Acupuncture Treatment Modulates the Connectivity of Key Regions of the Descending Pain Modulation and Reward Systems in Patients with Chronic Low Back Pain
Abstract
:1. Introduction
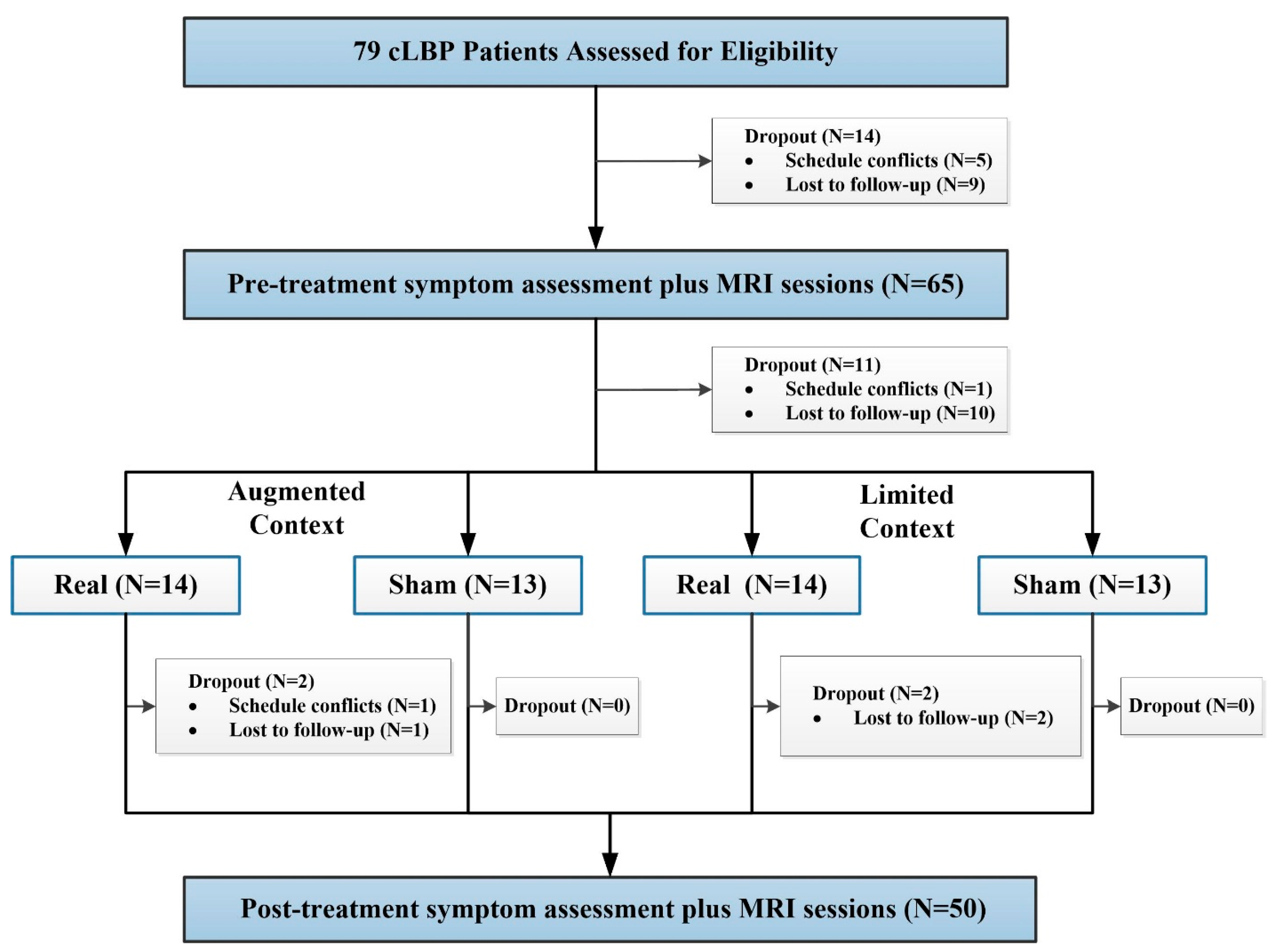
2. Materials and Methods
3. Participants
4. Experimental Procedures
5. Acupuncture Treatment
6. High- and Low-Context Manipulation
7. Clinical Outcomes and Data Analysis
8. fMRI Data Acquisition and Data Analysis
9. Results
10. Clinical Outcomes
11. Functional Connectivity Results
12. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Andersson, G. The epidemiology of spinal disorders. In Adult Spine Principles & Practice; Lippincott-Raven Publishers: Philadelphia, PA, USA, 1997. [Google Scholar]
- Sehgal, N.; Manchikanti, L.; Smith, H.S. Prescription opioid abuse in chronic pain: A review of opioid abuse predictors and strategies to curb opioid abuse. Pain Physician 2012, 15, ES67. [Google Scholar] [PubMed]
- Krebs, E.E.; Gravely, A.; Nugent, S.; Jensen, A.C.; DeRonne, B.; Goldsmith, E.S.; Kroenke, K.; Bair, M.J.; Noorbaloochi, S. Effect of opioid vs. nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: The SPACE randomized clinical trial. JAMA 2018, 319, 872–882. [Google Scholar] [CrossRef]
- Ballantyne, J.C. Avoiding opioid analgesics for treatment of chronic low back pain. JAMA 2016, 315, 2459–2460. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, I.A.; Persaud, N.; Juurlink, D.N. Facing up to the prescription opioid crisis. BMJ 2011, 343, d5142. [Google Scholar] [CrossRef] [Green Version]
- Paulozzi, L.J.; Jones, C.M.; Mack, K.A.; Rudd, R.A. Vital signs: Overdoses of prescription opioid pain relievers—United States, 1999–2008. Morb. Mortal. Wkly. Rep. 2011, 60, 1487–1492. [Google Scholar]
- Vickers, A.J.; Cronin, A.M.; Maschino, A.C.; Lewith, G.; MacPherson, H.; Foster, N.E.; Sherman, K.J.; Witt, C.M.; Linde, K.; on behalf of the Acupuncture Trialists’ Collaboration. Acupuncture for chronic pain: Individual patient data meta-analysis. Arch. Intern. Med. 2012, 172, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Vertosick, E.A.; Lewith, G.; MacPherson, H.; Foster, N.E.; Sherman, K.J.; Irnich, D.; Witt, C.M.; Linde, K.; Collaboration, A.T. Acupuncture for chronic pain: Update of an individual patient data meta-analysis. J. Pain 2018, 19, 455–474. [Google Scholar] [CrossRef] [Green Version]
- Qaseem, A.; Wilt, T.J.; Mclean, R.M.; Forciea, M.A. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2017, 167, 833. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Lao, L.; Ren, K.; Berman, B.M. Mechanisms of acupuncture—Electroacupuncture on persistent pain. Anesthesiol. J. Am. Soc. Anesthesiol. 2014, 120, 482–503. [Google Scholar] [CrossRef] [Green Version]
- Han, J.-S. Acupuncture: Neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003, 26, 17–22. [Google Scholar] [CrossRef]
- Zhao, Z.-Q. Neural mechanism underlying acupuncture analgesia. Prog. Neurobiol. 2008, 85, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Young, R.F.; Chambi, V.I. Pain relief by electrical stimulation of the periaqueductal and periventricular gray matter. Evidence for a non-opioid mechanism. J. Neurosurg. 1987, 66, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Murotani, T.; Ishizuka, T.; Nakazawa, H.; Wang, X.; Mori, K.; Sasaki, K.; Ishida, T.; Yamatodani, A. Possible involvement of histamine, dopamine, and noradrenalin in the periaqueductal gray in electroacupuncture pain relief. Brain Res. 2010, 1306, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Fields, H.L. Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annu. Rev. Neurosci. 1984, 7, 309–338. [Google Scholar] [CrossRef]
- Pinto, M.; Castro, A.R.; Tshudy, F.; Wilson, S.P.; Lima, D.; Tavares, I. Opioids modulate pain facilitation from the dorsal reticular nucleus. Mol. Cell. Neurosci. 2008, 39, 508–518. [Google Scholar] [CrossRef]
- Neugebauer, V.; Li, W. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J. Neurophysiol. 2003, 89, 716. [Google Scholar] [CrossRef]
- Millan, M.J.; Członkowski, A.; Pilcher, C.W.; Almeida, O.F.; Millan, M.H.; Colpaert, F.C.; Herz, A. A model of chronic pain in the rat: Functional correlates of alterations in the activity of opioid systems. J. Neurosci. Off. J. Soc. Neurosci. 1987, 7, 77. [Google Scholar] [CrossRef] [Green Version]
- Terayama, R.; Guan, Y.; Dubner, R.; Ren, K. Activity-induced plasticity in brain stem pain modulatory circuitry after inflammation. Neuroreport 2000, 11, 1915–1919. [Google Scholar] [CrossRef]
- Tu, Y.; Jung, M.; Gollub, R.L.; Napadow, V.; Gerber, J.; Ortiz, A.; Lang, C.; Mawla, I.; Shen, W.; Chan, S.-T. Abnormal medial prefrontal cortex functional connectivity and its association with clinical symptoms in chronic low back pain. Pain 2019, 160, 1308–1318. [Google Scholar] [CrossRef]
- Fields, H. State-dependent opioid control of pain. Nat. Rev. Neurosci. 2004, 5, 565. [Google Scholar] [CrossRef]
- Yu, R.; Gollub, R.L.; Spaeth, R.; Napadow, V.; Wasan, A.; Kong, J. Disrupted functional connectivity of the periaqueductal gray in chronic low back pain. Neuroimage Clin. 2014, 6, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navratilova, E.; Porreca, F. Reward and motivation in pain and pain relief. Nat. Neurosci. 2014, 17, 1304. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Gandhi, W.; Schweinhardt, P. Cerebral interactions of pain and reward and their relevance for chronic pain. Neurosci. Lett. 2012, 520, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Becker, S.; Schweinhardt, P.; Cahill, C. Mesolimbic dopamine signaling in acute and chronic pain: Implications for motivation, analgesia, and addiction. Pain 2016, 157, 1194. [Google Scholar] [CrossRef] [Green Version]
- Navratilova, E.; Xie, J.Y.; Okun, A.; Qu, C.; Eyde, N.; Ci, S.; Ossipov, M.H.; King, T.; Fields, H.L.; Porreca, F. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc. Natl. Acad. Sci. USA 2012, 109, 20709–20713. [Google Scholar] [CrossRef] [Green Version]
- Björklund, A.; Dunnett, S.B. Dopamine neuron systems in the brain: An update. Trends Neurosci. 2007, 30, 194–202. [Google Scholar] [CrossRef]
- Murty, V.P.; Shermohammed, M.; Smith, D.V.; Carter, M.K.; Huettel, S.A.; Adcock, R.A. Resting state networks distinguish human ventral tegmental area from substantia nigra. Neuroimage 2014, 100, 580–589. [Google Scholar] [CrossRef] [Green Version]
- Peterson, A.C.; Zhang, S.; Hu, S.; Chao, H.H.; Li, C.R. The Effects of Age, from Young to Middle Adulthood, and Gender on Resting State Functional Connectivity of the Dopaminergic Midbrain. Front. Hum. Neurosci. 2017, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Haber, S.N.; Fudge, J.L. The Primate Substantia Nigra and VTA: Integrative Circuitry and Function. Crit. Rev. Neurobiol. 1997, 11, 323–342. [Google Scholar] [CrossRef]
- Haber, S.N.; Knutson, B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2010, 35, 4–26. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Li, W.; Shen, W.; Edwards, R.R.; Gollub, R.L.; Wilson, G.; Park, J.; Ortiz, A.; Cao, J.; Gerber, J.; et al. Impaired mesocorticolimbic connectivity underlies increased mechanical pain sensitivity in chronic low back pain. Neuroimage 2020. [Google Scholar] [CrossRef] [PubMed]
- Porreca, F.; Navratilova, E. Reward, motivation and emotion of pain and its relief. Pain 2017, 158, S43. [Google Scholar] [CrossRef] [PubMed]
- Mitsi, V.; Zachariou, V. Modulation of pain, nociception, and analgesia by the brain reward center. Neuroscience 2016, 338, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chen, L.; Chen, X.; Hu, K.; Tu, Y.; Lin, M.; Huang, J.; Liu, W.; Wu, J.; Qiu, Z. Modulatory effects of different exercise modalities on the functional connectivity of the periaqueductal grey and ventral tegmental area in patients with knee osteoarthritis: A randomised multimodal magnetic resonance imaging study. Br. J. Anaesth. 2019, 123, 506–518. [Google Scholar] [CrossRef] [Green Version]
- Street, R.L., Jr.; Cox, V.; Kallen, M.A.; Suarez-Almazor, M.E. Exploring communication pathways to better health: Clinician communication of expectations for acupuncture effectiveness. Patient Educ. Couns. 2012, 89, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Suarez-Almazor, M.E.; Looney, C.; Liu, Y.; Cox, V.; Pietz, K.; Marcus, D.M.; Street, R.L., Jr. A randomized controlled trial of acupuncture for osteoarthritis of the knee: Effects of patient-provider communication. Arthritis Care Res. 2010, 62, 1229–1236. [Google Scholar] [CrossRef] [Green Version]
- Gollub, R.L.; Kong, J. For placebo effects in medicine, seeing is believing. Sci. Transl. Med. 2011, 3, 70ps5. [Google Scholar] [CrossRef]
- Kong, J.; Kaptchuk, T.J.; Polich, G.; Kirsch, I.; Vangel, M.; Zyloney, C.; Rosen, B.; Gollub, R. Expectancy and treatment interactions: A dissociation between acupuncture analgesia and expectancy evoked placebo analgesia. Neuroimage 2009, 45, 940–949. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.; Kaptchuk, T.J.; Polich, G.; Kirsch, I.; Vangel, M.; Zyloney, C.; Rosen, B.; Gollub, R.L. An fMRI study on the interaction and dissociation between expectation of pain relief and acupuncture treatment. Neuroimage 2009, 47, 1066–1076. [Google Scholar] [CrossRef] [Green Version]
- Kaptchuk, T.J.; Kelley, J.M.; Conboy, L.A.; Davis, R.B.; Kerr, C.E.; Jacobson, E.E.; Kirsch, I.; Schyner, R.N.; Nam, B.H.; Nguyen, L.T. Components of placebo effect: Randomised controlled trial in patients with irritable bowel syndrome. BMJ 2008, 336, 999–1003. [Google Scholar] [CrossRef] [Green Version]
- Tu, Y.; Ortiz, A.; Gollub, R.L.; Cao, J.; Gerber, J.; Lang, C.; Park, J.; Wilson, G.; Shen, W.; Chan, S.-T.; et al. Multivariate resting-state functional connectivity predicts responses to real and sham acupuncture treatment in chronic low back pain. Neuroimage Clin. 2019, 23, 101885. [Google Scholar] [CrossRef] [PubMed]
- Werneke, M.W.; Hart, D.L. Categorizing patients with occupational low back pain by use of the Quebec Task Force Classification system versus pain pattern classification procedures: Discriminant and predictive validity. Phys. Ther. 2004, 84, 243–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, K.; Cherkin, D. Developing methods for acupuncture research: Rationale for and design of a pilot study evaluating the efficacy of acupuncture for chronic low back pain. Altern. Ther. Health Med. 2003, 9, 54. [Google Scholar] [PubMed]
- Ratcliffe, J.; Thomas, K.; MacPherson, H.; Brazier, J. A randomised controlled trial of acupuncture care for persistent low back pain: Cost effectiveness analysis. BMJ 2006, 333, 626. [Google Scholar] [CrossRef] [Green Version]
- Hsu, M.; Bhatt, M.; Adolphs, R.; Tranel, D.; Camerer, C.F. Neural systems responding to degrees of uncertainty in human decision-making. Science 2005, 310, 1680–1683. [Google Scholar] [CrossRef] [Green Version]
- Sharot, T.; Korn, C.W.; Dolan, R.J. How unrealistic optimism is maintained in the face of reality. Nat. Neurosci. 2011, 14, 1475. [Google Scholar] [CrossRef]
- Wasan, A.D.; Davar, G.; Jamison, R. The association between negative affect and opioid analgesia in patients with discogenic low back pain. Pain 2005, 117, 450–461. [Google Scholar] [CrossRef]
- Wasan, A.D.; Kong, J.; Pham, L.-D.; Kaptchuk, T.J.; Edwards, R.; Gollub, R.L. The impact of placebo, psychopathology, and expectations on the response to acupuncture needling in patients with chronic low back pain. J. Pain 2010, 11, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Cherkin, D.C.; Sherman, K.J.; Avins, A.L.; Erro, J.H.; Ichikawa, L.; Barlow, W.E.; Delaney, K.; Hawkes, R.; Hamilton, L.; Pressman, A. A randomized trial comparing acupuncture, simulated acupuncture, and usual care for chronic low back pain. Arch. Intern. Med. 2009, 169, 858–866. [Google Scholar] [CrossRef] [Green Version]
- Dunn, K.M.; Croft, P.R. Classification of low back pain in primary care: Using “bothersomeness” to identify the most severe cases. Spine 2005, 30, 1887–1892. [Google Scholar] [CrossRef]
- Patrick, D.L.; Deyo, R.A.; Atlas, S.J.; Singer, D.E.; Chapin, A.; Keller, R.B. Assessing health-related quality of life in patients with sciatica. Spine 1995, 20, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Cherkin, D.C.; Deyo, R.A.; Street, J.H.; Barlow, W. Predicting poor outcomes for back pain seen in primary care using patients′ own criteria. Spine 1996, 21, 2900–2907. [Google Scholar] [CrossRef] [PubMed]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012, 2, 125–141. [Google Scholar] [CrossRef] [Green Version]
- Power, J.D.; Barnes, K.A.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012, 59, 2142–2154. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.Y.; Chao, H.T.; Tu, C.H.; Lin, M.W.; Li, W.C.; Low, I.; Shen, H.D.; Chen, L.F.; Hsieh, J.C. The BDNF Val66Met polymorphism is associated with the functional connectivity dynamics of pain modulatory systems in primary dysmenorrhea. Sci. Rep. 2016, 6, 23639. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.; Loggia, M.L.; Zyloney, C.; Tu, P.; Laviolette, P.; Gollub, R.L. Exploring the brain in pain: Activations, deactivations and their relation. Pain 2010, 148, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Adcock, R.A.; Thangavel, A.; Whitfield-Gabrieli, S.; Knutson, B.; Gabrieli, J.D. Reward-motivated learning: Mesolimbic activation precedes memory formation. Neuron 2006, 50, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.-Y.; Chao, H.-T.; Tu, C.-H.; Li, W.-C.; Low, I.; Chuang, C.-Y.; Chen, L.-F.; Hsieh, J.-C. Changes in functional connectivity of pain modulatory systems in women with primary dysmenorrhea. Pain 2016, 157, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Liu, M.; Lan, L.; Zeng, F.; Makris, N.; Liang, Y.; Guo, T.; Wu, F.; Gao, Y.; Dong, M. Altered periaqueductal gray resting state functional connectivity in migraine and the modulation effect of treatment. Sci. Rep. 2016, 6, 20298. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.X.; Li, B.; Xu, Y.K.; Ji, T.T.; Li, L.; Zhao, C.J.; Chen, L.; Zhuo, Z.Z. Altered functional connectivity of the periaqueductal gray in chronic neck and shoulder pain. Neuroreport 2017, 28, 720–725. [Google Scholar] [CrossRef]
- Behbehani, M.M. Functional characteristics of the midbrain periaqueductal gray. Prog. Neurobiol. 1995, 46, 575–605. [Google Scholar] [CrossRef]
- Cao, J.; Tu, Y.; Orr, S.P.; Lang, C.; Park, J.; Vangel, M.; Chen, L.; Gollub, R.; Kong, J. Analgesic Effects Evoked by Real and Imagined Acupuncture: A Neuroimaging Study. Cereb. Cortex 2019, 29, 3220–3231. [Google Scholar] [CrossRef] [PubMed]
- Gollub, R.L.; Kirsch, I.; Maleki, N.; Wasan, A.D.; Edwards, R.R.; Tu, Y.; Kaptchuk, T.J.; Kong, J. A functional neuroimaging study of expectancy effects on pain response in patients with knee osteoarthritis. J. Pain 2018, 19, 515–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, J.; Wang, Z.; Leiser, J.; Minicucci, D.; Edwards, R.; Kirsch, I.; Wasan, A.D.; Lang, C.; Gerber, J.; Yu, S. Enhancing treatment of osteoarthritis knee pain by boosting expectancy: A functional neuroimaging study. Neuroimage Clin. 2018, 18, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Leknes, S.; Tracey, I. A common neurobiology for pain and pleasure. Nat. Rev. Neurosci. 2008, 9, 314. [Google Scholar] [CrossRef]
- Howe, L.C.; Goyer, J.P.; Crum, A.J. Harnessing the placebo effect: Exploring the influence of physician characteristics on placebo response. Health Psychol. 2017, 36, 1074. [Google Scholar] [CrossRef]
- Colloca, L.; Tinazzi, M.; Recchia, S.; Le Pera, D.; Fiaschi, A.; Benedetti, F.; Valeriani, M. Learning potentiates neurophysiological and behavioral placebo analgesic responses. Pain 2008, 139, 306–314. [Google Scholar] [CrossRef]
- Hui, K.K.; Liu, J.; Marina, O.; Napadow, V.; Haselgrove, C.; Kwong, K.K.; Kennedy, D.N.; Makris, N. The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. Neuroimage 2005, 27, 479–496. [Google Scholar] [CrossRef]
- Tracey, I.; Mantyh, P.W. The Cerebral Signature for Pain Perception and Its Modulation. Neuron 2007, 55, 377. [Google Scholar] [CrossRef] [Green Version]
- Neugebauer, V.; Li, W.; Bird, G.C.; Han, J.S. The amygdala and persistent pain. Neuroscientist 2004, 10, 221–234. [Google Scholar] [CrossRef]
- Morris, J.S.; Friston, K.J.; Büchel, C.; Frith, C.D.; Young, A.W.; Calder, A.J.; Dolan, R.J. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain J. Neurol. 1998, 121, 47–57. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J. The emotional brain, fear, and the amygdala. Cell. Mol. Neurobiol. 2003, 23, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Tian, J.; Bai, L.; Pan, X.; Yang, L.; Chen, P.; Dai, J.; Ai, L.; Zhao, B.; Gong, Q. FMRI connectivity analysis of acupuncture effects on an amygdala-associated brain network. Mol. Pain 2008, 4, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napadow, V.; Kettner, N.; Liu, J.; Li, M.; Kwong, K.; Vangel, M.; Makris, N.; Audette, J.; Hui, K. Hypothalamus and amygdala response to acupuncture stimuli in carpal tunnel syndrome. Pain 2007, 130, 254–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Wang, Z.; Liu, J.; Chen, J.; Liu, X.; Nie, G.; Byun, J.-S.; Liang, Y.; Park, J.; Huang, R. Repeated acupuncture treatments modulate amygdala resting state functional connectivity of depressive patients. Neuroimage Clin. 2016, 12, 746–752. [Google Scholar] [CrossRef] [Green Version]
- Greenwald, J.D.; Shafritz, K.M. An Integrative Neuroscience Framework for the Treatment of Chronic Pain: From Cellular Alterations to Behavior. Front. Integr. Neurosci. 2018, 12, 18. [Google Scholar] [CrossRef]
- Madan, C.R.; Fujiwara, E.; Caplan, J.B.; Sommer, T. Emotional arousal impairs association-memory: Roles of amygdala and hippocampus. NeuroImage 2017, 156, 14–28. [Google Scholar] [CrossRef] [Green Version]
- Turk, D.C.; Wilson, H.D. Fear of Pain as a Prognostic Factor in Chronic Pain: Conceptual Models, Assessment, and Treatment Implications. Curr. Pain Headache Rep. 2010, 14, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Vlaeyen, J.W.; Linton, S.J. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain 2012, 153, 1144–1147. [Google Scholar] [CrossRef]
- Meier, M.L.; Staempfli, P.; Humphreys, B.K.; Vrana, A.; Seifritz, E.; Schweinhardt, P. The impact of pain-related fear on neural pathways of pain modulation in chronic low back pain. Pain Rep. 2017, 2, e601. [Google Scholar] [CrossRef]
- Mainero, C.; Boshyan, J.; Hadjikhani, N. Altered functional MRI resting-state connectivity in periaqueductal gray networks in migraine. Ann. Neurol. 2011, 70, 838. [Google Scholar] [CrossRef] [Green Version]
- Linnman, C.; Beucke, J.C.; Jensen, K.B.; Gollub, R.L.; Kong, J. Sex similarities and differences in pain-related periaqueductal gray connectivity. Pain 2012, 153, 444–454. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, T.A.; Ennis, M.; Behbehani, M.M.; Shipley, M.T. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: Topography and reciprocity. J. Comp. Neurol. 2010, 303, 121–131. [Google Scholar] [CrossRef]
- Connell, K.; Bolton, N.; Olsen, D.; Piomelli, D.; Hohmann, A.G. Role of the basolateral nucleus of the amygdala in endocannabinoid-mediated stress-induced analgesia. Neurosci. Lett. 2006, 397, 180–184. [Google Scholar] [CrossRef] [Green Version]
- Rea, K.; Olango, W.M.; Harhen, B.; Kerr, D.M.; Galligan, R.; Fitzgerald, S.; Moore, M.; Roche, M.; Finn, D.P. Evidence for a role of GABAergic and glutamatergic signalling in the basolateral amygdala in endocannabinoid-mediated fear-conditioned analgesia in rats. Pain 2013, 154, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Segerdahl, A.R.; Mezue, M.; Okell, T.W.; Farrar, J.T.; Tracey, I. The dorsal posterior insula subserves a fundamental role in human pain. Nat. Neurosci. 2015, 18, 499. [Google Scholar] [CrossRef]
- Apkarian, A.V.; Bushnell, M.C.; Treede, R.-D.; Zubieta, J.-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 2005, 9, 463–484. [Google Scholar] [CrossRef]
- Kucyi, A.; Moayedi, M.; Weissman-Fogel, I.; Goldberg, M.B.; Freeman, B.V.; Tenenbaum, H.C.; Davis, K.D. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J. Neurosci. 2014, 34, 3969–3975. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.; Tu, P.-C.; Zyloney, C.; Su, T.-P. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav. Brain Res. 2010, 211, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Linnman, C.; Moulton, E.A.; Barmettler, G.; Becerra, L.; Borsook, D. Neuroimaging of the periaqueductal gray: State of the field. Neuroimage 2012, 60, 505–522. [Google Scholar] [CrossRef] [Green Version]
- Cheriyan, J.; Sheets, P.L. Altered Excitability and Local Connectivity of mPFC-PAG Neurons in a Mouse Model of Neuropathic Pain. J. Neurosci. 2018, 38, 4829–4839. [Google Scholar] [CrossRef]
- Zhang, B.L.; Jung, M.Y.; Tu, Y.H.; Gollub, R.L.; Lang, C.A.; Ortiz, A.; Park, J.; Wilson, G.J.; Gerber, J.; Mawla, I.; et al. Identifying brain regions associated with the neuropathology of chronic low back pain: A resting-state amplitude of low-frequency fluctuation study. Br. J. Anaesth. 2019, 123, e303–e311. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, F.; Yin, T.; Lan, L.; Makris, N.; Jorgenson, K.; Guo, T.; Wu, F.; Gao, Y.; Dong, M. Acupuncture modulates the abnormal brainstem activity in migraine without aura patients. Neuroimage Clin. 2017, 15, 367–375. [Google Scholar] [CrossRef]
- Redgrave, P.; Gurney, K.J. What is reinforced by phasic dopamine signals? Brain Res. Rev. 2008, 58, 322–339. [Google Scholar] [CrossRef]
- Craig, A.D. A new view of pain as a homeostatic emotion. Trends Neurosci. 2003, 26, 303–307. [Google Scholar] [CrossRef]
- Denton, D.A.; Mckinley, M.J.; Farrell, M.; Egan, G.F. The role of primordial emotions in the evolutionary origin of consciousness. Conscious. Cogn. 2009, 18, 500–514. [Google Scholar] [CrossRef]
- Dennis, S.G.; Melzack, R. Effects of cholinergic and dopaminergic agents on pain and morphine analgesia measured by three pain tests. Exp. Neurol. 1983, 81, 167–176. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Liu, J.; Chen, J.; Liu, X.; Nie, G.; Jorgenson, K.; Sohn, K.C.; Huang, R.; Liu, M. Acupuncture treatment modulates the corticostriatal reward circuitry in major depressive disorder. J. Psychiatr. Res. 2017, 84, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.S.; Wallraven, C.; Kong, J.; Chang, D.S.; Lee, H.; Park, H.J.; Chae, Y. When pain is not only pain: Inserting needles into the body evokes distinct reward-related brain responses in the context of a treatment. Physiol. Behav. 2015, 140, 148–155. [Google Scholar] [CrossRef]
- Ridderinkhof, K.R.; Wp, V.D.W.; Segalowitz, S.J.; Carter, C.S. Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004, 56, 129–140. [Google Scholar] [CrossRef]
- Bryden, D.W.; Johnson, E.E.; Tobia, S.C.; Kashtelyan, V.; Roesch, M.R. Attention for learning signals in anterior cingulate cortex. J. Neurosci. 2011, 31, 18266. [Google Scholar] [CrossRef] [Green Version]
- Navratilova, E.; Atcherley, C.W.; Porreca, F. Brain Circuits Encoding Reward from Pain Relief. Trends Neurosci. 2015, 38, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Hashmi, J.A.; Baria, A.T.; Baliki, M.N.; Huang, L.; Schnitzer, T.J.; Apkarian, A.V. Brain networks predicting placebo analgesia in a clinical trial for chronic back pain. Pain 2012, 153, 2393–2402. [Google Scholar] [CrossRef]
- Borsook, D.; Becerra, L.R. Breaking down the barriers: fMRI applications in pain, analgesia and analgesics. Mol. Pain 2006, 2, 30. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.; Wolcott, E.; Wang, Z.; Jorgenson, K.; Harvey, W.F.; Tao, J.; Rones, R.; Wang, C. Altered resting state functional connectivity of the cognitive control network in fibromyalgia and the modulation effect of mind-body intervention. Brain Imaging Behav. 2019, 13, 492. [Google Scholar] [CrossRef]
- Beissner, F.; Nöth, U.; Schockert, T. The problem of metal needles in acupuncture-fMRI studies. Evid. Based Complementary Altern. Med. 2011, 2011, 808203. [Google Scholar] [CrossRef] [Green Version]



| Item | Real Acupuncture | Sham Acupuncture | Real vs. Sham | Augmented vs. Limited | ||||
|---|---|---|---|---|---|---|---|---|
| Augmented Real (12) | Limited Real (12) | Augmented Sham (13) | Limited Sham (13) | T/X2 | p | T/X2 | p | |
| Age | 43.00 (11.09) | 34.98 (13.16) | 40.02 (13.51) | 39.51 (14.40) | −2.71 | 0.787 | 1.05 | 0.225 |
| Female/male | 8/4 | 8/4 | 8/5 | 7/6 | 0.43 | 0.514 † | 0.09 | 0.771 |
| Beck Depression Inventory | 6.66 (6.05) | 12.18 (11.43) # | 6.69 (5.58) | 6.61 (5.09) | 1.41 | 0.16 | −1.21 | 0.23 |
| Baseline pain bothersomeness | 5.97 (1.60) | 6.23 (1.81) | 5.22 (1.78) | 5.33 (1.69) | 1.73 | 0.091 | −0.39 | 0.711 |
| Post-treatment pain bothersomeness | 3.59 (2.16) | 3.03 (2.59) | 3.60 (2.47) | 3.51 (2.51) | 0.37 | 0.715 | 0.47 | 0.692 |
| Change in pain bothersomeness | −2.38 (1.45) | −3.21 (2.45) | −1.62 (2.41) | −1.81 (2.26) | −1.75 | 0.043 * | 0.81 | 0.210 * |
| Seed | Contrast | Brain Regions | Cluster Size (Voxels) | MNI Coordinates (x, y, z) | Peak z-Value |
|---|---|---|---|---|---|
| VTA | Real > sham (post minus pre) | Bilateral ACC/mPFC * | 118 | −2, 28, −20 | 4.38 |
| Bilateral mPFC * | 51 | 2, 48, −12 | 4.09 | ||
| Left amygdala * | 19 | −24, −8, −18 | 3.25 | ||
| Sham > real (post minus pre) | Left SPL/AG | 119 | −22, −66, 62 | 4.84 | |
| Left precuneus/SPL | 156 | −8, −56, 64 | 3.71 | ||
| Left SPL/AG | 134 | −30, −50, 50 | 4.58 | ||
| Right anterior insula * | 39 | 38, 0, 6 | 3.49 | ||
| PAG | Real > sham (post minus pre) | RVM * | 83 | 6, −36, −48 | 3.82 |
| Right amygdala * | 33 | 20, −16, −14 | 3.72 | ||
| Left amygdala * | 28 | −24, −10, −18 | 3.72 | ||
| Sham > real (post minus pre) | Right precuneus/SPL | 190 | 14, −66, 40 | 4.19 | |
| Left insula * | 23 | −38, −14, 0 | 4.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Ortiz, A.; Gollub, R.L.; Wilson, G.; Gerber, J.; Park, J.; Huang, Y.; Shen, W.; Chan, S.-T.; Wasan, A.D.; et al. Acupuncture Treatment Modulates the Connectivity of Key Regions of the Descending Pain Modulation and Reward Systems in Patients with Chronic Low Back Pain. J. Clin. Med. 2020, 9, 1719. https://doi.org/10.3390/jcm9061719
Yu S, Ortiz A, Gollub RL, Wilson G, Gerber J, Park J, Huang Y, Shen W, Chan S-T, Wasan AD, et al. Acupuncture Treatment Modulates the Connectivity of Key Regions of the Descending Pain Modulation and Reward Systems in Patients with Chronic Low Back Pain. Journal of Clinical Medicine. 2020; 9(6):1719. https://doi.org/10.3390/jcm9061719
Chicago/Turabian StyleYu, Siyi, Ana Ortiz, Randy L. Gollub, Georgia Wilson, Jessica Gerber, Joel Park, Yiting Huang, Wei Shen, Suk-Tak Chan, Ajay D. Wasan, and et al. 2020. "Acupuncture Treatment Modulates the Connectivity of Key Regions of the Descending Pain Modulation and Reward Systems in Patients with Chronic Low Back Pain" Journal of Clinical Medicine 9, no. 6: 1719. https://doi.org/10.3390/jcm9061719





