, T.G.; Bhawan, J.; Gonthier, R.; et al. Characteristics of men who report persistent sexual symptoms after finasteride use for hair loss. J. Clin. Endocrinol. Metab. 2016, 101, 4669–4680. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Arrones, O.M.; Becerra, A.; Vano-Galvan, S. Therapeutic experience with oral finasteride for androgenetic alopecia in female-to-male transgender patients. Clin. Exp. Dermatol. 2017, 42, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, M.O.; Wixon, N.; Safer, J.D. Scalp hair regrowth in hormone-treated transgender woman. Transgend. Health 2016, 1, 202–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachner, T.D.; Coleman, R.; Hadji, P.; Hofbauer, L.C. Bone health during endocrine therapy for cancer. Lancet Diabetes Endocrinol. 2018, 6, 901–910. [Google Scholar] [CrossRef]
- Shorter, K.; Farjo, N.P.; Picksley, S.M.; Randall, V.A. Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil. FASEB J. 2008, 22, 1725–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messenger, A.G.; Rundegren, J. Minoxidil: Mechanisms of action on hair growth. Br. J. Dermatol 2004, 150, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: A review. Drug Des. Devel. Ther. 2019, 13, 2777–2786. [Google Scholar] [CrossRef] [Green Version]
- Marks, D.H.; Senna, M.M. Androgenetic alopecia in gender minority patients. Dermatol. Clin. 2020, 38, 239–247. [Google Scholar] [CrossRef]
- Ingprasert, S.; Tanglertsampan, C.; Tangphianphan, N.; Reanmanee, C. Efficacy and safety of minoxidil 3% lotion for beard enhancement: A randomized, double-masked, placebo-controlled study. J. Dermatol. 2016, 43, 968–969. [Google Scholar] [CrossRef]
- Martin, K.A.; Anderson, R.R.; Chang, R.J.; Ehrmann, D.A.; Lobo, R.A.; Murad, M.H.; Pugeat, M.M.; Rosenfield, R.L. Evaluation and treatment of hirsutism in premenopausal women: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1233–1257. [Google Scholar] [CrossRef]
- Smith, S.R.; Piacquadio, D.J.; Beger, B.; Littler, C. Eflornithine cream combined with laser therapy in the management of unwanted facial hair growth in women: A randomized trial. Dermatol. Surg. 2006, 32, 1237–1243. [Google Scholar]
- Hamzavi, I.; Tan, E.; Shapiro, J.; Lui, H. A randomized bilateral vehicle-controlled study of eflornithine cream combined with laser treatment versus laser treatment alone for facial hirsutism in women. J. Am. Acad. Dermatol. 2007, 57, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Bultynck, C.; Pas, C.; Defreyne, J.; Cosyns, M.; den Heijer, M.; T’Sjoen, G. Self-perception of voice in transgender persons during cross-sex hormone therapy. Laryngoscope 2017, 127, 2796–2804. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocchetti, C.; Ristori, J.; Romani, A.; Maggi, M.; Fisher, A.D. Hormonal Treatment Strategies Tailored to Non-Binary Transgender Individuals. J. Clin. Med. 2020, 9, 1609. https://doi.org/10.3390/jcm9061609
Cocchetti C, Ristori J, Romani A, Maggi M, Fisher AD. Hormonal Treatment Strategies Tailored to Non-Binary Transgender Individuals. Journal of Clinical Medicine. 2020; 9(6):1609. https://doi.org/10.3390/jcm9061609
Chicago/Turabian StyleCocchetti, Carlotta, Jiska Ristori, Alessia Romani, Mario Maggi, and Alessandra Daphne Fisher. 2020. "Hormonal Treatment Strategies Tailored to Non-Binary Transgender Individuals" Journal of Clinical Medicine 9, no. 6: 1609. https://doi.org/10.3390/jcm9061609
APA StyleCocchetti, C., Ristori, J., Romani, A., Maggi, M., & Fisher, A. D. (2020). Hormonal Treatment Strategies Tailored to Non-Binary Transgender Individuals. Journal of Clinical Medicine, 9(6), 1609. https://doi.org/10.3390/jcm9061609



