Role of Advanced Glycation End Products on Aortic Calcification in Patients with Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Experimental Section
2.1. Subjects and Methods
2.2. Variables Outcomes
2.3. Serum Advanced Glycation End Products (AGEs) Determination
2.4. Lateral Lumbar Radiography of Abdominal Aorta
2.5. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
3.2. Laboratory Analysis Parameters
3.3. Abdominal Aortic Calcification and AGEs Levels
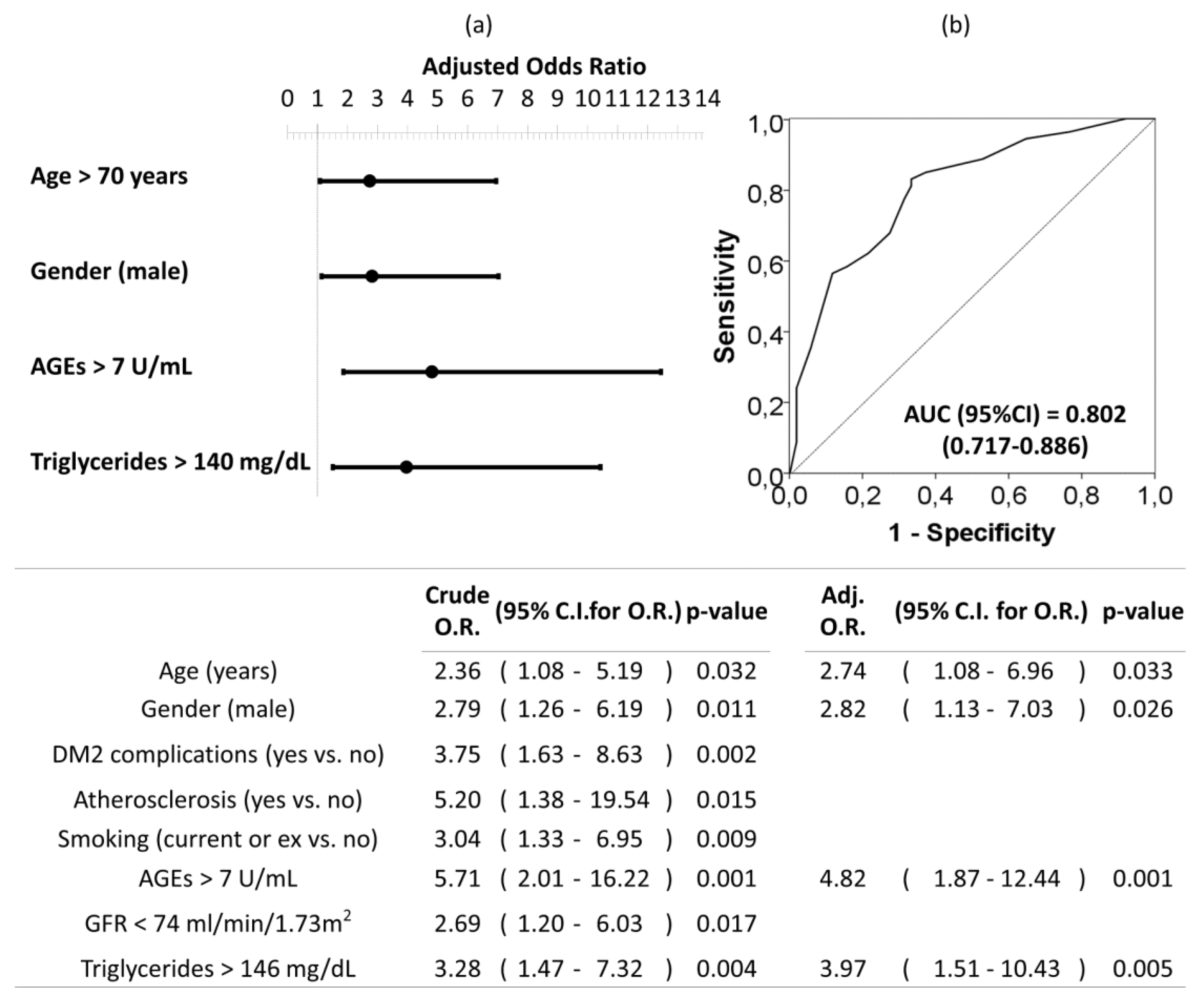
3.4. Cardiovascular (CV) Risk Factors Associated with Abdominal Aortic Calcification (AAC)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Hear. J. 2019, 41, 255–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendix, E.F.; Johansen, E.; Ringgaard, T.; Wolder, M.; Starup-Linde, J. Diabetes and Abdominal Aortic Calcification—A Systematic Review. Curr. Osteoporos. Rep. 2018, 16, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Kalofoutis, C.; Piperi, C.; Kalofoutis, A.; Harris, F.; Phoenix, D.; Singh, J. Type II diabetes mellitus and cardiovascular risk factors: Current therapeutic approaches. Exp. Clin. Cardiol. 2007, 12, 17–28. [Google Scholar] [PubMed]
- Gonçalves, F.B.; Voûte, M.; Hoeks, S.E.; Chonchol, M.B.; Boersma, E.E.; Stolker, R.J.; Verhagen, H.J.M. Calcification of the abdominal aorta as an independent predictor of cardiovascular events: A meta-analysis. Heart 2012, 98, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Jung, H.Y.; Park, H.C.; Oh, J.; Kim, J.; Lee, Y.-K. Relationship between abdominal aortic calcification on plain radiograph and coronary artery calcification detected by computed tomography in hemodialysis patients. Clin. Nephrol. 2020, 93, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cai, H.; Zhu, M.; Zhan, Y.; Che, M.; Lin, X.; Zhang, W.; Ni, Z. Association of abdominal aortic calcification estimated by plain radiography with outcomes in haemodialysis patients: A 6-year follow-up study. Nephrology 2019. [Google Scholar] [CrossRef]
- Oishi, H.; Horibe, H.; Yamase, Y.; Ueyama, C.; Takemoto, Y.; Shigeta, T.; Hibino, T.; Kondo, T.; Suzuki, S.; Ishii, H.; et al. Predictive value of abdominal aortic calcification index for mid-term cardiovascular events in patients with acute coronary syndrome. Hear. Vessel. 2019, 35, 620–629. [Google Scholar] [CrossRef]
- Schousboe, J.T.; Vo, T.N.; Langsetmo, L.; Adabag, S.; Szulc, P.; Lewis, J.R.; Kats, A.M.; Taylor, B.C.; Ensrud, K.E. Abdominal aortic calcification (AAC) and ankle-brachial index (ABI) predict health care costs and utilization in older men, independent of prevalent clinical cardiovascular disease and each other. Atherosclerosis 2020, 295, 31–37. [Google Scholar] [CrossRef]
- Giachelli, C.M. Vascular Calcification Mechanisms. J. Am. Soc. Nephrol. 2004, 15, 2959–2964. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Rementer, C.; Giachelli, C.M. Vascular calcification: An update on mechanisms and challenges in treatment. Calcif. Tissue Int. 2013, 93, 365–373. [Google Scholar] [CrossRef]
- Demer, L.L.; Tintut, Y. Vascular Calcification. Circulation 2008, 117, 2938–2948. [Google Scholar] [CrossRef] [PubMed]
- Pescatore, L.A.; Gamarra, L.F.; Liberman, M. Multifaceted Mechanisms of Vascular Calcification in Aging. Arter. Thromb. Vasc. Boil. 2019, 39, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Harper, E.; Forde, H.; Davenport, C.; Rochfort, K.; Smith, D.; Cummins, P.M. Vascular calcification in type-2 diabetes and cardiovascular disease: Integrative roles for OPG, RANKL and TRAIL. Vasc. Pharmacol. 2016, 82, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Schlieper, G.; Schurgers, L.; Brandenburg, V.; Reutelingsperger, C.; Floege, J. Vascular calcification in chronic kidney disease: An update. Nephrol. Dial. Transplant. 2015, 31, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.X.; Moe, S.M. Pathophysiology of Vascular Calcification. Curr. Osteoporos. Rep. 2015, 13, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Setiawati, R.; di Chio, F.; Rahardjo, P.; Nasuto, M.; Dimpudus, F.J.; Guglielmi, G.; Information, P.E.K.F.C. Quantitative Assessment of Abdominal Aortic Calcifications Using Lateral Lumbar Radiograph, Dual-Energy X-ray Absorptiometry, and Quantitative Computed Tomography of the Spine. J. Clin. Densitom. 2016, 19, 242–249. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Salvayre, R.; Augé, N.; Pamplona, R.; Portero-Otín, M. Hyperglycemia and Glycation in Diabetic Complications. Antioxid. Redox Signal. 2009, 11, 3071–3109. [Google Scholar] [CrossRef]
- Peppa, M.; Vlassara, H. Advanced glycation end products and diabetic complications: A general overview. Hormones 2006, 4, 28–37. [Google Scholar] [CrossRef]
- Brownlee, M.M. Advanced protein glycosylation in diabetes and aging. Annu. Rev. Med. 1995, 46, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Aronson, D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J. Hypertens. 2003, 21, 3–12. [Google Scholar] [CrossRef]
- Bierhaus, A.; Humpert, P.M.; Stern, D.M.; Arnold, B.; Nawroth, P.P. Advanced Glycation End Product Receptor?Mediated Cellular Dysfunction. Ann. N. Y. Acad. Sci. 2005, 1043, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.M.; Simpson, C.L.; Stewart, J.A. The Role of AGE/RAGE Signaling in Diabetes-Mediated Vascular Calcification. J. Diabetes Res. 2016, 2016, 6809703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taki, K.; Takayama, F.; Tsuruta, Y.; Niwa, T. Oxidative stress, advanced glycation product, and coronary artery calcification in hemodialysis patients. Kidney Int. 2006, 70, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Nasrallah, M.M.; El-Shehaby, A.R.; Osman, N.A.; Salem, M.M.; Nassef, A.; El Din, U.A.A.S. Erratum to: Endogenous soluble receptor of advanced glycation end-products (esRAGE) is negatively associated with vascular calcification in non-diabetic hemodialysis patients. Int. Urol. Nephrol. 2011, 44, 1201–1202. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Shao, H.; Wei, Q.; Sun, Z.; Liu, N. Advanced Glycation End-products Enhance Calcification in Vascular Smooth Muscle Cells. J. Int. Med Res. 2009, 37, 847–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanikawa, T.; Okada, Y.; Tanikawa, R.; Tanaka, Y. Advanced Glycation End Products Induce Calcification of Vascular Smooth Muscle Cells through RAGE/p38 MAPK. J. Vasc. Res. 2009, 46, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Suga, T.; Iso, T.; Shimizu, T.; Tanaka, T.; Yamagishi, S.-I.; Takeuchi, M.; Imaizumi, T.; Kurabayashi, M. Activation of receptor for advanced glycation end products induces osteogenic differentiation of vascular smooth muscle cells. J. Atheroscler. Thromb. 2011, 18, 670–683. [Google Scholar] [CrossRef] [Green Version]
- Gawdzik, J.; Mathew, L.; Kim, G.; Puri, T.S.; Bowman, M.H. Vascular remodeling and arterial calcification are directly mediated by S100A12 (EN-RAGE) in chronic kidney disease. Am. J. Nephrol. 2011, 33, 250–259. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Ma, W.-Q.; Han, X.-Q.; Wang, Y.; Wang, X.; Liu, N.-F. Advanced glycation end products accelerate calcification in VSMCs through HIF-1α/PDK4 activation and suppress glucose metabolism. Sci. Rep. 2018, 8, 13730. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [Green Version]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [CrossRef]
- Honkanen, E.; Kauppila, L.; Wikström, B.; Rensma, P.L.; Krzesinski, J.-M.; Aasarod, K.; Verbeke, F.; Jensen, P.B.; Mattelaer, P.; Volck, B.; et al. Abdominal aortic calcification in dialysis patients: Results of the CORD study. Nephrol. Dial. Transplant. 2008, 23, 4009–4015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schousboe, J.T.; Wilson, K.E.; Hangartner, T.N. Detection of Aortic Calcification during Vertebral Fracture Assessment (VFA) Compared to Digital Radiography. PLoS ONE 2007, 2, e715. [Google Scholar] [CrossRef] [PubMed]
- Saremi, A.; Howell, S.; Schwenke, D.C.; Bahn, G.; Beisswenger, P.J.; Reaven, P.D. Advanced Glycation End Products, Oxidation Products, and the Extent of Atherosclerosis During the VA Diabetes Trial and Follow-up Study. Diabetes Care 2017, 40, 591–598. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.C.; Woodward, M.; Neal, B.; Li, Q.; Pickering, R.; Marre, M.; Williams, B.; Perkovic, V.; Cooper, M.E.; Zoungas, S.; et al. Relationship Between Levels of Advanced Glycation End Products and Their Soluble Receptor and Adverse Outcomes in Adults With Type 2 Diabetes. Diabetes Care 2015, 38, 1891–1897. [Google Scholar] [CrossRef] [Green Version]
- Fishman, S.L.; Sonmez, H.; Basman, C.; Singh, V.; Poretsky, L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol. Med. 2018, 24, 59. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, I.U.; Díaz-Díaz, E.; Castro, J.S.; Ramos, J.P.; León, M.C.; Ríos, J.A.A.; Bautista, J.C.A.; Correa-Rotter, R.; Salinas, C.A.A.; Larrea, F. Circulating Concentrations of Advanced Glycation end Products, its Association With the Development of Diabetes Mellitus. Arch. Med Res. 2017, 48, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Wautier, M.P.; Massin, P.; Guillausseau, P.J.; Huijberts, M.; Levy, B.; Boulanger, E.; Laloi-Michelin, M.; Wautier, M. N(carboxymethyl)lysine as a biomarker for microvascular complications in type 2 diabetic patients. Diabetes Metab. 2003, 29, 44–52. [Google Scholar] [CrossRef]
- Boehm, B.O.; Schilling, S.; Rösinger, S.; Lang, G.; Lang, G.; Kientsch-Engel, R.; Stahl, P. Elevated serum levels of N?-carboxymethyl-lysine, an advanced glycation end product, are associated with proliferative diabetic retinopathy and macular oedema. Diabetology 2004, 47, 1376–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fosmark, D.S.; Torjesen, P.A.; Kilhovd, B.K.; Berg, T.J.; Sandvik, L.; Hanssen, K.F.; Agardh, C.-D.; Agardh, E. Increased serum levels of the specific advanced glycation end product methylglyoxal-derived hydroimidazolone are associated with retinopathy in patients with type 2 diabetes mellitus. Metaolism 2006, 55, 232–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Chen, L.-J.; Yu, J.; Wang, H.-J.; Zhang, F.; Liu, Q.; Wu, J. Involvement of Advanced Glycation End Products in the Pathogenesis of Diabetic Retinopathy. Cell. Physiol. Biochem. 2018, 48, 705–717. [Google Scholar] [CrossRef]
- Shimoike, T.; Inoguchi, T.; Umeda, F.; Nawata, H.; Kawano, K.; Ochi, H. The meaning of serum levels of advanced glycosylation end products in diabetic nephropathy. Metabolism 2000, 49, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Prevost, G.; Fajardy, I.; Besmond, C.; Balkau, B.; Tichet, J.; Fontaine, P.; Danze, P.M.; Marre, M.; DESIR Studies. Polymorphisms of the receptor of advanced glycation endproducts (RAGE) and the development of nephropathy in type 1 diabetic patients. Diabetes Metab. 2005, 31, 35–39. [Google Scholar] [CrossRef]
- Bucala, R.; Makita, Z.; Vega, G.; Grundy, S.; Koschinsky, T.; Cerami, A.; Vlassara, H. Modification of low-density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc. Natl. Acad. Sci. USA 1994, 91, 9441–9445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meerwaldt, R.; Links, T.P.; Zeebregts, C.; Tio, R.; Hillebrands, J.-L.; Smit, A.J. The clinical relevance of assessing advanced glycation endproducts accumulation in diabetes. Cardiovasc. Diabetol. 2008, 7, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawandi, S.; Gangawane, S.; Chakrabarti, A.; Kedare, S.; Bantwal, K.; Wadhe, V.; Kulkarni, A.; Kulkarni, S.; Rajan, M.G.R. A Study of Microalbuminuria (MAU) and Advanced Glycation End Products (AGEs) Levels in Diabetic and Hypertensive Subjects. Indian J. Clin. Biochem. 2017, 33, 81–85. [Google Scholar] [CrossRef]
- Singh, R.; Barden, A.; Mori, T.A.; Beilin, L. Advanced glycation end-products: A review. Diabetology 2001, 44, 129–146. [Google Scholar] [CrossRef] [Green Version]
- Brownlee, M. The pathological implications of protein glycation. Clin. Investig. Med. 1995, 18, 275–281. [Google Scholar]
- Sell, D.R.; Monnier, V.M. Molecular Basis of Arterial Stiffening: Role of Glycation? A Mini-Review. Gerontology 2012, 58, 227–237. [Google Scholar] [CrossRef]
- Kilhovd, B.K.; Berg, T.J.; Birkeland, K.I.; Thorsby, P.M.; Hanssen, K.F. Serum levels of advanced glycation end products are increased in patients with type 2 diabetes and coronary heart disease. Diabetes Care 1999, 22, 1543–1548. [Google Scholar] [CrossRef]
- Koska, J.; Saremi, A.; Howell, S.; Bahn, G.; De Courten, B.; Ginsberg, H.; Beisswenger, P.J.; Reaven, P.D.; For the VADT Investigators. Advanced Glycation End Products, Oxidation Products, and Incident Cardiovascular Events in Patients with Type 2 Diabetes. Diabetes Care 2017, 41, 570–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yozgatli, K.; Brouwer, T.; Jager, J.; Cabezas, M.C.; Lefrandt, J.D.; Noordzij, M.J.; Oomen, P.H.N.; Smit, A.J. Accumulation of advanced glycation end products is associated with macrovascular events and glycaemic control with microvascular complications in Type 2 diabetes mellitus. Diabet. Med. 2018, 35, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, M.M.; El-Shehaby, A.R.; Osman, N.A.; Salem, M.M.; Nassef, A.; El Din, U.A.A.S. Endogenous soluble receptor of advanced glycation end-products (esRAGE) is negatively associated with vascular calcification in non-diabetic hemodialysis patients. Int. Urol. Nephrol. 2011, 44, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Guerin-Dubourg, A.; Cournot, M.; Planesse, C.; Debussche, X.; Meilhac, O.; Rondeau, P.; Bourdon, E. Association between Fluorescent Advanced Glycation End-Products and Vascular Complications in Type 2 Diabetic Patients. BioMed Res. Int. 2017, 2017, 7989180. [Google Scholar] [CrossRef]
- Hangai, M.; Takebe, N.; Honma, H.; Sasaki, A.; Chida, A.; Nakano, R.; Togashi, H.; Nakagawa, R.; Oda, T.; Matsui, M.; et al. Association of Advanced Glycation End Products with coronary Artery Calcification in Japanese Subjects with Type 2 Diabetes as Assessed by Skin Autofluorescence. J. Atheroscler. Thromb. 2016, 23, 1178–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simm, A.; Wagner, J.; Gursinsky, T.; Nass, N.; Friedrich, I.; Schinzel, R.; Czeslik, E.; Silber, R.; Scheubel, R. Advanced glycation endproducts: A biomarker for age as an outcome predictor after cardiac surgery? Exp. Gerontol. 2007, 42, 668–675. [Google Scholar] [CrossRef]
- Okura, T.; Ueta, E.; Nakamura, R.; Fujioka, Y.; Sumi, K.; Matsumoto, K.; Shoji, K.; Matsuzawa, K.; Izawa, S.; Nomi, Y.; et al. High Serum Advanced Glycation End Products Are Associated with Decreased Insulin Secretion in Patients with Type 2 Diabetes: A Brief Report. J. Diabetes Res. 2017, 2017, 5139750. [Google Scholar] [CrossRef] [PubMed]
- Assiri, A.M.A.; Kamel, H.F.; Alrefai, A. Critical Appraisal of Advanced Glycation End Products (AGEs) and Circulating Soluble Receptors for Advanced Glycation End Products (sRAGE) as a Predictive Biomarkers for Cardiovascular Disease in Hemodialysis Patients. Med. Sci. 2018, 6, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Eupen, M.G.; Schram, M.T.; Colhoun, H.M.; Scheijen, J.L.; Stehouwer, C.D.; Schalkwijk, C.G. Plasma levels of advanced glycation end products are associated with type 1 diabetes and coronary artery calcification. Cardiovasc. Diabetol. 2013, 12, 149. [Google Scholar] [CrossRef] [Green Version]
- Saleh, J. Glycated hemoglobin, and its spinoffs: Cardiovascular disease markers or risk factors? World J. Cardiol. 2015, 7, 449–453. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.-G.; Wu, L.-T.; Kim, J.S.; Kim, E.-D.; Yoon, S.J. Relationship between Smoking and Abdominal Aorta Calcification on Computed Tomography. Korean J. Fam. Med. 2019, 40, 248–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kauppila, L.; Polak, J.F.; Cupples, L.A.; Hannan, M.T.; Kiel, D.P.; Wilson, P.W. New indices to classify location; severity and progression of calcific lesions inthe abdominal aorta: A 25-year follow-up study. Atherosclerosis 1997, 132, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Allison, M.; Criqui, M.H.; Wright, C.M. Patterns and Risk Factors for Systemic Calcified Atherosclerosis. Arter. Thromb. Vasc. Boil. 2004, 24, 331–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, P.T.; Coche, E.; Goffin, E.; Beguin, C.; Vlassenbroek, A.; Devuyst, O.; Robert, A.; Jadoul, M. Prevalence and Determinants of Coronary and Aortic Calcifications Assessed by Chest CT in Renal Transplant Recipients. Am. J. Nephrol. 2007, 27, 329–335. [Google Scholar] [CrossRef]
- Quispe, R.; Martin, S.S.; Jones, S.R. Triglycerides to high-density lipoprotein–cholesterol ratio, glycemic control, and cardiovascular risk in obese patients with type 2 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 150–156. [Google Scholar] [CrossRef]
- Egeland, G.M.; Igland, J.; Sulo, G.; Nygård, O.; Ebbing, M.; Tell, G.S. Non-fasting triglycerides predict incident acute myocardial infarction among those with favourable HDL-cholesterol: Cohort Norway. Eur. J. Prev. Cardiol. 2014, 22, 872–881. [Google Scholar] [CrossRef]
- Sarwar, N.; Danesh, J.; Eiriksdottir, G.; Sigurdsson, G.; Wareham, N.; Bingham, S.; Boekholdt, S.M.; Khaw, K.-T.; Gudnason, V. Triglycerides and the Risk of Coronary Heart Disease. Circulation 2007, 115, 450–458. [Google Scholar] [CrossRef]
- Nichols, G.A.; Philip, S.; Reynolds, K.; Granowitz, C.B.; Fazio, S. Increased Cardiovascular Risk in Hypertriglyceridemic Patients with Statin-Controlled LDL Cholesterol. J. Clin. Endocrinol. Metab. 2018, 103, 3019–3027. [Google Scholar] [CrossRef] [Green Version]
- Leopold, J.A. Vascular calcification: Mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc. Med. 2014, 25, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Brownlee, M. Biochemistry, and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Yamagishi, S.-I.; Matsui, T.; Yamagish, S.-I. Therapeutic Potential of DNA-aptamers Raised Against AGE-RAGE Axis in Diabetes-related Complications. Curr. Pharm. Des. 2018, 24, 2802–2809. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, P.; Rivera, R.; Berga, F.; Fortuny, R.; Adrover, M.; Costa-Bauzà, A.; Grases, F.; Masmiquel, L. Phytate Decreases Formation of Advanced Glycation End-Products in Patients with Type II Diabetes: Randomized Crossover Trial. Sci. Rep. 2018, 8, 9619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Palomeque, C.; Grau, A.; Perelló, J.; Sanchis, P.; Isern, B.; Prieto, R.M.; Costa-Bauzá, A.; Caldés, O.J.; Bonnin, O.; García-Raja, A.; et al. Relationship between Urinary Level of Phytate and Valvular Calcification in an Elderly Population: A Cross-Sectional Study. PLoS ONE 2015, 10, e0136560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchis, P.; Buades, J.M.; Berga, F.; Gelabert, M.M.; Molina, M.; Íñigo, M.V.; García, S.; Gonzalez, J.; Bernabeu, M.R.; Costa-Bauzá, A.; et al. Protective Effect of Myo-Inositol Hexaphosphate (Phytate) on Abdominal Aortic Calcification in Patients With Chronic Kidney Disease. J. Ren. Nutr. 2016, 26, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Grases, F. Phytate reduces age-related cardiovascular calcification. Front. Biosci. 2008, 13, 7115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekki, M.; Tahara, N.; Tahara, A.; Igata, S.; Honda, A.; Sugiyama, Y.; Nakamura, T.; Sun, J.; Kumashiro, Y.; Matsui, T.; et al. Switching Dipeptidyl Peptidase-4 Inhibitors to Tofogliflozin, a Selective Inhibitor of Sodium-Glucose Cotransporter 2 Improve Arterial Stiffness Evaluated by Cardio-Ankle Vascular Index in Patients with Type 2 Diabetes: A Pilot Study. Curr. Vasc. Pharmacol. 2019, 17, 411–420. [Google Scholar] [CrossRef]
- Vlassara, H. Advanced Glycation in Health and Disease: Role of the Modern Environment. Ann. N. Y. Acad. Sci. 2005, 1043, 452–460. [Google Scholar] [CrossRef]
- Yamagishi, S.-I.; Matsui, T. Pathologic role of dietary advanced glycation end products in cardiometabolic disorders, and therapeutic intervention. J. Nutr. 2016, 32, 157–165. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; Kauppila, L.I.; O’Donnell, C.J.; Kiel, D.P.; Hannan, M.; Polak, J.M.; Cupples, A. Abdominal Aortic Calcific Deposits Are an Important Predictor of Vascular Morbidity and Mortality. Circulation 2001, 103, 1529–1534. [Google Scholar] [CrossRef]



| Low AGEs | Intermediate AGEs | High AGEs | p-Value | |
|---|---|---|---|---|
| (< 6.5 U/mL) | (6.5–10.4 U/mL) | (> 10.4 U/mL) | ||
| (n = 35) | (n = 34) | (n = 35) | ||
| Age (years) | 67.5 ± 7.3 | 66.9 ± 6.9 | 73.7 ± 7.9 a,b | < 0.001 |
| Gender (male) | 12 (34.3%) | 17 (50.0%) | 21 (60.0%) | 0.095 |
| Body Mass Index (kg/m2) | 31.6 ± 4.8 | 31.1 ± 4.4 | 31.4 ± 8.1 | 0.689 |
| Waist circumference (cm) | 110 ± 10 | 110 ± 11 | 108 ± 12 | 0.466 |
| Systolic blood pressure (mmHg) | 145 ± 17 | 145 ± 21 | 147 ± 19 | 0.827 |
| Diastolic blood pressure (mmHg) | 77.4 ± 10.7 | 76.5 ± 9.8 | 75.1 ± 12 | 0.827 |
| Pulse pressure (mmHg) | 67.5 ± 16.1 | 68.8 ± 17.5 | 72.3 ± 15.8 | 0.533 |
| Heart rate (pulse/min) | 78.1 ± 12.6 | 77.9 ± 13.7 | 76.4 ± 14.2 | 0.830 |
| Time from diagnosis DM2 | ||||
| Less than 5 years | 4 (11.4%) | 2 (5.9%) | 3 (8.6%) | 0.726 |
| Between 5 and 10 years | 10 (28.6%) | 11 (32.4%) | 7 (20.0%) | |
| More than 5 years | 21 (60.0%) | 21 (61.8%) | 25 (71.4%) | |
| Comorbidities, toxics and clinical history | ||||
| Hypertension | 31 (88.6%) | 30 (88.2%) | 32 (91.4%) | 0.893 |
| Smoking | 9 (25.7%) | 17 (50.0%) | 14 (40.0%) | 0.114 |
| Alcohol | 5 (14.3%) | 5 (14.7%) | 3 (8.6%) | 0.688 |
| Prior cardiovascular disease | 15 (42.9%) | 13 (38.2%) | 20 (57.1%) | 0.258 |
| Chronic kidney disease (CKD) | 27 (77.1%) | 19 (55.9%) | 32 (91.4%) b | 0.003 |
| 2 (GFR 89–60 mL/min/1.73 m2) | 17 (48.6%) | 9 (26.5%) | 17 (48.6%) | 0.100 |
| 3a (GFR 59–45 mL/min/1.73 m2) | 5 (14.3%) | 5 (14.7%) | 8 (22.9%) | 0.566 |
| 3b (GFR 44–30 mL/min/1.73 m2) | 5 (14.3%) | 5 (14.7%) | 7 (20.0%) | 0.772 |
| Diabetic retinopathy | 9 (25.7%) | 13 (38.2%) | 14 (40.0%) | 0.393 |
| Ischemic heart disease | 4 (11.4%) | 5 (14.7%) | 9 (25.7%) | 0.255 |
| Cerebral stroke | 3 (8.6%) | 3 (8.8%) | 8 (22.9%) | 0.135 |
| Diabetic polyneuropathy | 7 (20.0%) | 6 (17.6%) | 11 (31.4%) | 0.135 |
| Diabetic foot | 1 (2.9%) | 1 (2.9%) | 3 (8.6%) | 0.442 |
| Intermittent claudication | 2 (5.7%) | 2 (5.9%) | 6 (17.1%) | 0.207 |
| Atherosclerosis | 3 (8.6%) | 2 (5.9%) | 11 (31.4%) a,b | 0.007 |
| Medication use | ||||
| Angiotensin-converting enzyme inhibitors/Angiotensin II receptor-blocking agents | 22 (62.9%) | 29 (85.3%) | 24 (68.6%) | 0.190 |
| Beta-blockers | 13 (37.1%) | 9 (26.5%) | 14 (40.0%) | 0.462 |
| Calcium antagonists | 12 (34.3%) | 9 (26.5%) | 20 (57.1%)b | 0.025 |
| Statins | 21 (60.0%) | 29 (85.3%) | 24 (68.6%) | 0.062 |
| Fibrates | 2 (5.7%) | 1 (2.9%) | 2 (5.7%) | 0.825 |
| Phosphate binders | 0 (0.0%) | 1 (2.9%) | 0 (0.0%) | 0.354 |
| 25-hydroxyvitamin D | 5 (14.3%) | 6 (17.6%) | 11 (31.4%) | 0.178 |
| Calcitriol or alphacalcidiol | 1 (2.9%) | 1 (2.9%) | 0 (0.0%) | 0.596 |
| Paricalcitol | 2 (5.7%) | 0 (0.0%) | 1 (2.9%) | 0.366 |
| Calcium supplement | 4 (11.4%) | 3 (8.8%) | 3 (8.6%) | 0.904 |
| Bisphosphonates | 3 (8.6%) | 0 (0.0%) | 2 (5.7%) | 0.239 |
| Steroids | 0 (0.0%) | 2 (5.9%) | 0 (0.0%) | 0.123 |
| Antiplatelets | 13 (37.1%) | 27 (79.4%) a | 22 (62.9%) a | 0.001 |
| Oral anticoagulants | 4 (11.4%) | 3 (8.8%) | 5 (14.3%) | 0.777 |
| Insulin | 19 (54.3%) | 17 (50.0%) | 20 (57.1%) | 0.836 |
| Oral antidiabetic | 30 (85.7%) | 30 (88.2%) | 26 (74.3%) | 0.262 |
| Uric acid medication (allopurinol or febuxostat) | 5 (14.3%) | 5 (14.7%) | 8 (22.9%) | 0.566 |
| Potassium citrate | 0 (0.0%) | 0 (0.0%) | 1 (2.9%) | 0.370 |
| Thiazides | 10 (28.6%) | 10 (29.4%) | 12 (34.3%) | 0.856 |
| Furosemide or triamterene | 8 (22.9%) | 9 (26.5%) | 11 (31.4%) | 0.719 |
| Spironolactone or eplerenone | 1 (2.9%) | 2 (5.9%) | 3 (8.6%) | 0.591 |
| Low AGEs | Intermediate AGEs | High AGEs | P-Value | |
|---|---|---|---|---|
| (<6.5 U/mL) | (6.5–10.4 U/mL) | (>10.4 U/mL) | ||
| (n = 35) | (n = 35) | (n = 35) | ||
| HbA1c (%) | 7.2 (6.0–8.1) | 7.2 (6.8–7.8) | 8.0 (7.0–8.5) a,b | 0.026 |
| Leukocytes (×109/L) | 7.9 (6.7–9.6) | 7.5 (6.3–9.2) | 7.5 (6.3–9.5) | 0.675 |
| Hemoglobin (g/dL) | 13.4 (12.5–14.5) | 13.2 (12.6–14.8) | 13.0 (11.4–13.8) a,b | 0.039 |
| Glucose (mg/dL) | 152.0 (125.0–194.0) | 148.5 (125.5–191.8) | 138.0 (110.0–230.0) | 0.847 |
| Urea (mg/dL) | 38.0 (30.0–52.0) | 41.0 (33.8–48.3) | 49.0 (37.0–66.0) a | 0.026 |
| Creatinine (mg/dL) | 0.8 (0.7–1.1) | 0.9 (0.7–1.2) | 1.1 (0.9–1.4) a,b | 0.018 |
| Urate (mg/dL) | 5.2 (4.5–6.2) | 5.4 (4.2–6.3) | 6.4 (4.8–7.4) a,b | 0.033 |
| Sodium (mg/L) | 140.8 (139.0–141.0) | 140.0 (138.7–141.2) | 140.0 (138.0–142.0) | 0.847 |
| Potassium (mg/L) | 4.5 (4.2–4.7) | 4.5 (4.3–4.9) | 4.7 (4.1–5.0) | 0.350 |
| Chloride (mg/L) | 104.7 (103.0–106.0) | 106.0 (102.8–107.2) | 104.8 (103.0–107.0) | 0.517 |
| Calcium (mg/dL) | 9.4 (9.1–9.9) | 9.5 (9.3–9.8) | 9.4 (9.2–9.6) | 0.714 |
| Magnesium (mg/dL) | 1.8 (1.7–1.9) | 1.8 (1.7–2.0) | 1.7 (1.7–1.9) | 0.326 |
| Phosphate (mg/dL) | 3.5 (3.3–3.8) | 3.6 (3.2–3.8) | 3.3 (3.1–3.7) | 0.274 |
| Total cholesterol (mg/dL) | 158.0 (138.0–180.0) | 154.0 (137.8–177.8) | 154.5 (141.0–179.0) | 0.991 |
| HDL cholesterol (mg/dL) | 42.5 (36.0–48.0) | 39.5 (31.0–46.8) | 38.0 (32.0–53.0) | 0.597 |
| LDL cholesterol mg/dL) | 93.0 (69.2–104.8) | 89.8 (66.3–101.6) | 84.9 (70.0–101.8) | 0.874 |
| Triglycerides (mg/dL) | 121.0 (84.0–191.0) | 143.5 (99.8–198.5) | 132.0 (103.0–203.0) | 0.354 |
| Albumin (g/dL) | 4.2 (4.0–4.4) | 4.1 (4.0–4.3) | 4.0 (3.8–4.2) | 0.106 |
| Alkaline phosphatase (u/L) | 78.0 (57.0–91.0) | 73.5 (58.5–93.3) | 81.5 (69.0–93.0) | 0.512 |
| PTHi (pg/mL) | 67.0 (48.9–99.6) | 73.0 (48.9–100.3) | 69.7 (51.8–100.3) | 0.993 |
| 25-hydroxyvitamin D (ng/mL) | 23.0 (17.0–37.2) | 26.8 (16.0–44.0) | 26.8 (17.9–33.3) | 0.670 |
| GFR (mL/min/1.73 m2) | 77.8 (55.7–88.9) | 83.1 (50.1–98.2) | 65.4 (46.0–79.1) a,b | 0.040 |
| Urinary creatinine (mg/dL) | 70.5 (44.9–89.7) | 68.7 (54.6–100.3) | 54.5 (36.5–88.1) | 0.115 |
| Urinary Alb/creat ratio (mg/g) | 9.5 (4.2–22.2) | 11.2 (5.5–98.6) | 24.5 (9.4–47.5) a | 0.017 |
| Urinary albumin (mg/dL) | 0.6 (0.5–3.3) | 0.8 (0.5–12.7) | 1.1 (0.5–7.7) | 0.069 |
| No-Mild AAC | Moderate-Severe AAC | p-Value | |
|---|---|---|---|
| (AAC < 6) | (AAC ≥ 6) | ||
| (n = 51) | (n = 53) | ||
| Age (years) | 67.3 ± 7.4 | 71.4 ± 8.1 | 0.011 |
| Sex (female) | 33 (64.7%) | 21 (39.6%) | 0.012 |
| Body Mass Index (kg/m2) | 31.6 ± 4.8 | 31.0 ± 7.0 | 0.174 |
| Waist circumference (cm) | 111 ± 10 | 108 ± 12 | 0.067 |
| Systolic blood pressure (mmHg) | 145 ± 17 | 147 ± 20 | 0.815 |
| Diastolic blood pressure (mmHg) | 77 ± 10 | 76 ± 11 | 0.951 |
| Pulse pressure (mmHg) | 69 ± 16 | 70 ± 17 | 0.682 |
| Heart rate (pulse/min) | 79 ± 12 | 76 ± 14 | 0.401 |
| Time from diagnosis of diabetes | 0.254 | ||
| Less than 5 years | 6 (11.8%) | 3 (5.7%) | |
| Between 5 and 10 years | 16 (31.4%) | 12 (22.6%) | |
| More than 5 years | 29 (56.9%) | 38 (71.7%) | |
| Diabetic complications (nº) | 0.002 | ||
| 1 | 10 (19.6%) | 16 (30.2%) | |
| 2 | 9 (17.6%) | 7 (13.2%) | |
| 3 or more | 4 (7.8%) | 17 (32.1%) | |
| Comorbidities, toxics and clinical history | |||
| Chronic kidney disease (stage 2 or upper) | 34 (66.7%) | 44 (86.3%) | 0.071 |
| Hypertension | 45 (88.2%) | 48 (94.1%) | 0.758 |
| Smoking (current or ex) | 13 (25.5%) | 27 (52.9%) | 0.009 |
| Alcohol (current or ex) | 8 (15.7%) | 5 (9.8%) | 0.386 |
| Prior cardiovascular disease | 20 (39.2%) | 28 (54.9%) | 0.175 |
| Diabetic retinopathy | 15 (29.4%) | 21 (39.6%) | 0.308 |
| Ischemic heart disease | 5 (9.8%) | 13 (24.5%) | 0.069 |
| Cerebral stroke | 5 (9.8%) | 9 (17.0%) | 0.391 |
| Diabetic polyneuropathy | 9 (17.6%) | 15 (28.3%) | 0.247 |
| Diabetic foot | 1 (2.0%) | 4 (7.5%) | 0.363 |
| Intermittent claudication | 2 (3.9%) | 8 (15.1%) | 0.930 |
| Atherosclerosis | 3 (5.9%) | 13 (24.5%) | 0.013 |
| Laboratory parameters | |||
| AGEs (U/mL) | 5.9 (4.5–10.1) | 9.7 (7.5–11.1) | 0.015 |
| HbA1c (%) | 7.2 (6.4–8.1) | 7.4 (6.9–8.4) | 0.171 |
| Leukocytes (×109/L) | 7.8 (6.7–9.3) | 7.5 (6.3–9.3) | 0.509 |
| Hemoglobin (g/dL) | 13.4 (12.5–14.5) | 13.0 (11.6–14.5) | 0.164 |
| Glucose (mg/dL) | 152 (125–180) | 139 (111–226) | 0.701 |
| Creatinine (mg/dL) | 0.8 (0.7–1.1) | 1.1 (0.8–1.4) | 0.004 |
| Urate (mg/dL) | 5.2 (4.5–6.0) | 6.2 (4.3–7.3) | 0.036 |
| Sodium (mg/L) | 140 (139–141) | 140 (138–142) | 0.536 |
| Potassium (mg/L) | 4.4 (4.2–4.7) | 4.7 (4.3–5.0) | 0.022 |
| Calcium (mg/dL) | 9.5 (9.2–9.8) | 9.4 (9.2–9.7) | 0.577 |
| Phosphate (mg/dL) | 3.5 (3.2–3.8) | 3.4 (3.1–3.8) | 0.320 |
| Total cholesterol (mg/dL) | 158 (134–180) | 154 (141–177) | 0.964 |
| HDL cholesterol (mg/dL) | 42.5 (36.0–49.0) | 38.0 (32.0–47.5) | 0.199 |
| LDL cholesterol mg/dL) | 93.0 (68.3–105.2) | 85.0 (67.8–100.9) | 0.599 |
| Triglycerides (mg/dL) | 121 (94–176) | 149 (103–203) | 0.048 |
| Albumin (g/dL) | 4.2 (4.0–4.4) | 4.0 (3.8–4.2) | 0.004 |
| Alkaline phosphatase (u/L) | 77 (57–91) | 82 (67–93) | 0.356 |
| PTHi (pg/mL) | 67.0 (48.9–94.6) | 74.2 (51.8–102.9) | 0.333 |
| 25-hydroxyvitamin D (ng/mL) | 23.0 (16.3–41.0) | 26.8 (18.0–34.0) | 0.547 |
| GFR (mL/min/sup) | 79.2 (57.4–95.3) | 68.0 (47.1–82.3) | 0.024 |
| Urinary creatinine (mg/dL) | 70.5 (52.4–89.7) | 60.3 (39.2–89.7) | 0.184 |
| Urinary Alb/creat ratio (mg/g) | 10 (5–29) | 25 (9–149) | 0.004 |
| Urinary albumin (mg/dL) | 0.6 (0.5–3.6) | 1.1 (0.5–17.1) | 0.012 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchis, P.; Rivera, R.; Fortuny, R.; Río, C.; Mas-Gelabert, M.; Gonzalez-Freire, M.; Grases, F.; Masmiquel, L. Role of Advanced Glycation End Products on Aortic Calcification in Patients with Type 2 Diabetes Mellitus. J. Clin. Med. 2020, 9, 1751. https://doi.org/10.3390/jcm9061751
Sanchis P, Rivera R, Fortuny R, Río C, Mas-Gelabert M, Gonzalez-Freire M, Grases F, Masmiquel L. Role of Advanced Glycation End Products on Aortic Calcification in Patients with Type 2 Diabetes Mellitus. Journal of Clinical Medicine. 2020; 9(6):1751. https://doi.org/10.3390/jcm9061751
Chicago/Turabian StyleSanchis, Pilar, Rosmeri Rivera, Regina Fortuny, Carlos Río, Miguel Mas-Gelabert, Marta Gonzalez-Freire, Felix Grases, and Luis Masmiquel. 2020. "Role of Advanced Glycation End Products on Aortic Calcification in Patients with Type 2 Diabetes Mellitus" Journal of Clinical Medicine 9, no. 6: 1751. https://doi.org/10.3390/jcm9061751
APA StyleSanchis, P., Rivera, R., Fortuny, R., Río, C., Mas-Gelabert, M., Gonzalez-Freire, M., Grases, F., & Masmiquel, L. (2020). Role of Advanced Glycation End Products on Aortic Calcification in Patients with Type 2 Diabetes Mellitus. Journal of Clinical Medicine, 9(6), 1751. https://doi.org/10.3390/jcm9061751





