Platelet-to-lymphocyte and Neutrophil-to-lymphocyte Ratios Predict Target Vessel Restenosis after Infrainguinal Angioplasty with Stent Implantation
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Blood Sampling
2.3. Measurement of Platelet, Neutrophil, Lymphocyte and Monocyte Count
2.4. Light transmission Aggregometry
2.5. Neutrophil-platelet Aggregate (NPA) Formation
2.6. Clinical Endpoints
2.7. Sample Size Calculation and Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Welten, G.M.; Schouten, O.; Hoeks, S.E.; Chonchol, M.; Vidakovic, R.; van Domburg, R.T.; Bax, J.J.; van Sambeek, M.R.; Poldermans, D. Long-Term prognosis of patients with peripheral arterial disease: A comparison in patients with coronary artery disease. J. Am. Coll. Cardiol. 2008, 51, 1588–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Bjorck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the diagnosis and treatment of Peripheral Arterial Diseases, in collaboration with the European society for vascular surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: The European stroke organization (ESO)The task force for the diagnosis and treatment of peripheral arterial diseases of the European society of cardiology (ESC) and of the European society for vascular surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gremmel, T.; Steiner, S.; Seidinger, D.; Koppensteiner, R.; Panzer, S.; Kopp, C.W. In vivo and protease-activated receptor-1-mediated platelet activation but not response to antiplatelet therapy predict two-year outcomes after peripheral angioplasty with stent implantation. Thromb. Haemost. 2014, 111, 474–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojkovic, S.; Jurisic, M.; Kopp, C.W.; Koppensteiner, R.; Huber, K.; Wojta, J.; Gremmel, T. Circulating microRNAs identify patients at increased risk of in-stent restenosis after peripheral angioplasty with stent implantation. Atherosclerosis 2018, 269, 197–203. [Google Scholar] [CrossRef]
- Gremmel, T.; Wadowski, P.P.; Mueller, M.; Kopp, C.W.; Koppensteiner, R.; Panzer, S. Serum cholinesterase levels are associated with 2-Year ischemic outcomes after angioplasty and stenting for peripheral artery disease. J. Endovasc. Ther. 2016, 23, 738–743. [Google Scholar] [CrossRef]
- Lee, S.; Koppensteiner, R.; Kopp, C.W.; Gremmel, T. alpha-Hydroxybutyrate dehydrogenase is associated with atherothrombotic events following infrainguinal angioplasty and stenting. Sci. Rep. 2019, 9, 18200. [Google Scholar] [CrossRef] [Green Version]
- Gary, T.; Pichler, M.; Belaj, K.; Eller, P.; Hafner, F.; Gerger, A.; Brodmann, M. Lymphocyte-to-monocyte ratio: A novel marker for critical limb ischemia in PAOD patients. Int. J. Clin. Pract. 2014, 68, 1483–1487. [Google Scholar] [CrossRef]
- Gary, T.; Pichler, M.; Belaj, K.; Hafner, F.; Gerger, A.; Froehlich, H.; Eller, P.; Pilger, E.; Brodmann, M. Neutrophil-to-lymphocyte ratio and its association with critical limb ischemia in PAOD patients. PLoS ONE 2013, 8, e56745. [Google Scholar] [CrossRef] [Green Version]
- Gary, T.; Pichler, M.; Belaj, K.; Hafner, F.; Gerger, A.; Froehlich, H.; Eller, P.; Rief, P.; Hackl, G.; Pilger, E.; et al. Platelet-to-lymphocyte ratio: A novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. PLoS ONE 2013, 8, e67688. [Google Scholar] [CrossRef] [Green Version]
- Falati, S.; Liu, Q.; Gross, P.; Merrill-Skoloff, G.; Chou, J.; Vandendries, E.; Celi, A.; Croce, K.; Furie, B.C.; Furie, B. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J. Exp. Med. 2003, 197, 1585–1598. [Google Scholar] [CrossRef]
- Gould, T.J.; Vu, T.; Swystun, L.L.; Dwivedi, D.; Mai, S.; Weitz, J.I.; Liaw, P.C. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1977–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horn, M.; Bertling, A.; Brodde, M.F.; Muller, A.; Roth, J.; van Aken, H.; Jurk, K.; Heilmann, C.; Peters, G.; Kehrel, B.E. Human neutrophil alpha-defensins induce formation of fibrinogen and thrombospondin-1 amyloid-like structures and activate platelets via glycoprotein IIb/IIIa. J. Thromb. Haemost. 2012, 10, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Mangold, A.; Alias, S.; Scherz, T.; Hofbauer, T.; Jakowitsch, J.; Panzenbock, A.; Simon, D.; Laimer, D.; Bangert, C.; Kammerlander, A.; et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ. Res. 2015, 116, 1182–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demyanets, S.; Stojkovic, S.; Mauracher, L.M.; Kopp, C.W.; Wojta, J.; Thaler, J.; Panzer, S.; Gremmel, T. Surrogate markers of neutrophil extracellular trap formation are associated with ischemic outcomes and platelet activation after peripheral angioplasty and stenting. J. Clin. Med. 2020, 9, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopp, C.W.; Gremmel, T.; Steiner, S.; Seidinger, D.; Minar, E.; Maurer, G.; Huber, K. Platelet-monocyte cross talk and tissue factor expression in stable angina vs. unstable angina/non ST-elevation myocardial infarction. Platelets 2011, 22, 530–536. [Google Scholar] [CrossRef]
- Gremmel, T.; Kopp, C.W.; Moertl, D.; Seidinger, D.; Koppensteiner, R.; Panzer, S.; Mannhalter, C.; Steiner, S. Influence of cytochrome 2C19 allelic variants on on-treatment platelet reactivity evaluated by five different platelet function tests. Thromb. Res. 2012, 129, 616–622. [Google Scholar] [CrossRef]
- Gremmel, T.; Calatzis, A.; Steiner, S.; Kaider, A.; Seidinger, D.; Koppensteiner, R.; Kopp, C.W.; Panzer, S. Is TRAP-6 suitable as a positive control for platelet reactivity when assessing response to clopidogrel? Platelets 2010, 21, 515–521. [Google Scholar] [CrossRef]
- Gremmel, T.; Durstberger, M.; Eichelberger, B.; Koppensteiner, R.; Kopp, C.W.; Panzer, S. Human neutrophil alpha-defensins are associated with adenosine diphosphate-inducible neutrophil-platelet aggregate formation and response to clopidogrel in patients with atherosclerosis. Transl. Res. 2014, 164, 202–208. [Google Scholar] [CrossRef]
- Gremmel, T.; Ay, C.; Riedl, J.; Kopp, C.W.; Eichelberger, B.; Koppensteiner, R.; Panzer, S. Platelet-specific markers are associated with monocyte-platelet aggregate formation and thrombin generation potential in advanced atherosclerosis. Thromb. Haemost. 2016, 115, 615–621. [Google Scholar] [CrossRef] [Green Version]
- Turak, O.; Ozcan, F.; Isleyen, A.; Tok, D.; Sokmen, E.; Buyukkaya, E.; Aydogdu, S.; Akpek, M.; Kaya, M.G. Usefulness of the neutrophil-to-lymphocyte ratio to predict bare-metal stent restenosis. Am. J. Cardiol. 2012, 110, 1405–1410. [Google Scholar] [CrossRef]
- Hartaigh, B.ó.; Bosch, J.A.; Thomas, G.N.; Lord, J.M.; Pilz, S.; Loerbroks, A.; Kleber, M.E.; Grammer, T.B.; Fischer, J.E.; Boehm, B.O.; et al. Which leukocyte subsets predict cardiovascular mortality? From the LUdwigshafen RIsk and Cardiovascular Health (LURIC) Study. Atherosclerosis 2012, 224, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamhane, U.U.; Aneja, S.; Montgomery, D.; Rogers, E.K.; Eagle, K.A.; Gurm, H.S. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am. J. Cardiol. 2008, 102, 653–657. [Google Scholar] [CrossRef]
- Tasoglu, I.; Cicek, O.F.; Lafci, G.; Kadirogullari, E.; Sert, D.E.; Demir, A.; Cavus, U.; Colak, N.; Songur, M.; Hodo, B. Usefulness of neutrophil/lymphocyte ratio as a predictor of amputation after embolectomy for acute limb ischemia. Ann. Vasc. Surg. 2014, 28, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Tasoglu, I.; Sert, D.; Colak, N.; Uzun, A.; Songur, M.; Ecevit, A. Neutrophil-lymphocyte ratio and the platelet-lymphocyte ratio predict the limb survival in critical limb ischemia. Clin. Appl. Thromb. Hemost. 2014, 20, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Mohri, Y.; Tanaka, K.; Ohi, M.; Yokoe, T.; Miki, C.; Kusunoki, M. Prognostic significance of host- and tumor-related factors in patients with gastric cancer. World J. Surg. 2010, 34, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.C.; Kim, S.H.; Oh, S.Y.; Lee, S.; Lee, J.H.; Choi, H.J.; Park, K.J.; Roh, M.S.; Kim, S.G.; Kim, H.J.; et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers 2012, 17, 216–222. [Google Scholar] [CrossRef]
- Bonaventura, A.; Montecucco, F.; Dallegri, F.; Carbone, F.; Luscher, T.F.; Camici, G.G.; Liberale, L. Novel findings in neutrophil biology and their impact on cardiovascular disease. Cardiovasc. Res. 2019, 115, 1266–1285. [Google Scholar] [CrossRef]
- Kaplan, M.J.; Radic, M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef] [Green Version]
- van Avondt, K.; Maegdefessel, L.; Soehnlein, O. Therapeutic targeting of neutrophil extracellular traps in atherogenic inflammation. Thromb. Haemost. 2019, 119, 542–552. [Google Scholar] [CrossRef] [Green Version]
- Pircher, J.; Engelmann, B.; Massberg, S.; Schulz, C. Platelet-Neutrophil crosstalk in atherothrombosis. Thromb. Haemost. 2019, 119, 1274–1282. [Google Scholar] [CrossRef]
- Gremmel, T.; Koppensteiner, R.; Kaider, A.; Eichelberger, B.; Mannhalter, C.; Panzer, S. Impact of variables of the P-selectin - P-selectin glycoprotein ligand-1 axis on leukocyte-platelet interactions in cardiovascular disease. Thromb. Haemost. 2015, 113, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.K.; Arthur, J.F.; Gardiner, E.E. Neutrophil extracellular traps (NETs) and the role of platelets in infection. Thromb. Haemost. 2014, 112, 659–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carestia, A.; Kaufman, T.; Schattner, M. Platelets: New bricks in the building of neutrophil extracellular traps. Front. Immunol. 2016, 7, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etulain, J.; Martinod, K.; Wong, S.L.; Cifuni, S.M.; Schattner, M.; Wagner, D.D. P-selectin promotes neutrophil extracellular trap formation in mice. Blood 2015, 126, 242–246. [Google Scholar] [CrossRef] [Green Version]
- Sreeramkumar, V.; Adrover, J.M.; Ballesteros, I.; Cuartero, M.I.; Rossaint, J.; Bilbao, I.; Nacher, M.; Pitaval, C.; Radovanovic, I.; Fukui, Y.; et al. Neutrophils scan for activated platelets to initiate inflammation. Science 2014, 346, 1234–1238. [Google Scholar] [CrossRef] [Green Version]
- Gawaz, M.; Langer, H.; May, A.E. Platelets in inflammation and atherogenesis. J. Clin. Investig. 2005, 115, 3378–3384. [Google Scholar] [CrossRef] [Green Version]
- Mammadova-Bach, E.; Ollivier, V.; Loyau, S.; Schaff, M.; Dumont, B.; Favier, R.; Freyburger, G.; Latger-Cannard, V.; Nieswandt, B.; Gachet, C.; et al. Platelet glycoprotein VI binds to polymerized fibrin and promotes thrombin generation. Blood 2015, 126, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Iso, Y.; Soda, T.; Sato, T.; Sato, R.; Kusuyama, T.; Omori, Y.; Shoji, M.; Koba, S.; Katagiri, T.; Kobayashi, Y.; et al. Impact of implanted bone marrow progenitor cell composition on limb salvage after cell implantation in patients with critical limb ischemia. Atherosclerosis 2010, 209, 167–172. [Google Scholar] [CrossRef]
- Stabile, E.; Kinnaird, T.; la Sala, A.; Hanson, S.K.; Watkins, C.; Campia, U.; Shou, M.; Zbinden, S.; Fuchs, S.; Kornfeld, H.; et al. CD8+ T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting CD4+ mononuclear cells through the expression of interleukin-16. Circulation 2006, 113, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Matetzky, S.; Shenkman, B.; Guetta, V.; Shechter, M.; Bienart, R.; Goldenberg, I.; Novikov, I.; Pres, H.; Savion, N.; Varon, D.; et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 2004, 109, 3171–3175. [Google Scholar] [CrossRef]
- Michelson, A.D.; Barnard, M.R.; Krueger, L.A.; Valeri, C.R.; Furman, M.I. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: Studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation 2001, 104, 1533–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badr Eslam, R.; Lang, I.M.; Koppensteiner, R.; Calatzis, A.; Panzer, S.; Gremmel, T. Residual platelet activation through protease-activated receptors (PAR)-1 and -4 in patients on P2Y12 inhibitors. Int. J. Cardiol. 2013, 168, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Wadowski, P.P.; Pultar, J.; Weikert, C.; Eichelberger, B.; Panzer, B.; Huber, K.; Lang, I.M.; Koppensteiner, R.; Panzer, S.; Gremmel, T. Protease-activated receptor-mediated platelet aggregation in acute coronary syndrome patients on potent P2Y12 inhibitors. Res. Pract. Thromb. Haemost. 2019, 3, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ollivier, V.; Roques, C.; Receveur, N.; Gratz, M.; Feldman, L.; Letourneur, D.; Gachet, C.; Mangin, P.H.; Jandrot-Perrus, M. Bioreactivity of stent material: Activation of platelets, coagulation, leukocytes and endothelial cell dysfunction in vitro. Platelets 2017, 28, 529–539. [Google Scholar] [CrossRef]

| Characteristics | Overall (n = 95) | noTVR (n = 63) | TVR (n = 32) | p |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 65 (58–74) | 64 (58–72) | 67 (60–79) | 0.14 |
| Male sex, n (%) | 57 (60) | 41 (65.1) | 16 (50) | 0.16 |
| Body mass index, kg/m2 | 26.5 (24.5–29.1) | 27.4 (24.8–29.4) | 25.5 (23.9–28.3) | 0.14 |
| Medical history | ||||
| Hypertension | 88 (92.6) | 58 (92.1) | 30 (93.8) | 1 |
| Hyperlipidemia | 89 (93.7) | 61 (96.8) | 28 (87.5) | 0.18 |
| Diabetes mellitus | 34 (35.8) | 19 (30.2) | 15 (46.9) | 0.11 |
| Active smoking | 42 (44.2) | 31 (49.2) | 11 (34.4) | 0.17 |
| Previous myocardial infarction | 18 (18.9) | 14 (22.2) | 4 (12.5) | 0.25 |
| Coronary artery disease | 31 (32.6) | 24 (38.1) | 7 (21.9) | 0.11 |
| Cerebrovascular disease | 22 (23.2) | 16 (25.4) | 6 (18.8) | 0.47 |
| Laboratory data | ||||
| Haemoglobin, g/dL | 13.7 (12.6–14.7) | 13.8 (12.6–14.9) | 13.5 (11.9–14.2) | 0.34 |
| Haematocrit, % | 40.5 (37.1–43.1) | 41.2 (37.5–43.8) | 39.3 (36.8–41.3) | 0.19 |
| White blood cell count, G/L | 8.9 (7–10.3) | 9.2 (6.8–10.3) | 8.7 (7.2–10.1) | 0.73 |
| Platelet count, G/L | 212 (183–250) | 210 (168–253) | 221 (203–249) | 0.14 |
| Serum creatinine, mg/dL | 1 (0.9–1.2) | 1 (0.9–1.1) | 1.1 (0.9–1.2) | 0.55 |
| High-sensitivity CRP, mg/dL | 1 (0.3–1.8) | 1.1 (0.4–1.8) | 0.8 (0.3–1.6) | 0.55 |
| Procedure | ||||
| Stent implantation | 95 (100) | 63 (100) | 32 (100) | 1 |
| Number of stents/patient | 2 (1–2) | 2 (1–2) | 2 (1–2) | 0.73 |
| Medication pre-intervention | ||||
| Clopidogrel | 95 (100) | 63 (100) | 32 (100) | 1 |
| Aspirin | 95 (100) | 63 (100) | 32 (100) | 1 |
| Statins | 86 (90.5) | 59 (93.7) | 27 (84.4) | 0.16 |
| ACE inhibitors/ARB | 81 (85.3) | 52 (82.5) | 29 (90.6) | 0.37 |
| Beta blockers | 58 (61.1) | 39 (61.9) | 19 (59.4) | 0.81 |
| Ratio | No TVR (n = 63) | TVR (n = 32) | p |
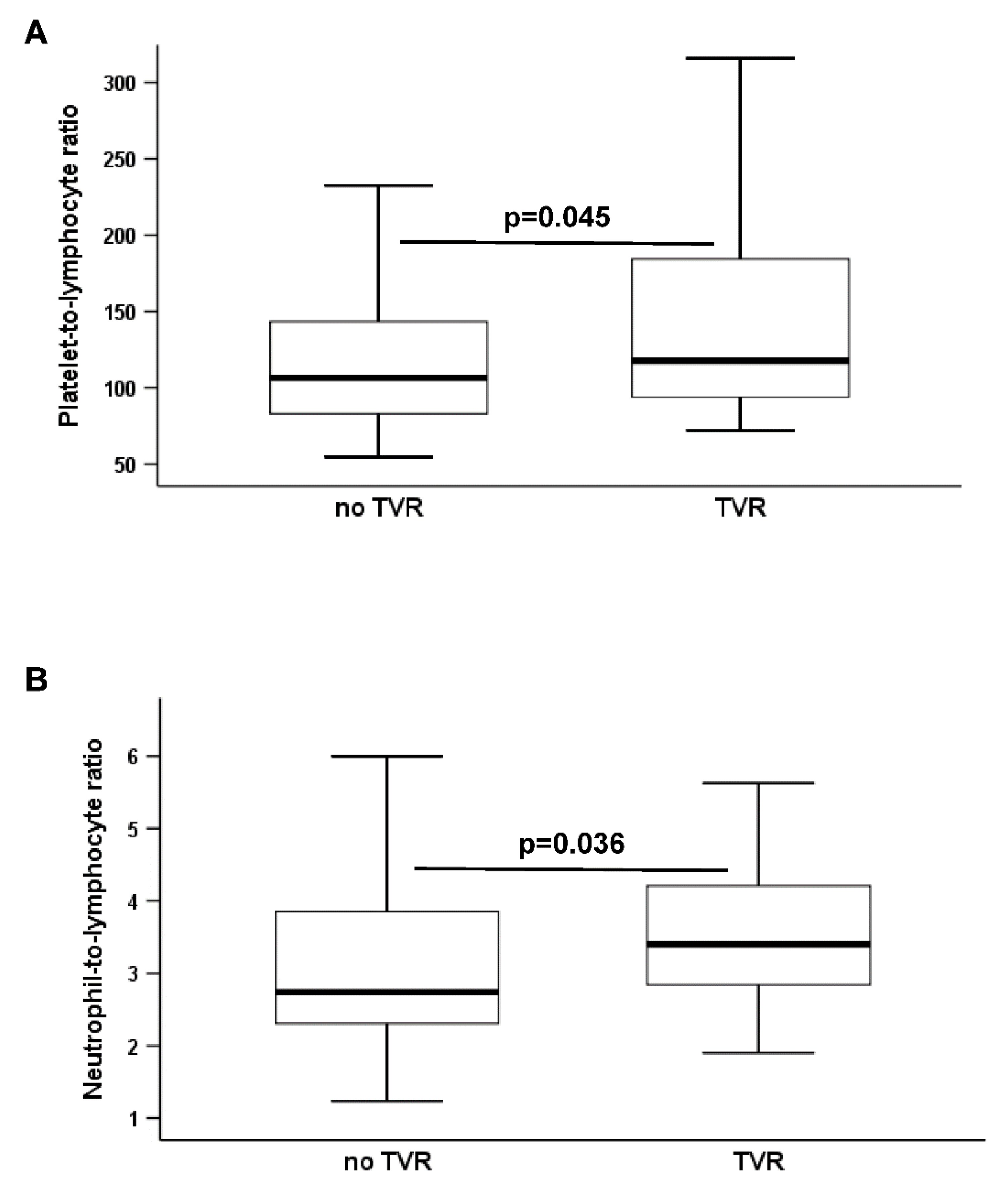
| PLR | 106.5 (82.7–146.8) | 117.7 (94–184.3) | 0.045 |
| NLR | 2.7 (2.3–4) | 3.4 (2.8–4.2) | 0.036 |
| LMR | 2.5 (1.8–3) | 2.8 (2–3.3) | 0.28 |
| Ratio | No AE (n = 88) | AE (n = 7) | p |
| PLR | 108.4 (89.1–155) | 150.6 (76.5–172.5) | 0.79 |
| NLR | 3.1 (2.4–4.2) | 2.8 (2.3–4) | 0.62 |
| LMR | 2.5 (1.8–3.1) | 3 (2–3.6) | 0.52 |
| (A) | |||
| Variable | Adjusted Hazard Ratio | 95% Confidence Interval | p-Value |
| High PLR | 3 | 1.1–8.5 | 0.04 |
| Diabetes | 1.7 | 0.8–3.5 | 0.16 |
| Hypertension | 0.6 | 0.1–2.7 | 0.53 |
| Hyperlipidemia | 0.4 | 0.1–2.3 | 0.12 |
| Smoking | 0.6 | 0.3–1.3 | 0.23 |
| (B) | |||
| Variable | Adjusted Hazard Ratio | 95% Confidence Interval | p-Value |
| High NLR | 3.1 | 1.3–7.7 | 0.01 |
| Diabetes | 1.5 | 0.7–3.1 | 0.27 |
| Hypertension | 0.7 | 0.2–3 | 0.62 |
| Hyperlipidemia | 0.5 | 0.2–1.5 | 0.23 |
| Smoking | 0.6 | 0.3–1.4 | 0.24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Hoberstorfer, T.; Wadowski, P.P.; Kopp, C.W.; Panzer, S.; Gremmel, T. Platelet-to-lymphocyte and Neutrophil-to-lymphocyte Ratios Predict Target Vessel Restenosis after Infrainguinal Angioplasty with Stent Implantation. J. Clin. Med. 2020, 9, 1729. https://doi.org/10.3390/jcm9061729
Lee S, Hoberstorfer T, Wadowski PP, Kopp CW, Panzer S, Gremmel T. Platelet-to-lymphocyte and Neutrophil-to-lymphocyte Ratios Predict Target Vessel Restenosis after Infrainguinal Angioplasty with Stent Implantation. Journal of Clinical Medicine. 2020; 9(6):1729. https://doi.org/10.3390/jcm9061729
Chicago/Turabian StyleLee, Silvia, Timothy Hoberstorfer, Patricia P. Wadowski, Christoph W. Kopp, Simon Panzer, and Thomas Gremmel. 2020. "Platelet-to-lymphocyte and Neutrophil-to-lymphocyte Ratios Predict Target Vessel Restenosis after Infrainguinal Angioplasty with Stent Implantation" Journal of Clinical Medicine 9, no. 6: 1729. https://doi.org/10.3390/jcm9061729





